Abstract
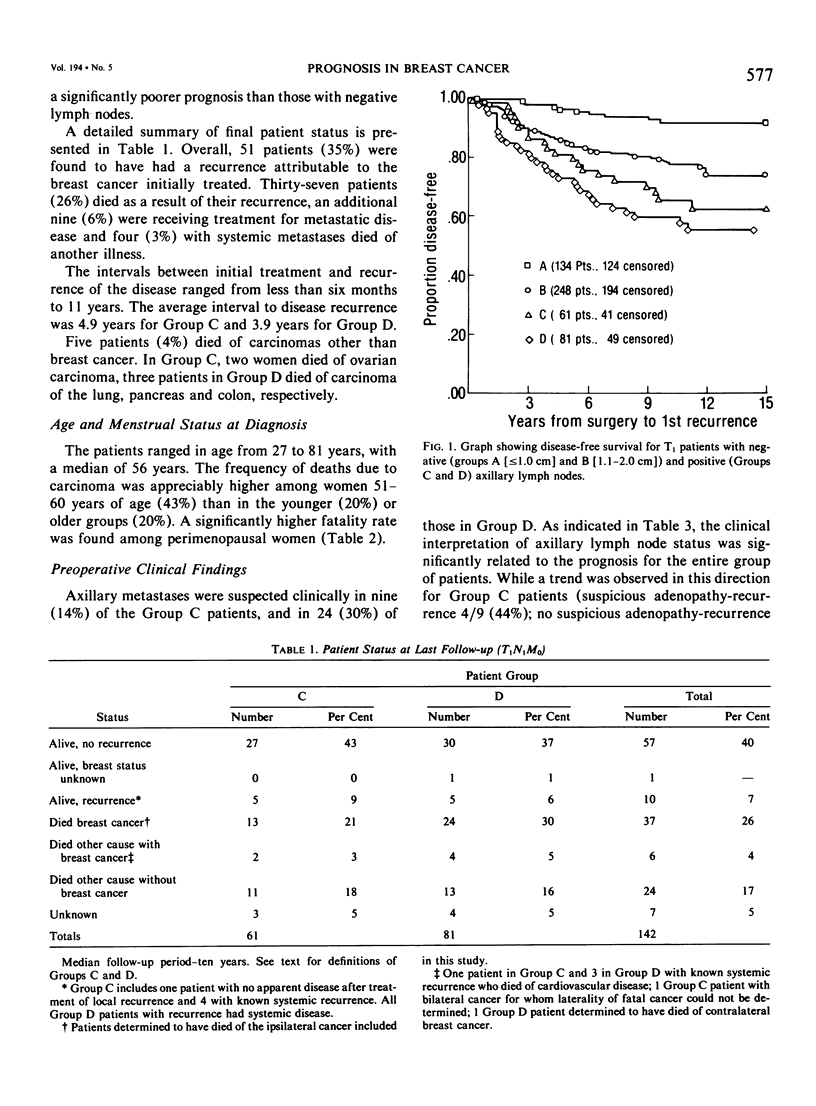
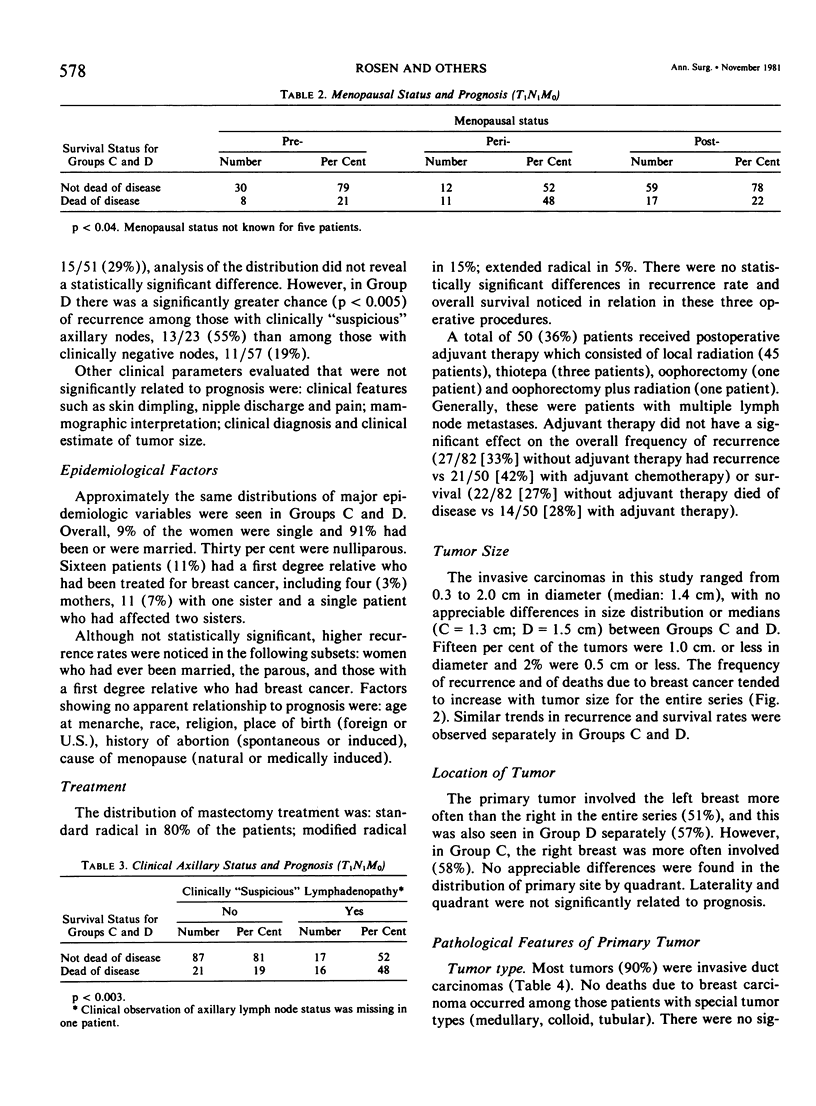
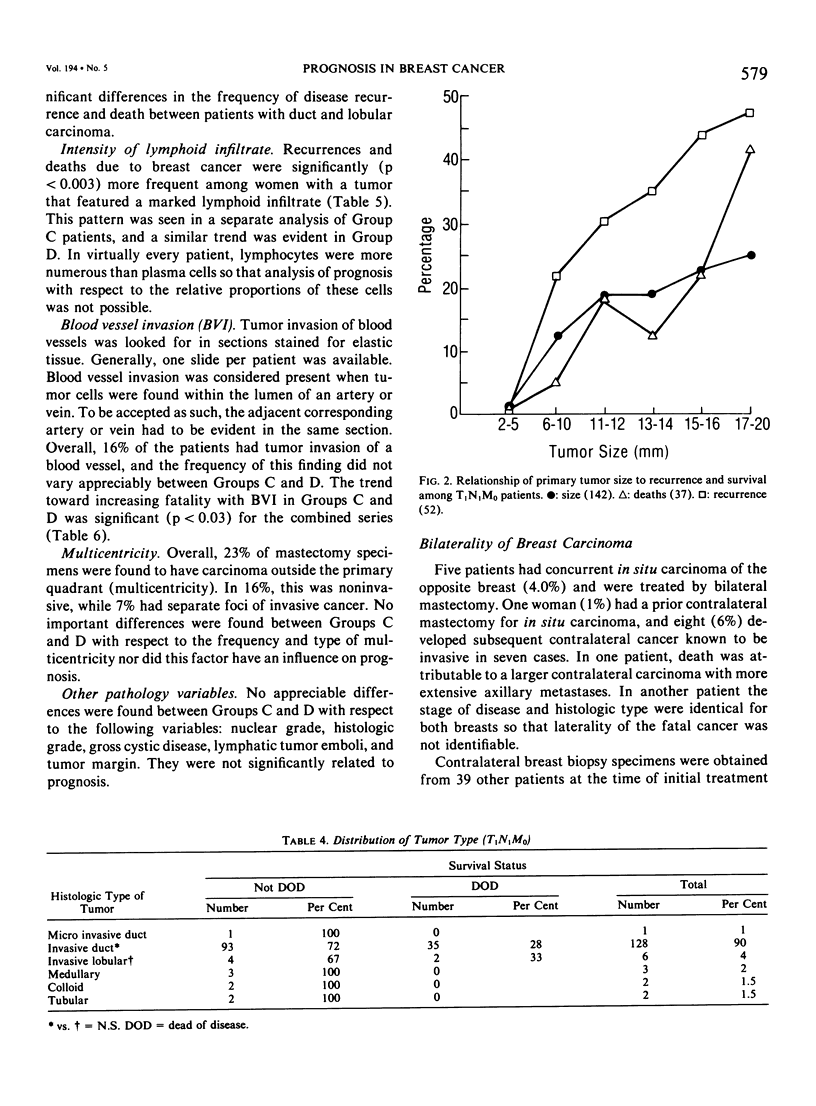
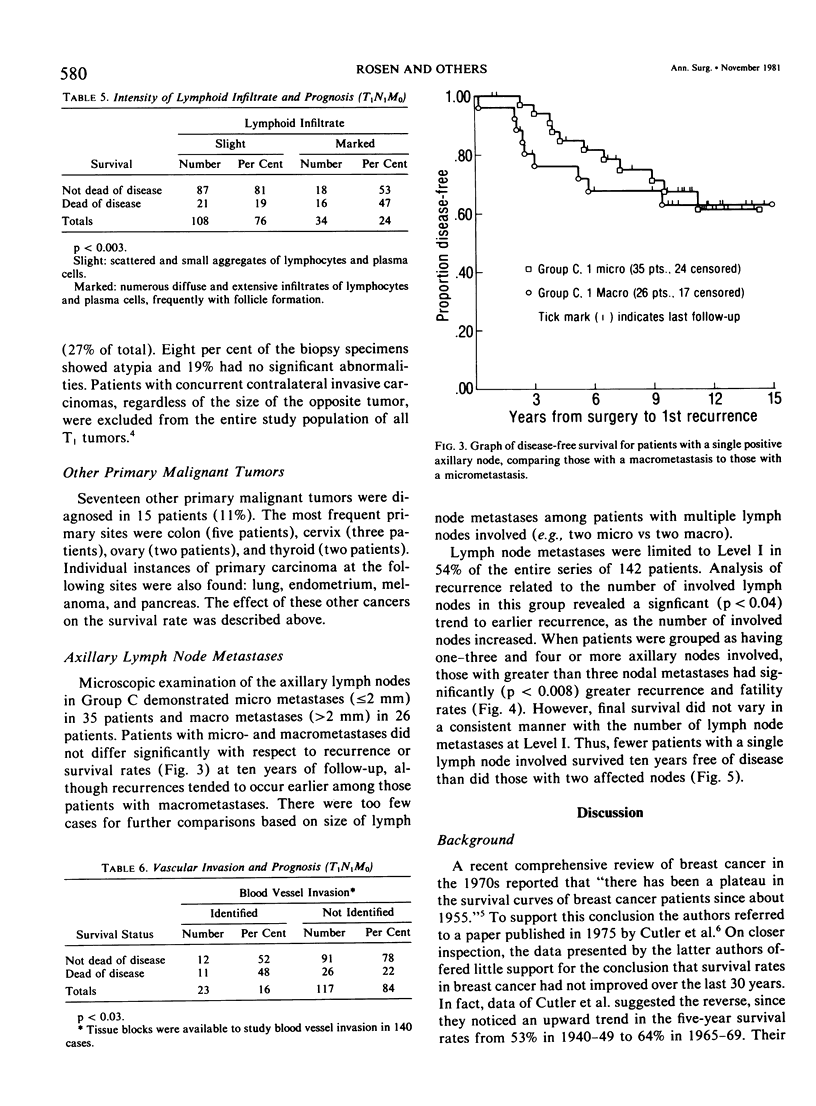
As part of a detailed study of prognostic factors in breast cancer, we have analyzed the ten year survival rates of 524 patients with primary invasive carcinomas 2.0 cm or less in diameter (T1). This report describes the subset of 142 patients (27%) who had metastases only in axillary lymph nodes (T1N1M0). All the patients were treated initially by at least a modified radical mastectomy. Factors associated with a significantly poorer prognosis were: axillary lymph node metastases suspected on clinical examination; perimenopausal menstrual status at diagnosis; tumor larger than 1.0 cm; prominent lymphoid reaction; infiltrating duct or lobular rather than medullary, colloid and tubular carcinoma; and blood vessel invasion. When compared with those patients with negative nodes (T1N0M0), the patients with one or more lymph node metastases had a significantly poorer prognosis. Generally, survival rates tended to diminish as the number of involved lymph nodes increased. In this respect, comparison of patients with one-three and four or more nodal metastases provided a significant discrimination of prognostic groups in the entire series. However, for patients with disease limited to Level I, the same discrimination was obtained comparing those with one-two and three or more