Abstract
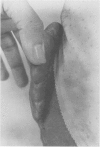
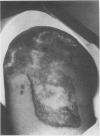
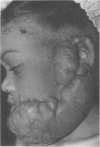
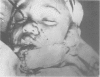
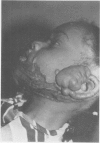
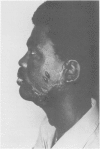
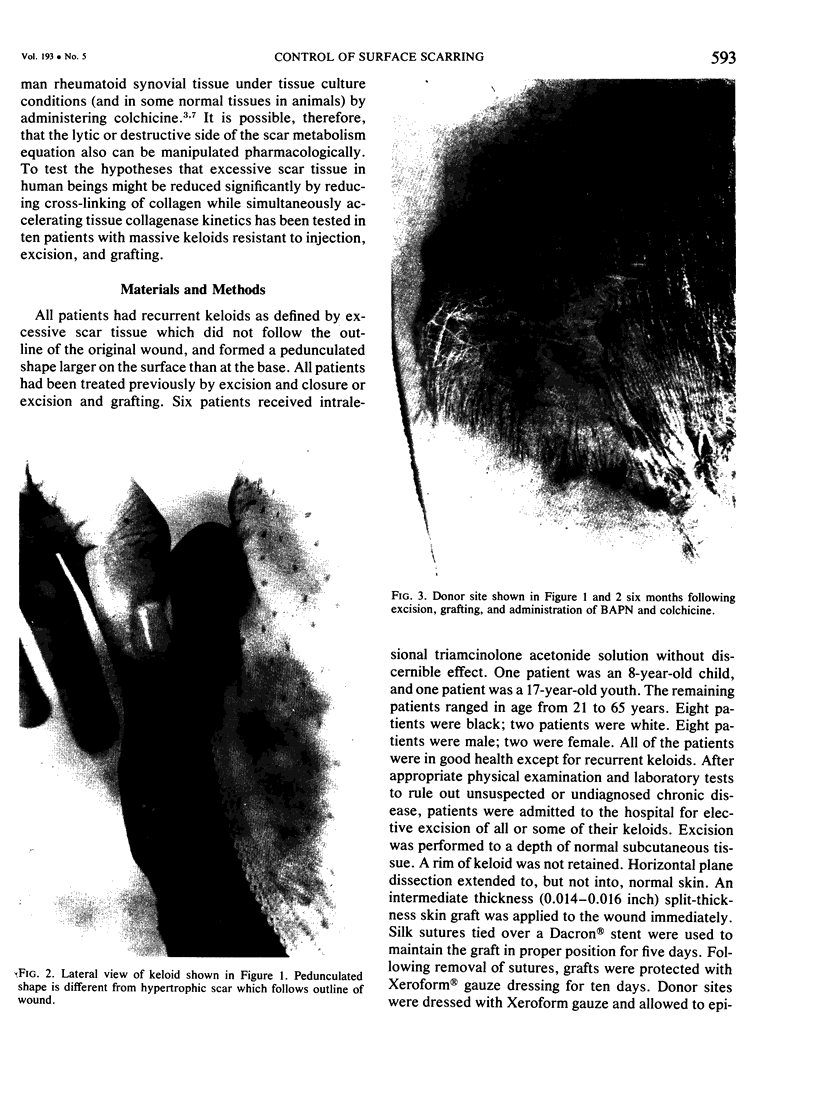
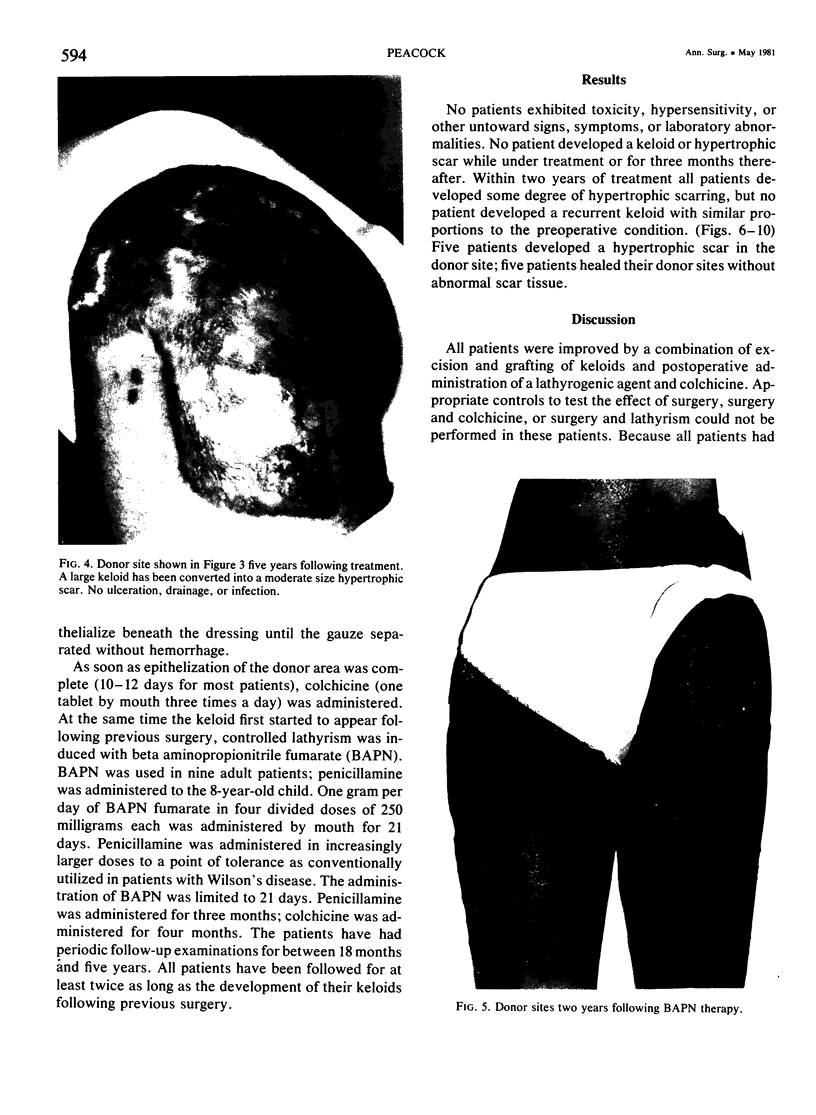
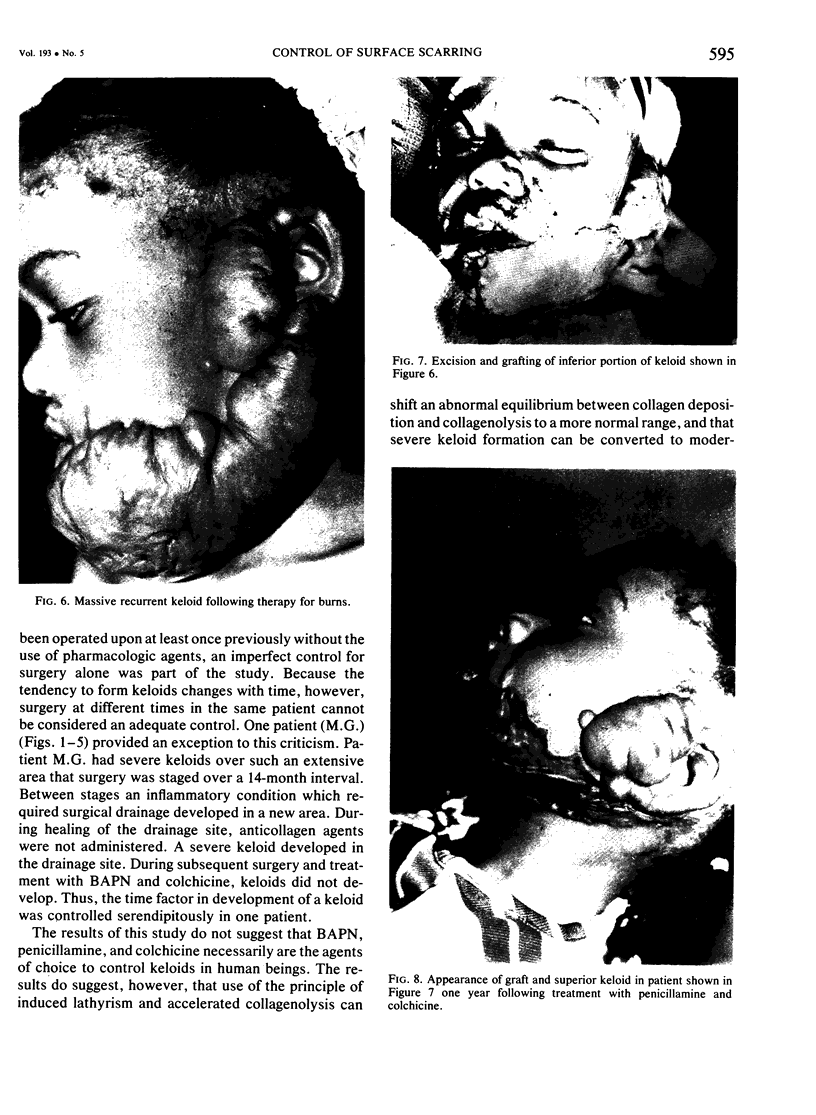
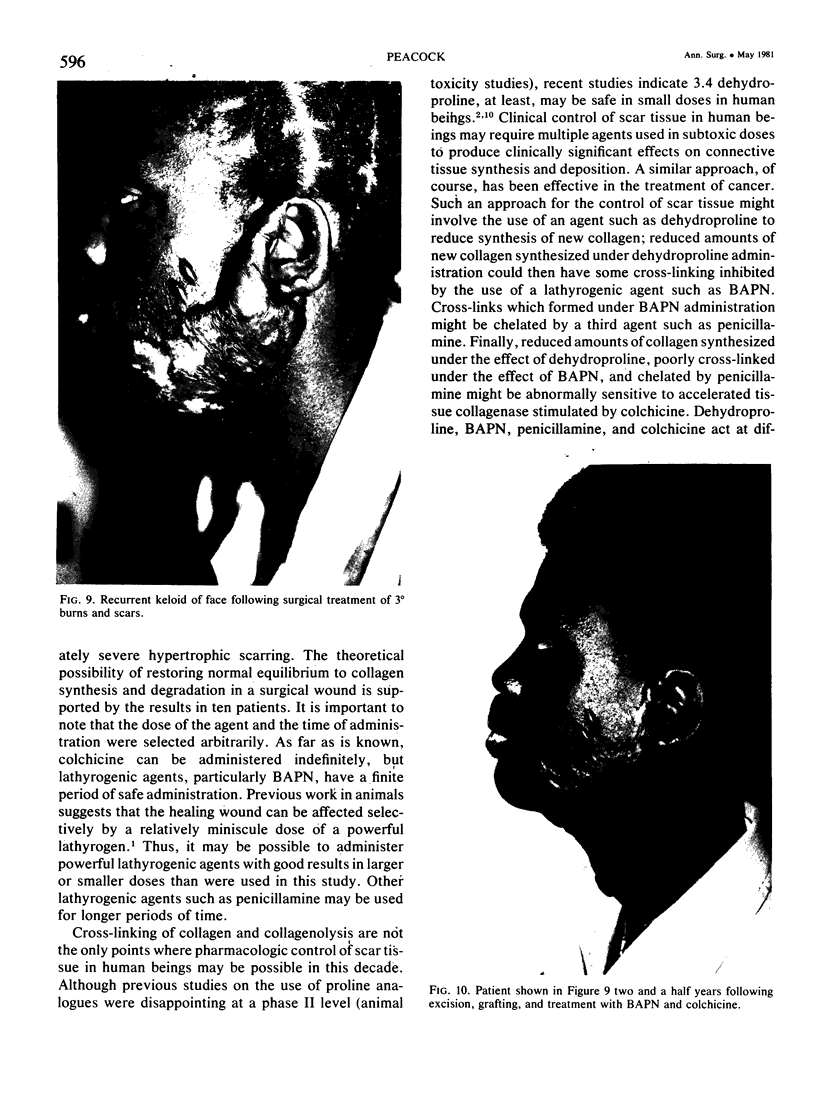
A hypothetical basis for control of surface scar in human beings is: lathyrism produces poorly cross-linked collagen in healing wounds; poorly corss-linked collagen is more susceptible to digestion by tissue collagenase than is normally cross-linked collagen; and colchicine stimulates tissue collagenase activity. Therefore, treatment of patients with abnormal deposits of surface scar by excising the scar, inducing lathyrism, and administering colchicine should tend to