Abstract
Defects in the mammalian Menkes and Wilson copper transporting P-type ATPases cause severe copper homeostasis disease phenotypes in humans. Here, we find that DmATP7, the sole Drosophila orthologue of the Menkes and Wilson genes, is vital for uptake of copper in vivo. Analysis of a DmATP7 loss-of-function allele shows that DmATP7 is essential in embryogenesis, early larval development, and adult pigmentation and is probably required for copper uptake from the diet. These phenotypes are analogous to those caused by mutation in the mouse and human Menkes genes, suggesting that like Menkes, DmATP7 plays at least two roles at the cellular level: delivering copper to cuproenzymes required for pigmentation and neuronal function and removing excess cellular copper via facilitated efflux. DmATP7 displays a dynamic and unexpected expression pattern in the developing embryo, implying novel functions for this copper pump and the lethality observed in DmATP7 mutant flies is the earliest seen for any copper homeostasis gene.
INTRODUCTION
Copper is a trace element essential for all aerobic organisms. It is required by copper-dependent enzymes involved in diverse metabolic processes, including cellular respiration, antioxidant defense, pigment formation, neurotransmitter production, and peptide biosynthesis (Danks, 1988). However, the redox potential that makes copper such an effective cofactor also makes it extremely toxic if levels are not properly controlled, a property that has resulted in tightly regulated homeostatic mechanisms, well conserved from yeast to humans (Halliwell and Gutteridge, 1984; Pena et al., 1999). A key aspect of metal homeostasis is regulated export, or efflux, of the metal from individual cells. In single-celled organisms such as yeast, metal export serves mainly a detoxification role. In a multicellular organism, however, essential metals such as copper that are absorbed from dietary sources must be distributed to different tissues throughout the body. Therefore, export of copper from individual cells is necessary both for detoxification and for systemic supply.
The primary human copper exporters are the Menkes and Wilson copper-transporting P-type ATPases. These proteins show 54% sequence identity and at the cellular level function in a similar way to transport copper across membranes (Lutsenko and Petris, 2002). However, the human diseases associated with disruption of their respective functions are very different and illustrate the dual nature of copper. Menkes disease is an X-linked recessive disorder that presents as a severe systemic copper deficiency with symptoms including growth failure, skeletal defects, and progressive degeneration of the central nervous system, resulting in death during early childhood (Danks, 1995). The Menkes disease protein MNK (or ATP7A) is expressed in all tissues except the liver and is thought to play two roles at the cellular level: at basal or low intracellular copper levels, it delivers copper to enzymes of the secretory pathway in the trans-Golgi network (TGN) (Yamaguchi et al., 1996; Petris et al., 2000); and at high intracellular copper concentrations, MNK translocates to the plasma membrane where it catalyzes copper efflux (Petris et al., 1996). MNK is expressed in the mucosal cells lining the intestine and is required in these cells for systemic absorption of copper. In patients with Menkes disease, copper is trapped in the intestinal mucosa and very little is delivered to peripheral organs and tissues (Danks et al., 1972). In contrast, Wilson disease is caused by accumulation of copper in the liver and brain, leading to chronic liver disease, cirrhosis, and neurological problems such as behavioral disturbances and movement disorders (Scheinberg and Sternlieb, 1984). The protein involved is WND (or ATP7B), and it is expressed mainly in the liver and delivers copper to apoceruloplasmin in the trans-Golgi network. At elevated copper levels, WND traffics to the biliary canalicular membrane where copper is incorporated into the bile for excretion from the body (Roelofsen et al., 2000). The failure of this copper excretion function in Wilson disease patients leads to toxic copper accumulation.
In both cases described above, disruption of copper transport causes serious disease, although the phenotypes are effectively opposite, and normally MNK and WND function in complementary tissues and together maintain copper homeostasis. Although mammals have two copper-translocating P-type ATPases performing complementary functions, lower multicellular animals such as insects (Drosophila melanogaster and Anopheles gambiae) and nematodes (Caenorhabditis elegans) have only one, raising the question as to whether this sole copper pump fulfills some or all of the functions of its mammalian orthologues. To better understand the function and regulation of these vital copper-transporting P-type ATPases, this study sought to characterize the in vivo role of the sole Drosophila orthologue, DmATP7. We had previously identified DmATP7 as an X-linked Drosophila gene predicted to encode a protein with strong homology to both MNK and WND. Importantly, all motifs shown to be essential for copper transport in the mammalian P-type ATPases are highly conserved in DmATP7 (Figure 1). Knockdown of the DmATP7 transcript in cultured Drosophila embryonic “S2” cells by RNA interference resulted in a significant increase in copper accumulation within these cells, confirming a role for DmATP7 in copper efflux analogous to that seen in other systems (Southon et al., 2004).
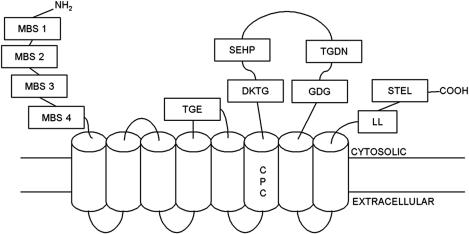
Figure 1.
Schematic diagram of DmATP7 based on the predicted MNK structure. The N-terminal domain comprises four GMxCxxC metal binding sites (MBS1-4; mammalian MNK and WND have six). The conserved cation transduction motif CPC lies in the sixth of eight transmembrane domains. The CPC motif and proposed ATP-binding site SEHP are conserved in transition metal transporting P-type ATPases. ATP hydrolysis requires the TGE, DKTG, TGDN, and GDGVNDSP (labeled GDG) sites characteristic of all P-type ATPases (identical to MNK/WND except MNK has GDGINDSP sequence). The C-terminal dileucine motif (LL; WND has three leucines at this location) is required for endocytosis and basolateral targeting, and STEL (DTAL in MNK) is a putative PDZ target motif required for localization to the basolateral membrane of polarized cells. A sequence alignment between MNK, WND, and DmATP7 is available in Supplemental Material 1.
This work investigates DmATP7 function in Drosophila, generating a loss-of-function DmATP7 mutant allele to analyze its role in embryogenesis, early larval development, and adult pigmentation formation. The embryonic DmATP7 expression pattern and localization of the DmATP7 protein in larval tissues is also examined, and the importance of key conserved residues is investigated in an in vivo overexpression assay. Our results reveal several vital functions for DmATP7 and establish Drosophila as an excellent animal model for investigating the regulation of these essential copper efflux pumps.
MATERIALS AND METHODS
Fly Stock Maintenance and Media
Fly stocks were maintained and experiments were performed at 25°C on standard laboratory medium, supplemented with either 100 μM bathocuproinedisulfonic acid (BCS; Sigma-Aldrich, St. Louis, MO) to make copper-deficient food medium, or 1 mM copper (CuSO4·5H2O; Merck, Whitehouse Station, NJ) to make copper-supplemented medium. Tetrathiomolybdate (TTM; Sigma-Aldrich) was used instead of BCS where indicated in figure legends. In the add-back experiments presented in Table 1, 50 μM BCS media was supplemented with either 50 μM copper, 200 μM zinc (ZnSO4-7H2O; Ajax Finechem, Seven Hills, Australia), or 100 μM iron (FeSO4·7H2O; BDH, Poole, Dorset, United Kingdom).
Table 1.
Rescue of DmATP7 overexpression-induced hypopigmentation by copper supplementation
| Cu (mM)
|
TTM (μM)
|
BCS (μM)
|
BCS (50 μM) + supplement
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-3 | NF | 2.5 | 5 | 10 | 5 | 10 | 20 | 50 | 100 | Cu | Zn | Fe | |
| DmATP7 overexpression | + | + | 1 | 2 | 3 | + | + | 1 | 2 | 3 | + | 2 | 2 |
| Wild type | + | + | + | + | + | + | + | + | + | + | − | − | − |
Abdominal pigmentation levels of DmATP7 overexpression (Act-GAL4; EP lineGS6038) and wild-type (Act-GAL4; +) adults raised on media indicated: + is wild-type pigmentation, and 1, 2, and 3 represent mild, moderate, and extreme hypopigmentation, respectively. Wild type was not screened on supplemented BCS media (−). NF is normal food media. Hypopigmentation is visible only in flies raised on copper-deficient media (TTM or BCS) and is rescued by addition of copper back into the media, but not addition of zinc or iron, indicating it is caused specifically by copper deficiency. The degree of hypopigmentation is proportional to the amount of chelator in the media; therefore, pigmentation is sensitive to the amount of available copper.