positive nodes. In the subset with a single lymph node metastasis, the size of the metastasis (micro or less than or equal to 2 mm vs macro or greater than 2 mm) was not significantly related to prognosis. Lymph node metastases were significantly less frequent among tumors smaller than 1 cm and special tumor types (medullary, colloid, lobular and tubular). However, no factor proved to be a reliable predictor of the presence of axillary metastases for the single largest group consisting of patients with infiltrating duct carcinoma 1-2 cm in diameter.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attiyeh F. F., Jensen M., Huvos A. G., Fracchia A. Axillary micrometastasis and macrometastasis in carcinoma of the breast. Surg Gynecol Obstet. 1977 Jun;144(6):839–842. [PubMed] [Google Scholar]
- Brian D. D., Melton L. J., 3rd, Goellner J. R., Williams R. L., O'Fallon W. M. Breast cancer incidence, prevalence, mortality, and survivorship in Rochester, Minnesota, 1935 to 1974. Mayo Clin Proc. 1980 Jun;55(6):355–359. [PubMed] [Google Scholar]
- Champion H. R., Wallace I. W., Prescott R. J. Histology in breast cancer prognosis. Br J Cancer. 1972 Apr;26(2):129–138. doi: 10.1038/bjc.1972.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S. J., Myers M. H., Green S. B. Trends in survival rates of patients with cancer. N Engl J Med. 1975 Jul 17;293(3):122–124. doi: 10.1056/NEJM197507172930305. [DOI] [PubMed] [Google Scholar]