correct abnormal balance between collagen synthesis and collagenolysis and result in a small scar with improved physical properties. Ten patients with massive keloids, resistant to conventional therapy by excision, grafting, and/or intralesional injection of steroids, have been treated by excising the keloid, grafting the defect, inducing lathyrism with Beta aminopropionitrile fumurate or penicillamine and administering colchicine. Patients were followed for 18 months to five years. No toxicity or untoward side effects from therapy were observed. No patients developed recurrent keloids while undergoing treatment. All patients showed some change in the amount of scar which persisted during the period of study. This data supports the hypothesis that lathyrism and colchicine therapy exert a measurable beneficial effect on surface scar in human beings.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arem A. J., Misiorowski R., Chvapil M. Effects of low-dose BAPN on wound healing. J Surg Res. 1979 Oct;27(4):228–232. doi: 10.1016/0022-4804(79)90134-3. [DOI] [PubMed] [Google Scholar]
- Chvapil M., Madden J. W., Carlson E. C., Peacock E. E., Jr Effect of cis-hydroxyproline on collagen and other proteins in skin wounds, granuloma tissue, and liver of mice and rats. Exp Mol Pathol. 1974 Jun;20(3):363–373. doi: 10.1016/0014-4800(74)90066-5. [DOI] [PubMed] [Google Scholar]
- Cohen I. K., Keiser H. R., Sjoerdsma A. Collagen synthesis in human keloid and hypertrophic scar. Surg Forum. 1971;22:488–489. [PubMed] [Google Scholar]
- Cohen I. K., McCoy B. J. The biology and control of surface overhealing. World J Surg. 1980 May;4(3):289–295. doi: 10.1007/BF02393384. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Farrell M. E. Resistance to collagenase: a characteristic of collagen fibrils cross-linked by formaldehyde. Biochim Biophys Acta. 1972 Aug 31;278(1):133–141. doi: 10.1016/0005-2795(72)90114-6. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Effects of colchicine on collagenase in cultures of rheumatoid synovium. Arthritis Rheum. 1971 Nov-Dec;14(6):669–684. doi: 10.1002/art.1780140602. [DOI] [PubMed] [Google Scholar]
- Peacock E. E., Jr, Madden J. W., Trier W. C. Biologic basis for the treatment of keloids and hypertrophic scars. South Med J. 1970 Jul;63(7):755–760. doi: 10.1097/00007611-197007000-00002. [DOI] [PubMed] [Google Scholar]
- Salvador R. A., Tsai I., Marcel R. J., Felix A. M., Kerwar S. S. The in vivo inhibition of collagen synthesis and the reduction of prolyl hydroxylase activity by 3,4-dehydroproline. Arch Biochem Biophys. 1976 Jun;174(2):381–392. doi: 10.1016/0003-9861(76)90366-0. [DOI] [PubMed] [Google Scholar]