Generation of DmATP7 Null Allele
A DmATP7 null allele (ΔP17) was generated using imprecise excision of a single P-element inserted ∼340 base pairs upstream of the DmATP7 transcription start site in the EP308 line (w1118 P{w+ = EP}ATP7EP308; BL10114, Bloomington Stock Center, Indiana University, Bloomington, IN). To mobilize the P-element, EP308 flies were crossed to a line containing a stable transposase source (Sb1 P{Delta2-3}99B/TM6B; BL1798; Bloomington Stock Center; Robertson et al., 1988), and F1 males containing both the insertion and the transposase source were then crossed to Binsinscy females. Finally, single w- Sb+ F2 females that had lost both the transposase source and the P-element were crossed to Binsinscy males to establish individual lines. Southern blot analysis followed by sequencing of a PCR product from a hemizygous lethal line identified a 1134-base pair deletion beginning at the EP308 insertion site that removed the transcription start site and the first three metal binding sites of the putative DmATP7 product, and real-time PCR confirmed loss of expression from DmATP7, whereas neighboring genes were unaffected (our unpublished data). This line was subsequently crossed to an FM7-GFP line for mutant analysis.
Germline and Somatic Clone Generation
To create embryos lacking maternal DmATP7 activity, DmATP7ΔP17 P{FRT(whs)}101/w* ovoD1 v24 P{FRT(whs)}101; P{hsFLP}38 females (ΔP17 is the DmATP7 loss of function allele described above) were heat shocked for 2 h at 37°C over three consecutive days during larval or pupal development. Adult females were then mated to FM7-GFP/Y males. Paternally rescued embryos were detected using the FM7-GFP chromosome. To generate adult mosaic flies containing DmATP7ΔP17/ΔP17 clones, DmATP7ΔP17 P{FRT(whs)}101/y1 w67c23 P{Ubi-GFP}ID-1 P{FRT(whs)}101; MKRS, P{hsFLP}86E/+ flies were heat shocked for 2 h at 37°C once during larval development.
Overexpression Fly Strains and Transgenics
GAL4 lines used were as follows: Pnr-GAL4/Sb, Ser (gift from E. Hafen, University of Zurich, Zurich, Switzerland); Act-GAL4; Tra-GAL4 (gift from P. Whitington, University of Melbourne, Melbourne, Australia); Ptc-GAL4 w*; P{GawB}ptc559.1 (BL2017; Bloomington Stock Center); GutSpecifc-GAL4 (P. Daborn, University of Melbourne, unpublished data); w* ovoD1 v24 P{FRT(whs)}101/C(1)DX, y1 f1/Y; P{hsFLP}38 (BL1813; Bloomington Stock Center); y1 w67c23 P{Ubi-GFP}ID-1 P{FRT(whs)}101 (BL5153; Bloomington Stock Center); w1118; MKRS, P{hsFLP}86E/TM6B, Tb1 (BL279; Bloomington Stock Center). MtnA5'-EYFP and Ctr1B5'-EYFP lines were a kind gift from W. Schaffner (University of Zurich). UAS lines generated were UAS-DmATP7 (CG1886), UAS-Ctr1A (CG3977), UAS-DmCCS (CG17753), and UAS-DmAtox1 (CG32446). In addition to the direct UAS-DmATP7 constructs generated here, an EP line from the Gene Search project (GS6038) was also used to overexpress DmATP7 from the endogenous locus. This line was used for the experiments in Table 1 and Figure 8B and caused considerably weaker overexpression phenotypes than the direct UAS constructs generated. Full-length cDNAs lacking the C-terminal STOP codon (according to FlyBase annotation; The FlyBase Consortium, 2003; http://flybase.org/) were generated for each gene by PCR amplification from S2 cell-derived cDNA using the following primer pairs (restriction site linkers in uppercase): DmATP7, F1 5′ gaGGTACCatgtccacggtgcgcctgcc 3′ and R1 5′ gcTCTAGAcagcttttgcagttcggtAct 3′; Ctr1A F1 5′ gaGGTACCatgcaccacgatcacagcg 3′ and R1 5′gcTCTAGAgtgacagtgctcggttacg 3′; DmCCS F1 5′ gcGGTACCatgagctccattaagatcgaat 3′ and R1 5′gcTCTAGAcagcttttgtgagcggtcct 3′; and DmAtox1 F1 5′ gaGGTACCatgacagtgcacgaattcaag 3′ and R1 5′ gcTCTAGAtttcttcaccccgacgtagg 3′.
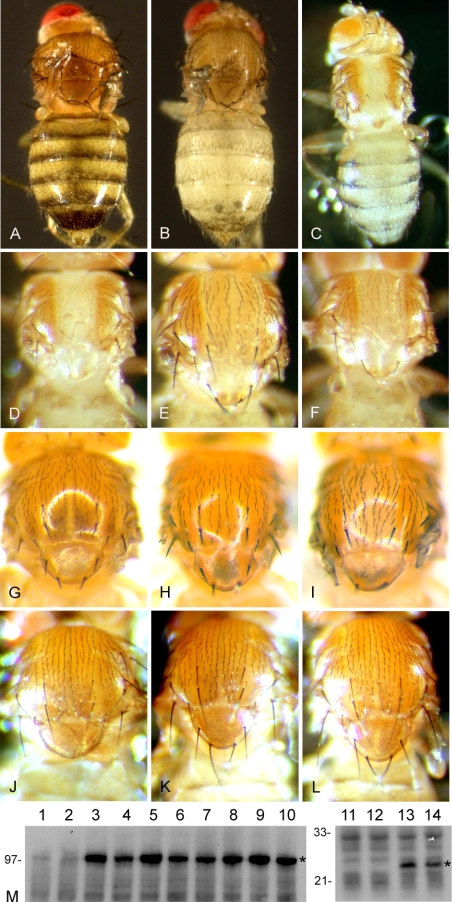
Figure 8.
(A and B) Act-GAL4/UAS-DmATP7wt flies grown on normal medium (A) and 10 mM TTM medium (B). In the DmATP7-overexpressing flies grown on copper-deficient media (B), reduction in pigmentation is seen throughout the adult abdominal cuticle, whereas increased pigmentation is observed in the head cuticle. Full-length (C) and close-up views (D) of Pnr-GAL4/UAS-DmATP7wt adults. Overexpression of DmATP7wt causes strong hypopigmentation in the Pnr expression domain of the thorax and abdomen and loss of thoracic bristles, reduction of the scutellum, and a cleft in the thorax. In contrast, overexpression of DmATP7mbs (E) and DmATP7cpc (F) under the same GAL4 driver causes hypopigmentation in the thorax and abdomen (albeit milder in the abdomen), but only mild loss of thoracic bristles, and mild or no thoracic cleft/scutellar reduction. (G) Expression of Ctr1A under Pnr-GAL4 control has no phenotypic effect. Coexpression of Ctr1A rescues the DmATP7wt overexpression phenotype (H) but not the DmATP7cpc overexpression phenotype (I). (J-L) Expression of DmATP7wt (J) under Ptc-GAL4 control causes reduction in the scutellum and loss of the major scutellar bristles, whereas expression of DmATP7tap (K) and DmATP7mbs (L) with the same GAL4 driver has no phenotypic effect. (M) Anti-FLAG Western blot showing expression in adult heads under GMR-GAL4 control of negative controls (1, 2, 11, and 12), DmATP7wt (3 and 4), DmATP7cpc (5 and 6), DmATP7tap (7 and 8), DmATP7mbs (9 and 10), and Ctr1A (13 and 14). Two independent insertion lines for each transgene are shown. Asterisk (*) shows DmATP7 and Ctr1A bands.