- Fisher B., Slack N. H., Bross I. D. Cancer of the breast: size of neoplasm and prognosis. Cancer. 1969 Nov;24(5):1071–1080. doi: 10.1002/1097-0142(196911)24:5<1071::aid-cncr2820240533>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Fracchia A. A., Rosen P. P., Ashikari R. Primary carcinoma of the breast without axillary lymph node metastases. Surg Gynecol Obstet. 1980 Sep;151(3):375–378. [PubMed] [Google Scholar]
- Henderson I. C., Canellos G. P. Cancer of the breast: the past decade (first of two parts). N Engl J Med. 1980 Jan 3;302(1):17–30. doi: 10.1056/NEJM198001033020104. [DOI] [PubMed] [Google Scholar]
- Huvos A. G., Hutter R. V., Berg J. W. Significance of axillary macrometastases and micrometastases in mammary cancer. Ann Surg. 1971 Jan;173(1):44–46. doi: 10.1097/00000658-197101000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Identification of breast cancer patients with high risk of early recurrence after radical mastectomy. II. Clinical and pathological correlations. A report of the Primary Therapy of Breast Cancer Study Group. Cancer. 1978 Dec;42(6):2809–2826. doi: 10.1002/1097-0142(197812)42:6<2809::aid-cncr2820420642>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Kister S. J., Sommers S. C., Haagensen C. D., Cooley E. Re-evaluation of blood-vessel invasion as a prognostic factor in carcinoma of the breast. Cancer. 