These full-length cDNAs were cloned into pUAST modified to contain an in-frame C-terminal FLAG epitope tag (MDYKDDDDKA). These pUAST constructs were injected into w1118 embryos and transformants selected on the basis of nonwhite eye color. DmATP7 primers were based on the FlyBase 2002 annotation, and the coding sequence has subsequently been updated and extended 10 aa 5′ to include MPSDERVEAT, lacking in the cDNA used here. In addition, sequencing this PCR product revealed an additional 105 base pairs between base pairs 2634 and base pairs 2635 of CG1886 (GTA CGC AAG TCC ATG GAG CTG AAC AAT CAG CAG TTG CTA TCC GAC TTG GTA TTG GAA CCG GAG GAA GAG CTC CTT ACG GAT CAG AAA ATC ATC GAT TCA CC CGA G) to be exonic sequence, rather than intronic as in the current FlyBase-NG annotation.
Generation of Mutated DmATP7
Wild-type DmATP7 cDNA was altered using the Transformer site-directed mutagenesis kit (BD Biosciences Clontech, Palo Alto, CA). Oligonucleotides used for mutagenesis are as follows, with altered bases in uppercase. The altered amino acids have been included in parentheses (numbering based on CG1886 FlyBase-NG annotation, November 2004): MBS1, gtgggcatgacttCccagtcgtCtgtgcgcaatatc (C23-S; C26-S), MBS2, gggcatgacctCccagtcgtCcgtgcgcaacatc (C104-S; C107-S); MBS3, ggcatgacgtCcgccagctCtgtggccgcc (C219-S; C222-S); MBS4, gggcatgacttCcgcctcctCcgtcaacaag (C295-S; C298-S); CPC, ggccattgcgGCtccaGCtgctttgggc (C702-A; C704-A); and TAP, ctgtggtcttcgCcaagaccggcac (D746-A).
All mutant DmATP7 versions were confirmed by sequencing. Only DmATP7MBS was not completely as expected, with the C298 (MBS4) remaining unmutated. These mutant DmATP7 versions were subcloned into pUAST and used to create transgenic transformants as described above.
Immunohistochemistry, Microscopy, and Western Blotting
Wild-type (w1118 or FM7-GFP) and DmATP7 -/Y embryos were staged, dechorionated in 50% sodium hypochlorite (Ajax Finechem), and fixed for 30 min in 1:1 (vol/vol) heptane (Merck) and 8% paraformaldehyde (PFA; Sigma-Aldrich). The vitelline membrane was removed by shaking in 1:1 methanol (Ajax Finechem): Phosphate-buffered saline (PBS) (Oxoid, Basingstoke, Hampshire, England), and then embryos were rehydrated in PBS. Third instars were dissected and then fixed 30 min in 8% PFA. Primary antibodies used were polyclonal rabbit anti-ATP7a raised against a region of human ATP7a cDNA coding for the six metal-binding sites (used at 1:200; Camakaris et al., 1995), monoclonal mouse anti-FLAG (KM5-1C7, used at 1:200; Walter and Eliza Hall Institute Biotechnology Centre, Melbourne, Australia), and monoclonal mouse anti-green fluorescent protein (GFP; used at 1:200). Secondary antibodies from the Alexa Fluor range of IgG-fluorophore conjugates (Invitrogen, Carlsbad, CA) were used to detect primary antibodies. Larval tissue images were recorded with a Zeiss Axioplan 2 fluorescence microscope attached to a Bio-Rad μRadiance control unit using LaserSharp2000 software. Embryonic images were recorded on a Zeiss microscope. Adult Drosophila were photographed with an Olympus digital camera through a Leica MZ16 stereomicroscope. Samples for Western blotting were prepared by homogenizing 30 adult fly heads in 60 μl of 2% SDS sample buffer and then lysing on ice for 1 h before clearing lysate by centrifugation. The equivalent of approximately eight fly heads per sample was run on NuPAGE 4-12% Bis-Tris gels (Invitrogen) before transfer to nitrocellulose membranes and Western blotting with monoclonal mouse anti-FLAG primary and polyclonal rabbit anti-mouse horseradish peroxidase-coupled secondary (DakoCytomation, Ely, Cambridgeshire, United Kingdom) antibodies.
Gene Expression Analysis
Wild-type and DmATP7 -/Y first instars were transferred within 4 h of hatching to copper-deficient, copper-supplemented, or normal food media for 24 h and then snap frozen in liquid nitrogen. Third instars were transferred to copper-deficient, copper-supplemented, or normal food media for 24 h before dissection in Ringer's solution and transferal of gut and fat bodies to TRIzol (Invitrogen). Total RNA was extracted using the RNeasy kit, including DNase treatment, according to the manufacturer's instructions (QIAGEN, Valencia, CA). cDNA was transcribed from 1 μg of total RNA using avian myeloblastosis virus reverse transcriptase in a 20-μl reaction according to the manufacturer's instructions (Promega, Madison, WI). Primers for real-time PCR were designed using Primer3 software (Rozen and Skaletsky, 2000) available at http://www.broad.mit.edu/genome_software/other/primer3.html. DmATP7 forward and reverse primers were CCCACTGACCTTCTTCGATACand GGTCTTTCCCTTGGCTATGTrespectively. Other primers have been described previously (Southon et al., 2004). Real-time PCR was performed using the Rotor Gene 3000 (Corbett Research, Mortlake, NSW, Australia). Twenty nanograms of reverse transcribed total RNA was amplified in a 25-μl reaction containing 1 μM of each primer and 12.5 μl of 2× QuantiTect SYBR Green PCR Master Mix (QIAGEN). The amount of gene product in each sample was determined using the comparative quantitation method used by the Rotor Gene 5.0 software (Corbett Research) and normalized to Actin42A as described previously (Southon et al., 2004).
Statistical Analysis
Statistical analysis was conducted using SPSS version 11 (SPSS, Chicago, IL). A one-sample Kolomogorov-Smirinov test was used to assess whether data were normally distributed. Statistical analyses are described in figure legends. Phosphate-buffered saline <0.05 was deemed statistically significant.
RESULTS
DmATP7 Exhibits a Dynamic Expression Pattern during D. melanogaster Embryonic Development
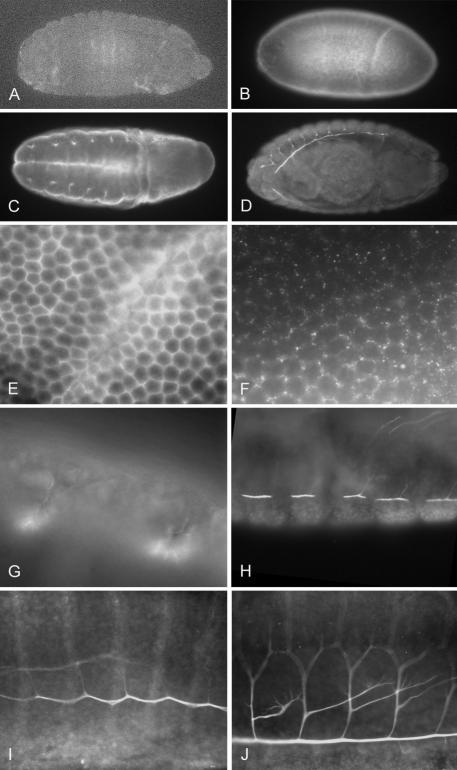
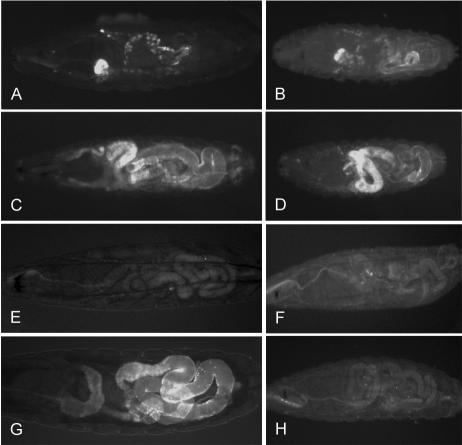
To determine the embryonic expression pattern of DmATP7, an anti-MNK antibody that cross-reacts specifically with DmATP7 was used to probe staged Drosophila embryos (Figure 2). Although no staining is observed either in DmATP7 mutant embryos (Figure 2A; see next section) or with preimmune serum (our unpublished data), clear DmATP7 expression is visible at the plasma membrane (PM) of all cells in early embryogenesis (Figure 2E), becoming brighter and taking on a punctate appearance from 2 to 4 h after egg laying (AEL; Figure 2F). From 4 h AEL, the PM staining is replaced by a diffuse cytoplasmic staining. At this time, expression is also seen in early tracheal placodes (Figure 2, C and G), at the edge of the tracheal pit. Expression is then observed in developing tracheae, starting in the trunk (Figure 2H) and extending into the tracheal branches as they develop (Figure 2, I and J). The lack of staining in DmATP7 mutant embryos or with preimmune serum and the early tracheal staining before tube formation demonstrate the specificity and veracity of this staining pattern. This antibody could not be used to examine DmATP7 expression in larval stages because it detects a nonspecific product not present in embryos.
Figure 2.
Mutant and wild-type embryos stained with anti-MNK antibody. (A) DmATP7 -/Y embryos show no staining with anti-MNK antibody. (B and E) Membranal staining is observed at 0-2 h AEL, becoming punctate by 4 h AEL (F). Staining is visible in the early tracheal placodes (C and G) from 4 h and remains visible in the developing tracheal dorsal trunk (H and I) and in branching trachea (D and J). Original magnification, 40× (A-D), 1000× (E-G), and 400× (H-J).
DmATP7 Is Essential for Early Larval Growth and Survival
To determine the in vivo requirement for DmATP7 function in Drosophila, imprecise P-element excision was used to generate a genomic deletion extending 798 base pairs into the DmATP7 coding sequence from the start codon. To confirm that this deletion was a loss-of-function allele, male DmATP7 -/Y embryos (distinguished from their female DmATP7 +/+ and DmATP7 +/- siblings using a GFP balancer chromosome) were probed with the anti-MNK antibody. Compared with their wild-type siblings, DmATP7 -/Y embryos did not show any signal, confirming that no detectable DmATP7 protein is produced from the disrupted locus (Figure 2A).
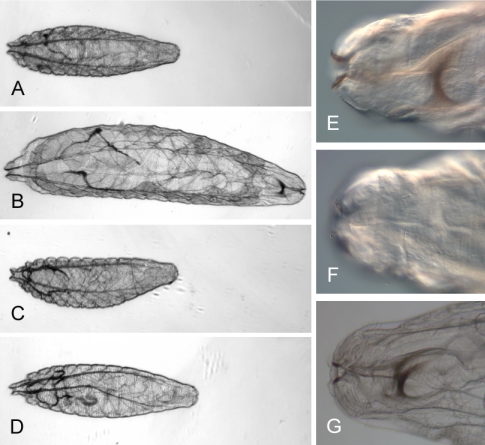
DmATP7 -/Y animals were examined for any defects arising from disruption of DmATP7 (Figure 3). Although at hatching there is no size difference between DmATP7 -/Y larvae and heterozygous/wild-type siblings, the mutant animals seem extremely lethargic in comparison with their wild-type siblings (see video in Supplemental Material 2), and their mouthparts are smaller and reduced in pigmentation (compare Figure 3, E and F). Unlike heterozygous and wild-type animals, DmATP7 -/Y larvae fail to grow and develop to the second instar (Figures 3, A-D), dying by 36 h after hatching. This lethality can be rescued by a full-length DmATP7 transgene (discussed below), which restores mouthpart size and pigmentation (Figure 3G), normal growth, and activity (see video in Supplemental Material 2), resulting in viable adults that are slightly lacking in cuticle pigment, indicating the phenotypes observed are solely the result of DmATP7 disruption.
Figure 3.
(A and B) Wild-type larvae <2 h after hatching (A) and 24 h after hatching (B). (C and D) DmATP7 -/Y larvae <2 h after hatching (C) and 24 h after hatching (D). DmATP7 -/Y larvae do not show significant growth in 24 h on normal media. (E and F) Mouth hooks of the larva shown in B (E) and the larva shown in D (F). DmATP7 -/Y mouth hooks lack normal pigmentation. (G) Mouth hooks of a DmATP7 -/Y larvae containing a UAS-DmATP7 transgene that rescues the mutant animals to adulthood—mouth hook pigmentation and structure are restored. These larvae are siblings developing on the same media. Original magnification, 40× (A-D) and 400× (E-G).
To determine whether DmATP7 -/Y larvae are dying from excess or insufficient copper, rescue experiments with copper-depleted and copper-supplemented media were attempted. DmATP7 -/Y larvae die at the same stage on all types of media tested. To exclude the trivial possibility that mutant larvae were dying simply because they could not feed, medium containing food-dye was used to show that DmATP7 -/Y larvae do feed, although considerably less than their wild-type siblings (our unpublished data).
Copper Is Likely to Accumulate in the Gut of DmATP7 Mutant Larvae
Direct detection of copper levels in first instars is technically difficult due to their small size, ∼1/100th the mass of third instars. Copper levels from 500 wild-type first instars were barely detectable by inductively coupled plasma mass spectrometry. Furthermore, we were unable to detect any activity of the copper-dependent enzyme tyrosinase in up to 2000 wild-type first instars, whereas five wild-type third instars showed considerable tyrosinase activity. Due to these technical constraints, we sought to use transcript levels of the copper-responsive genes Ctr1B and MtnA, B, C, and D as proxy markers of copper accumulation in wild-type and mutant animals. Ctr1B, one of the three Drosophila copper uptake genes, is up-regulated in response to copper starvation (Zhou et al., 2003; Selvaraj et al., 2005), whereas all four Drosophila metallothionein genes (MtnA-D) are known to be up-regulated in high copper conditions (Lastowski-Perry et al., 1985; Mokdad et al., 1987; Egli et al., 2003). First, gut (the primary uptake organ) and fat bodies (the key detoxification organ of the insect, with functional similarities to the mammalian liver; Sondergaard, 1993) were dissected from wild-type third instars and analyzed for transcript levels (Table 2A). Strong up-regulation of MtnA-D was observed in the gut and fat body tissues of larvae fed a copper-supplemented diet. Up-regulation of Ctr1B in response to copper starvation and down-regulation in response to copper (previously demonstrated only in whole larvae) was predominant in gut tissues but also observed in fat bodies.
Table 2.