1966 Sep;19(9):1213–1216. doi: 10.1002/1097-0142(196609)19:9<1213::aid-cncr2820190906>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lesser M. L., Rosen P. P., Senie R. T., Duthie K., Menendez-Botet C., Schwartz M. K. Estrogen and progesterone receptors in breast carcinoma: correlations with epidemiology and pathology. Cancer. 1981 Jul 15;48(2):299–309. doi: 10.1002/1097-0142(19810715)48:2<299::aid-cncr2820480215>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Nemoto T., Vana J., Bedwani R. N., Baker H. W., McGregor F. H., Murphy G. P. Management and survival of female breast cancer: results of a national survey by the American College of Surgeons. Cancer. 1980 Jun 15;45(12):2917–2924. doi: 10.1002/1097-0142(19800615)45:12<2917::aid-cncr2820451203>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- PICKREN J. W. Significance of occult metastases. A study of breast cancer. Cancer. 1961 Nov-Dec;14:1266–1271. doi: 10.1002/1097-0142(196111/12)14:6<1266::aid-cncr2820140617>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Payne W. S., Taylor W. F., Khonsari S., Snider J. H., Harrison E. G., Jr, Golenzer H., Clagett O. T. Surgical treatment of breast cancer. Trends and factors affecting survival. Arch Surg. 1970 Aug;101(2):105–113. doi: 10.1001/archsurg.1970.01340260009002. [DOI] [PubMed] [Google Scholar]
- Ridolfi R. L., Rosen P. P., Port A., Kinne D., Miké V. Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow-up. Cancer. 