Gene expression
| Ctr1A | Ctr1B | MtnA | MtnB | MtnC | MtnD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. Gene expression in gut and fat bodies of wild-type third instars (relative to respective NF data points) | ||||||||||||
| Fat body BCS | 1.29 ± 0.25 | 1.57 ± 0.13a | 1.26 ± 0.41 | 1.08 ± 0.30 | 1.62 ± 0.27 | 0.96 ± 0.14 | ||||||
| Fat body NF | 1.00 ± 0.06 | 1.00 ± 0.05 | 1.00 ± 0.09 | 1.00 ± 0.06 | 1.00 ± 0.11 | 1.00 ± 0.14 | ||||||
| Fat body Cu | 1.30 ± 0.36 | 0.47 ± 0.13a | 3.86 ± 0.78a | 9.50 ± 3.52 | 12.8 ± 3.10a | 6.10 ± 2.02 | ||||||
| Gut BCS | 0.78 ± 0.13 | 5.14 ± 1.29b | 0.49 ± 0.09b | 0.78 ± 0.12 | 0.87 ± 0.11 | 0.39 ± 0.06b | ||||||
| Gut NF | 1.00 ± 0.06 | 1.00 ± 0.11 | 1.00 ± 0.13 | 1.00 ± 0.06 | 1.00 ± 0.05 | 1.00 ± 0.10 | ||||||
| Gut Cu | 1.05 ± 0.14 | 0.22 ± 0.04b | 4.45 ± 0.41b | 23.8 ± 5.30b | 29.3 ± 5.60b | 3.95 ± 0.64b | ||||||
| B. Gene expression (relative to WT NF) in wild-type and DmATP7−/γ first instars | ||||||||||||
| WT BCS | 3.17 ± 0.37c | 9.94 ± 1.95c | 0.59 ± 0.15c | 0.70 ± 0.17 | 0.74 ± 0.08 | 0.56 ± 0.01c | ||||||
| WT NF | 1.00 ± 0.06 | 1.00 ± 0.14 | 1.00 ± 0.08 | 1.00 ± 0.41 | 1.00 ± 0.38 | 1.00 ± 0.19 | ||||||
| WT Cu | 2.06 ± 0.39 | 1.24 ± 0.06 | 8.67 ± 1.04 | 39.7 ± 14.0 | 111 ± 55.5 | 21.3 ± 3.88 | ||||||
| DmATP7− BCS | 0.46 ± 0.12d | 0.50 ± 0.10d | 0.50 ± 0.16 | 0.66 ± 0.34 | 0.21 ± 0.12d | 0.46 ± 0.08 | ||||||
| DmATP7− NF | 0.51 ± 0.08c | 0.76 ± 0.21 | 0.79 ± 0.14 | 0.75 ± 0.38 | 1.38 ± 0.78 | 0.43 ± 0.02c | ||||||
| DmATP7− Cu | 0.52 ± 0.12 | 0.55 ± 0.10e | 5.21 ± 2.28 | 6.11 ± 3.21e | 11.3 ± 6.19 | 1.84 ± 0.37e,f | ||||||
(A) Values from fat bodies and gut tissues are expressed relative to the same tissue exposed to normal food media (NF) and are the mean ± SEM of eight replicates from two independent experiments. Gene expression was normally distributed and a one-sample t test was used to determine significant differences. (B) Values are expressed relative to control larvae exposed to normal food and are the mean ± SEM of four replicates from two independent experiments. Ctr1A expression was normally distributed, and a one-way ANOVA with Games-Howell post hoc test was used to determine significant differences. Ctr1B, MtnA, MtnB, MtnC, and MtnD expression levels were not normally distributed and a Mann-Whitney test was used to determine significant differences.
p < 0.05 compared with fat bodies exposed to normal food.
p < 0.05 compared with gut tissue exposed to normal food.
p < 0.05 compared with control flies exposed to normal food.
p < 0.05 compared with control larvae exposed to copper-deficient media.
p < 0.05 compared with control flies exposed to copper-supplemented media.
p < 0.05 compared with DmATP7 −/γ flies exposed to normal food.
Because DmATP7 -/Y larvae do not survive until third instar, and first instars are too small to dissect, transcript levels were compared between whole knockout and wild-type first instars (Table 2B). Like dissected third instars, wild-type first instars showed up-regulation of MtnA-D in response to high copper and up-regulation of Ctr1B in response to copper starvation. MtnA-D gene expression also increased in DmATP7 -/Y larvae in response to a high copper diet, indicating copper is taken up at least into the gut. Importantly, no up-regulation of Ctr1B was seen in response to copper starvation in the mutant larvae. Up-regulation of Ctr1B is presumably a response to low copper levels in the gut cells (where strong Ctr1B up-regulation was observed in third instars) where an increase in the amount of uptake protein would optimize copper absorption from copper-deficient food. The fact that no up-regulation is observed in DmATP7 -/Y larvae suggests that, even under copper-limiting conditions, sufficient copper levels are present in the gut cells of these larvae to avoid the starvation response. This situation is analogous to that of human Menkes disease patients, where copper can be taken up into gut cells (inducing Mtn up-regulation) but remains trapped in these cells (thus no Ctr1B up-regulation) and cannot be transported to other tissues of the body.
To confirm that the changes in gene regulation we had quantified in first instars by reverse transcription-PCR were indeed confined predominantly to the gut region, we monitored expression of two enhanced yellow fluorescent protein (EYFP) fusion constructs, one under the control of the MtnA 5′ regulatory region, and the other under the control of the Ctr1B 5′ regulatory region (Figure 4). These results clearly show that MtnA and Ctr1B expression is confined almost exclusively to the gut region in first instar and that DmATP7 -/Y mutant larvae do respond to high copper exposure (MtnA-EYFP up-regulation) but do not respond to copper starvation (Ctr1B-EYFP up-regulation), supporting our argument that in the mutant animals, copper is taken into the gut cells but is retained there and is unable to reach the rest of the animal, resulting in lethality.
Figure 4.
(A-D) Expression of MtnA5′-EYFP in wild-type (A and C) and DmATP7 -/Y (B and D) first instars raised for 6 h on normal food (A and B) or 1 mM copper food (C and D). Basal MtnA5′-EYFP expression is higher in mutant larvae, but both wild-type and mutant larvae respond robustly to a high copper diet. (E-H) Expression of Ctr1B5′-EYFP in wild-type (E and G) and DmATP7 -/Y (F and H) first instars raised for 12 h on 1 mM copper food (E and F) or 100 μM BCS food (G and H). Only wild-type larvae respond to copper starvation on BCS food by up-regulation of Ctr1B5′-EYFP.
Expression Pattern of a DmATP7 Rescue Transgene Supports Copper Uptake Role in the Gut
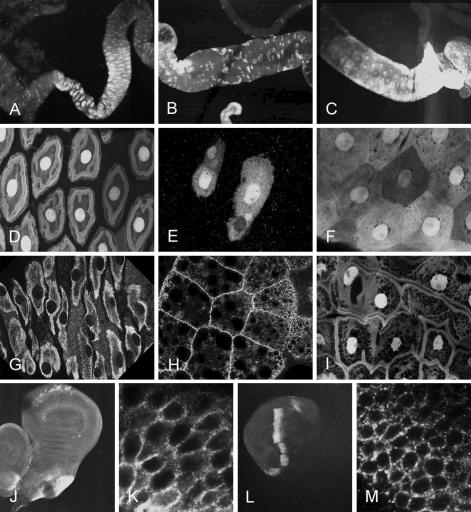
In the course of overexpression experiments described later, a UAS-DmATP7 transgene was found to serendipitously rescue DmATP7 -/Y animals to adulthood in the absence of a GAL4 driver, indicating that the transgene had inserted near an endogenous enhancer that drives sufficient DmATP7 expression for survival. Attempts to rescue DmATP7 -/Y animals with numerous constitutive, gut, and fat body-specific GAL4 drivers all failed, indicating a very specific DmATP7 expression pattern is required for survival. The expression pattern of the rescuing UAS-DmATP7 transgene was investigated in third instars by immunostaining using the FLAG-epitope tag attached to the transgenic DmATP7, providing some evidence of the tissue-specific requirements for DmATP7 (Figure 5). Low level cytoplasmic DmATP7-FLAG expression was observed in the imaginal discs, whereas strong nuclear/cytoplasmic staining was seen in discrete sections of the gut, including the foregut and hindgut imaginal rings, the large acid-secreting “copper cells” (Dubreuil, 2004) of the middle midgut, (Figure 5, A and D), the posterior midgut adjoining the hindgut (Figure 5, C and F), and all of the hindgut. The strong expression of this rescuing transgene in the midgut is consistent with a significant role for DmATP7 in copper absorption from the diet, but a DmATP7 regulatory region reporter construct is needed to make any definitive statements regarding the tissue-specific requirements of DmATP7.
Figure 5.
(A-F, and J) Expression of transgenic DmATP7wt-FLAG in rescued DmATP7 -/Y; UAS-DmATP7wt-FLAG third instars. All shown anterior to left. (A-F) DmATP7wt-FLAG expression in middle midgut (A, B, D, and E) and posterior midgut (C and F). In all cases, DmATP7wt-FLAG is observed in both the nuclei and the cytoplasm of these gut cells. (G) DmATP7wt-FLAG in middle midgut cells under a strong gut enhancer GAL4 control; expression is excluded from the nucleus in this case. (H) Ectopic DmATP7wt-FLAG in fat body cells; clear PM localization is observed. (I) DmATP7wt-FLAG expression in posterior midgut of larvae exposed to high copper diet; occasional relocalization to PM is observed compared with F. (J) Expression of DmATP7wt-FLAG under Pnr-GAL4 control in the Pnr domain of the third instar wing imaginal disk. (K) High-magnification view of J; strong staining is observed in a punctate pattern localized or proximal to the PM. (L) Expression of DmATP7wt-FLAG under Ptc-GAL4 control in the Ptc domain of the third instar wing imaginal disk. (M) High-magnification view of L; strong staining is observed in a punctate pattern localized to the PM. When either DmATP7tap-FLAG, DmATP7mbs-FLAG, or DmATP7cpc-FLAG expression is driven by Pnr-GAL4 or Ptc-GAL4, localization of the mutant protein is the same as that seen for the wild-type overexpressed protein (our unpublished data). Original magnification, 100× (A-C, J, and L) and 630× (D-J, K, and M).
Unlike the mammalian MNK protein that clearly translocates from the TGN to the PM upon copper stimulation in certain cultured mammalian cells, PM staining of DmATP7-FLAG was only very rarely seen in larvae fed a copper-supplemented diet (compare Figure 5, I with F) and never in larvae fed a normal or copper-deficient diet. However, when overexpressed under GAL4 control, DmATP7-FLAG staining is excluded from the nucleus (Figure 5G), and overexpression also causes clear DmATP7-FLAG staining in the PM of fat body cells (Figure 5H). Therefore, under artificially high expression levels DmATP7 can localize to the PM, suggesting it contains appropriate PM trafficking/retention information (putative trafficking motifs are outlined in Figure 1). Generation of a DmATP7-specific antibody is required to further investigate the localization of the endogenous protein in these key tissues and to avoid artifacts caused by overexpression.
There Is a Significant Maternal Contribution of DmATP7
Germline clone-derived embryos devoid of maternal DmATP7 product were examined to investigate the degree of maternal contribution from the DmATP7 locus. Despite the finding that DmATP7 is strongly expressed in embryonic tracheae (Figure 2), tracheal development in these embryos seems normal (Figure 6), suggesting this expression may reflect a functional rather than a structural role for DmATP7 in the tracheae. These mutant embryos, although alive and apparently fully developed, are unable to hatch. This more extreme phenotype reveals a significant maternal contribution that is required for hatching but not for oogenesis. Because DmATP7 -/Y larvae have hypopigmented mouth hooks (Figure 3), the more extreme maternal phenotype could be due to weak mouth hooks unable to penetrate the chorion and facilitate hatching. This phenotype is not affected by raising parents on either copper-deficient or copper-supplemented media (our unpublished data). This is the first evidence of such an early requirement for correct copper homeostasis in Drosophila and demonstrates a striking requirement for DmATP7 before feeding has commenced, for essential processes in addition to absorption of copper via the gut.
Figure 6.
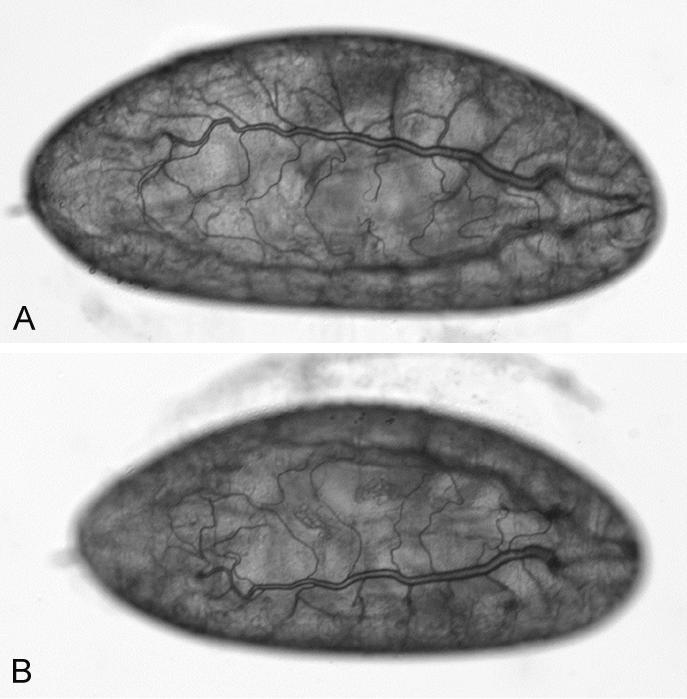
Wild-type embryo (A) and DmATP7 -/- germline cloned-derived embryo (B) devoid of maternal DmATP7 at 50× magnification. Mutant embryos have visibly normal tracheal development, despite the absence of DmATP7 expression in this tissue. No other developmental defects are visible, but the larvae fail to hatch.
DmATP7 Is Required for Adult Cuticle Pigmentation
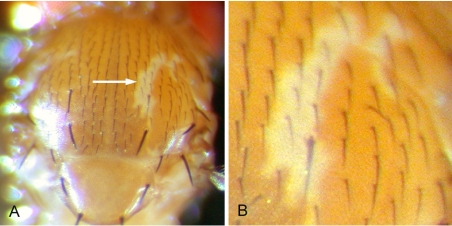
In mammals, the MNK protein is thought to supply copper to the pigment-forming enzyme tyrosinase (Petris et al., 2000), and mammals with reduced MNK function display hypopigmented hair/fur (Menkes et al., 1962; Hamza et al., 2001). Similarly, copper deficiency as a result of reduced Ctr1B copper uptake activity results in hypopigmented cuticle in Drosophila (Zhou et al., 2003). Because DmATP7 -/Y mutants die early in larval development, heterozygous adults containing DmATP7-/- clones were generated using the flippase-FRT system (Xu and Rubin, 1993) to examine any role of DmATP7 in pigment formation. Patches of depigmented tissue were observed in the thorax (Figure 7) and abdomen (our unpublished data), but no morphological defects were observed, indicating that DmATP7, like MNK in mammals, is essential for adult pigment formation.
Figure 7.
An example of a DmATP7 -/- clone in the adult Drosophila thorax at 500× (A) and 1000× (B) magnification. Although the clones have no cuticular markers, the depigmentation phenotype seen is not observed in control clones (our unpublished data), indicating it is due to DmATP7 -/- tissue.
Overexpression of Wild-Type DmATP7
To examine the effects of increased expression of a copper-transporting P-type ATPase in a multicellular organism, the GAL4-UAS system was used to drive expression of wild-type or mutant UAS-DmATP7 transgenes in a tissue-specific manner in Drosophila.
Overexpression of DmATP7 Results in Hypopigmentation
Unexpectedly, ubiquitous overexpression of DmATP7 resulted in dramatic hypopigmentation of the abdomen of animals grown on copper-deficient medium (Figure 8, A and B), a phenotype previously observed in copper-starved Ctr1B +/- flies (Zhou et al., 2003), whereas darker pigmentation was observed on the head cuticle of these flies. Add-back experiments supplementing this medium with copper, zinc, or iron showed that the phenotype is due specifically to lack of copper (Table 1). Thus, overexpression of DmATP7 seems to be creating a functional copper deficiency in certain cuticular cells, phenocopying the effect of loss of DmATP7 expression. When UAS-DmATP7 expression was driven under control of the Pannier (Pnr)-GAL4 driver, dramatic hypopigmentation was observed only in the Pnr expression domain, a strip of cuticular cells down the center of the adult thorax and abdomen (Figure 8C). A strong cleft in the thorax, reduction of the scutellum, and loss of thoracic bristles (Figure 8, C and D) was also observed. Unlike the Act-GAL4/UAS-DmATP7 hypopigmentation, the Pnr-GAL4/UAS-DmATP7 hypopigmentation is independent of dietary copper levels, indicating a more extreme functional copper deficiency.