1977 Oct;40(4):1365–1385. doi: 10.1002/1097-0142(197710)40:4<1365::aid-cncr2820400402>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Rosen P. P., Fracchia A. A., Urban J. A., Schottenfeld D., Robbins G. F. "Residual" mammary carcinoma following simulated partial mastectomy. Cancer. 1975 Mar;35(3):739–747. doi: 10.1002/1097-0142(197503)35:3<739::aid-cncr2820350329>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Rosen P. P., Menendez-Botet C. J., Nisselbaum J. S., Urban J. A., Miké V., Fracchia A., Schwartz M. K. Pathological review of breast lesions analyzed for estrogen receptor protein. Cancer Res. 1975 Nov;35(11 Pt 1):3187–3194. [PubMed] [Google Scholar]
- Rosen P. P., Saigo P. E., Braun D. W., Jr, Weathers E., DePalo A. Predictors of recurrence in stage I (T1N0M0) breast carcinoma. Ann Surg. 1981 Jan;193(1):15–25. doi: 10.1097/00000658-198101000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampat M. B., Sirsat M. V., Gangadharan P. Prognostic significance of blood vessel invasion in carcinoma of the breast in women. J Surg Oncol. 1977;9(6):623–632. doi: 10.1002/jso.2930090613. [DOI] [PubMed] [Google Scholar]
- Schottenfeld D., Robbins G. F. Breast cancer in elderly women. Geriatrics. 1971 Mar;26(3):121–131. [PubMed] [Google Scholar]
- Valagussa P., Bonadonna G., Veronesi U. Patterns of relapse and survival following radical mastectomy. Analysis of 716 consecutive patients. Cancer. 1978 Mar;41(3):1170–1178. doi: 10.1002/1097-0142(197803)41:3<1170::aid-cncr2820410355>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]