The Pnr-GAL4/UAS-DmATP7 hypopigmentation phenotype is almost completely rescued by simultaneous overexpression of the copper uptake gene Ctr1A (Figure 8H) but not by cooverexpression of either DmAtox1 or DmCCS (Drosophila orthologues of the mammalian Atox1 and CCS copper chaperone genes; Southon et al., 2004), suggesting it is the result of a copper deficiency caused by increased efflux and that copper balance is restored by increasing the rate of copper uptake. Overexpression of Ctr1A, DmAtox1, or DmCCS alone has no effect on pigmentation (Figure 8G; our unpublished data). Although the hypopigmentation caused by DmATP7 overexpression in cuticular cells is suggestive of increased copper efflux from these cells, we wanted to further explore this phenotype by generating UAS-DmATP7 transgenes with mutations in key catalytic residues. According to in vitro studies with mammalian, yeast and bacterial P-type ATPases, ablation of the DKTG transient acyl phosphorylation site (TAP; Figure 1) or the transmembrane CPC motif (CPC; Figure 1) should render DmATP7 catalytically inactive, unable to transport copper across membranes, and therefore unable to export copper from the cell (Forbes and Cox, 1998; Voskoboinik et al., 2001). Ablation of the metal binding sites (MBS; Figure 1) should have the same effect under copper limiting conditions and compromise activity under normal conditions (Lutsenko and Petris, 2002; Voskoboinik and Camakaris, 2002).
Surprisingly, expression of the three mutated forms of DmATP7 under Pnr-GAL4 control still resulted in dramatic hypopigmentation, although the morphological defects caused by wild-type DmATP7 overexpression were reduced or absent (Figure 8, E and F). Importantly, however, coexpression of DmCtr1A fails to rescue the hypopigmentation caused by these mutated forms of DmATP7 (Figure 8I) despite their less severe phenotype, indicating that separate mechanisms are causing the hypopigmentation phenotype in the wild-type and mutant cases. Under control of the Patched (Ptc)-GAL4 driver, expression of wild-type DmATP7 resulted in a reduction of the scutellum and partial or complete loss of the scutellar bristles (Figure 8J), whereas expression of the inactive mutants had no effect (Figure 8, K and L), reinforcing the differing activities of these transgenes. Similarly, overexpression of wild-type DmATP7 in the tracheae resulted in late larval/early pupal death (our unpublished data), a phenotype not caused by catalytically inactive DmATP7. At least three independent insertion lines were tested for each transgene to rule out possible insertion effects, and expression levels for two insertion lines of each construct are shown by Western blot (Figure 8M) to demonstrate that the mutations introduced are not affecting protein stability.
The subcellular localization of the overexpressed DmATP7 forms was examined to determine whether this might explain the differences in the observed phenotypes. Predominantly PM staining was observed with all four transgenes when expressed in the wing imaginal disk either under Pnr-GAL4 or Ptc-GAL4 control (Figure 5, J-M). This staining is in stark contrast to the cytoplasmic staining observed in imaginal discs and gut cells of DmATP7 -/Y larvae rescued by low levels of transgene-derived DmATP7 expression (Figure 5, A-I), suggesting that high levels of expression of the DmATP7 protein result in a partial shift in subcellular localization, and this shift could be responsible for the over-expression phenotype.
DISCUSSION
Recently, the power of Drosophila genetics has been bought to bear on the investigation of copper homeostasis in a highly productive manner (Egli et al., 2003; Zhou et al., 2003; Southon et al., 2004). The metal-responsive transcription factor MTF-1 has been shown to be necessary in Drosophila for response to both excess and copper-limiting conditions, with MTF-1 mutants dying on high copper load and undergoing developmental arrest in copper-limiting conditions (Egli et al., 2003). It has subsequently been shown that a mutation in one of the three Drosophila copper import genes, Ctr1B, also causes sensitivity to both copper deficiency and copper load. Ctr1B mutants undergo developmental arrest and death at early second instar when grown in copper-limiting conditions, and die at late pupal stages when raised under high copper conditions (Zhou et al., 2003).
The current study presents data on the role of the third key component of Drosophila copper homeostasis, the sole Drosophila orthologue of the mammalian MNK and WND copper-transporting P-type ATPases. We have previously described a requirement for DmATP7 in copper efflux from cultured Drosophila embryonic S2 cells (Southon et al., 2004). This study demonstrates conservation of function between DmATP7 and its mammalian orthologues and shows that DmATP7 function is absolutely required in vivo for completion of embryogenesis, early larval growth and development, and adult pigmentation of the insect. Strikingly, un-like Ctr1B and MTF-1, the early embryonic requirement for DmATP7 is independent of dietary copper levels and is observed even before feeding stages.
Copper-dependent phenol oxidases such as tyrosinase are involved in the production of biogenic amines needed for cuticle sclerotization and pigmentation, neurotransmitter production, and protein and chitin cross-linking at eclosion and molting (Wright, 1987). The range of DmATP7 mutant phenotypes described here correlates well with disruption to all of these pathways. First, disruption of endogenous DmATP7 activity bleaches both yellow+ and yellow- cuticle, indicating that DmATP7 activity is required for production of all three pigment components, Dopa melanin, dopamine melanin, and NBAD sclerotin, in agreement with the proposed requirement of copper for phenol oxidase activity (Wright, 1987). Second, the majority of mutations in the genes encoding two central components of the pigmentation pathway, Dopa decarboxylase (Ddc) and tyrosine hydroxylase (pale), result in active larvae that are unable to eclose and have hypopigmented mouthparts (Jurgens et al., 1984), an identical phenotype to that seen in the DmATP7 mutants lacking maternal contribution. Third, several alleles in the Ddc gene cluster result in thoracic and bristle defects similar to those seen in DmATP7 overexpressing mutants (Wright, 1987).
Finally, the lethargic behavior of DmATP7 -/Y larvae could be explained by impaired neuronal function as a result of loss of copper-dependent Ddc-mediated neurotransmitter production or could reflect a role for DmATP7 similar to that of MNK in N-methyl-d-aspartate receptor-dependent neuronal activation (Schlief et al., 2005). Alternatively, the discovery of DmATP7 expression in developing tracheae suggests that lethargy could also be explained by a respiratory defect, a possibility supported by the lethal effect of DmATP7 overexpression when driven in the tracheae. Egli et al. (2003) postulate a role for copper in cellular respiration, because it is essential for cytochrome c oxidase function. A combination of improperly functioning tracheae and low cytochrome c oxidase activity as a result of functional copper deficiency would very likely cause lethargy and eventual lethality.
One unexpected outcome of this study was the similarity in DmATP7 loss-of-function and overexpression phenotypes in the adult cuticle, with both situations resulting in the loss of pigmentation. By analogy to MNK in mammalian cells, endogenous DmATP7 may normally reside in the TGN, with a fraction cycling constitutively between the TGN and the PM (Petris et al., 1996). We postulate that overexpressed DmATP7 might also accumulate at the PM, as previously seen for MNK in mammalian cells (Greenough and Camakaris, personal communication), where it would constitutively pump copper from the cell, resulting in a depletion of cellular copper levels. Indeed we have observed both wild-type and mutant DmATP7-FLAG localized to the PM. Copper levels can be restored by increasing copper uptake with Ctr1A overexpression, as we have observed. Catalytically inactive DmATP7 causes a similar but less severe phenotype. We postulate that this is a dominant negative effect whereby some of the overexpressed mutant DmATP7 is displacing endogenous wild-type DmATP7 from its TGN docking sites. The inactive DmATP7 cannot however transport copper into secretory pathway, thus depriving tyrosinase of its essential cofactor. In this scenario, increasing copper uptake would have no affect on tyrosinase activity, because the mutant DmATP7 would still be displacing endogenous DmATP7 at the TGN. This is consistent with our observations that Ctr1A coexpression only rescues wild-type DmATP7 overexpression.
A key characteristic of the MNK and WND proteins is their ability to undergo copper-stimulated trafficking from the TGN to the PM or cytoplasmic vesicles (Petris et al., 1996). We have been unable to demonstrate definitively that DmATP7 undergoes similar trafficking events. We have, however, observed both cytoplasmic and PM localization of endogenous DmATP7 in the embryo, compatible with the ability of DmATP7 to traffic. In addition, increased expression of transgene-derived DmATP7 results in a shift in localization from the cytoplasm to the PM, further demon-strating that DmATP7 contains PM localization signals. Resolution of this issue will require a DmATP7-specific antibody or live imaging with a GFP-DmATP7 fusion protein.
Using the genetic tools developed here, we can now start to decipher the complex transcriptional and posttranslational regulatory mechanisms required to maintain copper homeostasis in Drosophila, establishing a model for how multicellular organisms deal with metals that are both toxic and essential.
Supplementary Material
Acknowledgments
We are grateful to Walter Schaffner, Ernst Hafen, and Paul Whitington for the gifts of fly stocks. We also thank Len Kelly for comments on the manuscript. This work was supported financially by the International Copper Association, the Australian Research Council through its Special Research Centre and Discovery Grants programs, and through a grant from the University of Melbourne Research Grants Scheme. R. B. was a recipient of a Peter Doherty Fellowship from the National Health and Medical Research Council and the J. N. Peters Bequest Fellowship from the University of Melbourne.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-06-0492) on October 26, 2005.
Abbreviations used: AEL, after egg laying; BCS, bathocuproinedisulfonic acid; PM, plasma membrane; TGN, trans-Golgi network; TTM, tetrathiomolybdate.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Camakaris, J., Petris, M. J., Bailey, L., Shen, P., Lockhart, P., Glover, T. W., Barcroft, C., Patton, J., and Mercer, J. F. (1995). Gene amplification of the Menkes (MNK; ATP7A) P-type ATPase gene of CHO cells is associated with copper resistance and enhanced copper efflux. Hum. Mol. Genet. 4, 2117-2123. [DOI] [PubMed] [Google Scholar]
- Danks, D. M. (1988). Copper deficiency in humans. Annu. Rev. Nutr. 8, 235-257. [DOI] [PubMed] [Google Scholar]
- Danks, D. M. (1995). Disorders of copper transport. In: The Metabolic and Molecular Basis of Inherited Disease, ed. C. R. Scriver, A. L. Beaudet, W. M. Sly, and D. Valle, New York: McGraw-Hill, 2211-2235.
- Danks, D. M., Campbell, P. E., Stevens, B. J., Mayne, V., and Cartwright, E. (1972). Menkes's kinky hair syndrome. An inherited defect in copper absorption with widespread effects. Pediatrics 50, 188-201. [PubMed] [Google Scholar]
- Dubreuil, R. R. (2004). Copper cells and stomach acid secretion in the Drosophila midgut. Int. J. Biochem. Cell Biol. 36, 745-752. [DOI] [PubMed] [Google Scholar]
- Egli, D., Selvaraj, A., Yepiskoposyan, H., Zhang, B., Hafen, E., Georgiev, O., and Schaffner, W. (2003). Knockout of `metal-responsive transcription factor' MTF-1 in Drosophila by homologous recombination reveals its central role in heavy metal homeostasis. EMBO J. 22, 100-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein, J. (1989). PHYLIP -phylogeny inference package (version 3.2). Cladistics 5, 164-166. [Google Scholar]
- Forbes, J. R., and Cox, D. W. (1998). Functional characterization of missense mutations in ATP7B: Wilson disease mutation or normal variant? Am. J. Hum. Genet. 63, 1663-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell, B., and Gutteridge, J. M. (1984). Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219, 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza, I., Faisst, A., Prohaska, J., Chen, J., Gruss, P., and Gitlin, J. D. (2001). The metallochaperone Atox1 plays a critical role in perinatal copper homeostasis. Proc. Natl. Acad. Sci. USA 98, 6848-6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens, G., Wieschaus, E., Nusslein-Volhard, C., and Kluding, H. (1984). Mutations affecting the pattern of larval cuticle in Drosophila melanogaster. II. Zygotic loci on the third chromosome. Wilhelm Roux's Arch. Dev. Biol. 193, 283-295. [DOI] [PubMed] [Google Scholar]
- Lastowski-Perry, D., Otto, E., and Maroni, G. (1985). Nucleotide sequence and expression of a Drosophila metallothionein. J. Biol. Chem. 260, 1527-1530. [PubMed] [Google Scholar]
- Lutsenko, S., and Petris, M. J. (2002). Function and regulation of the mammalian copper-transporting ATPases: insights from biochemical and cell biological approaches. J. Membr. Biol. 191, 1-12. [DOI] [PubMed] [Google Scholar]
- Menkes, J. H., Alter, M., Steigleder, G. K., Weakley, D. R., and Sung, J. H. (1962). A sex-linked recessive disorder with retardation of growth, peculiar hair, and focal cerebral and cerebellar degeneration. Pediatrics 29, 764-779. [PubMed] [Google Scholar]
- Mokdad, R., Debec, A., and Wegnez, M. (1987). Metallothionein genes in Drosophila melanogaster constitute a dual system. Proc. Natl. Acad. Sci. USA 84, 2658-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena, M.M.O., Lee, J., and Thiele, D. J. (1999). A delicate balance: homeostatic control of copper uptake and distribution. J. Nutr. 129, 1251-1260. [DOI] [PubMed] [Google Scholar]
- Petris, M. J., Mercer, J. F., Culvenor, J. G., Lockhart, P., Gleeson, P. A., and Camakaris, J. (1996). Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J. 15, 6084-6095. [PMC free article] [PubMed] [Google Scholar]
- Petris, M. J., Strausak, D., and Mercer, J. F. (2000). The Menkes copper transporter is required for the activation of tyrosinase. Hum. Mol. Genet. 9, 2845-2851. [DOI] [PubMed] [Google Scholar]
- Robertson, H. M., Preston, C. R., Phillis, R. W., Johnson-Schlitz, D. M., Benz, W. K., and Engels, W. R. (1988). A stable genomic source of P element transposase in Drosophila melanogaster. Genetics 118, 461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofsen, H., Wolters, H., Van Luyn, M. J., Miura, N., Kuipers, F., and Vonk, R. J. (2000). Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology 119, 782-793. [DOI] [PubMed] [Google Scholar]
- Rozen, S., and Skaletsky, H. (2000). Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132, 365-386. [DOI] [PubMed] [Google Scholar]
- Scheinberg, I. H., and Sternlieb, I. (1984). Wilson's disease, Philadelphia: WB Saunders.
- Schlief, M. L., Craig, A. M., and Gitlin, J. D. (2005). NMDA receptor activation mediates copper homeostasis in hippocampal neurons. J. Neurosci. 25, 239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj, A., Balamurugan, K., Yepiskoposyan, H., Zhou, H., Egli, D., Georgiev, O., Thiele, D. J., and Schaffner, W. (2005). Metal-responsive transcription factor (MTF-1) handles both extremes, copper load and copper starvation, by activating different genes. Genes Dev. 19, 891-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondergaard, L. (1993). Homology between the mammalian liver and the Drosophila fat body. Trends Genet. 9, 193. [DOI] [PubMed] [Google Scholar]
- Southon, A., Burke, R., Norgate, M., Batterham, P., and Camakaris, J. (2004). Copper homoeostasis in Drosophila melanogaster S2 cells. Biochem. J. 383, 303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The FlyBase Consortium. (2003). The FlyBase database of the Drosophila genome projects and community literature. Nucleic Acids Res. 31, 172-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskoboinik, I., and Camakaris, J. (2002). Menkes copper-translocating P-type ATPase (ATP7A): biochemical and cell biology properties, and role in Menkes disease. J. Bioenerg. Biomembr. 34, 363-371. [DOI] [PubMed] [Google Scholar]
- Voskoboinik, I., Mar, J., Strausak, D., and Camakaris, J. (2001). The regulation of catalytic activity of the Menkes copper-translocating P-type ATPase. Role of high affinity copper-binding sites. J. Biol. Chem. 276, 28620-28627. [DOI] [PubMed] [Google Scholar]
- Wright, T. R. (1987). The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv. Genet. 24, 127-222. [PubMed] [Google Scholar]
- Xu, T., and Rubin, G. M. (1993). Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223-1237. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, Y., Heiny, M. E., Suzuki, M., and Gitlin, J. D. (1996). Biochemical characterization and intracellular localization of the Menkes disease protein. Proc. Natl. Acad. Sci. USA 93, 14030-14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H., Cadigan, K. M., and Thiele, D. J. (2003). A copper-regulated transporter required for copper acquisition, pigmentation, and specific stages of development in Drosophila melanogaster. J. Biol. Chem. 278, 48210-48218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.