Abstract
We have earlier proposed that a cell-cell interaction, mediated by the specific binding of molecules of the β-amyloid precursor protein (β-APP) on one cell surface with molecules of presenilin (PS) on the other cell surface, is a required initial step in the ultimate production of β-amyloid (Aβ) from β-APP. Aβ is widely believed to be the neurotoxic agent in Alzheimer's disease. In this paper, we test this proposal by modifying cells to express surface β-APP but no PS, and other cells to express surface PS but no β-APP. Coculturing these two cell populations at appropriate cell densities produces substantial amounts of Aβ that appear both in cell extracts and culture media. Such Aβ production could occur only if the two cell types interacted with one another to provide the β-APP and the PS required for the generation of Aβ. The addition to the coculture, from the start, of the soluble specific N-terminal domain of the appropriate PS significantly reduces the amount of Aβ produced. These and related experiments, therefore, suggest a very different mechanism for Aβ production than the one that is currently widely accepted.
Keywords: Alzheimer's disease, neurodegeneration, presenilin, β-amyloid precursor protein
The proposal that the β-amyloid (Aβ) oligopeptides are directly involved in the etiology of Alzheimer's disease (AD) was originally based on the critical discovery of the oligopeptides Aβ in amyloid deposits of AD victims (1). Aβ was soon shown to be the product of a ubiquitous integral membrane precursor protein, β-amyloid precursor protein (β-APP), (2-4) that underwent two proteolytic cleavages. The system was then further complicated by the finding that two previously unknown ubiquitous polytopic integral membrane proteins, the presenilins (PS-1 and PS-2), were also critical to the AD disease process (5-7). The question then arose, what were the respective roles of the β-APP and PS proteins in AD?
In considering the question in 1995, we recognized certain parallels between the AD system and, a quite different system, the cellular development of the ommatidium in the Drosophila eye (8, 9). This developmental process was shown to require a specific intercellular adhesion between a pre-R7 epithelial cell and its adjacent R8 neuronal cell to convert the former into the R7 neuronal cell. This cell-cell adhesion involved the specific intercellular binding of the protein sevenless (sev) on the pre-R7 cell surface to another named bride of sevenless (boss) on the R8 cell surface. Sev is an integral membrane tyrosine kinase with a single transmembrane (TM) hydrophobic domain (similar to the membrane topography of β-APP). Boss and PS were initially assigned a similar seven-helix TM (7-TM) topography in their respective membranes.
The subsequent steps in the downstream behavior of the (sev:boss) system are taken up in the Discussion. Here, it is sufficient to say that a double-membrane patch is formed between the pre-R7 and R8 cells, with its sev molecules collected in a membrane patch on the pre-R7 cell, attached to a membrane patch on the R8 cell by its sev-bound and collected boss molecules. The two membrane patches are endocytosed together into the pre-R7 cell as a double-membrane vesicle. This vesicle ultimately winds up internalized within a multivesicular body in the pre-R7 cell. The vesicle contents are then subject to degradation by enzymes within the multivesicular body.
By analogy to this system, we then proposed (10) that the β-APP and PS-1 or PS-2 molecules are related to one another as a specific receptor-ligand pair, and that the β-APP molecules on one cell surface, and the PS molecules on another cell surface, become specifically bound together and then collected by diffusion in their respective membranes to form a double-membrane patch [probably by a process called “mutual capping” (11)]. This patch is the site of the cell-cell adhesion, which is then endocytosed as one or more vesicles into the β-APP-contributing cell inside which the vesicle(s) are ultimately en-gulfed in lysosomes. The endocytosed β-APP is then cleaved by the enzymes β-secretase and γ-secretase in the lysosome to form Aβ. The Aβ is then released into the cell or secreted out of it. We proposed that this specific cell-cell interaction is on the major, if not the only, pathway to Aβ production in vivo. To the contrary, Aβ production is currently widely believed to occur entirely within single cells (12, 13).
Meanwhile, other investigators provided strong evidence that PS is required for the γ-secretase proteolytic cleavage of β-APP, either because PS itself is the enzyme molecule, or because it is an essential component of a γ-secretase enzyme complex. (For a review, see ref. 14.)
We recognized that there are many different aspects of our proposal that are amenable to experimental test. A relatively simple one is to determine whether or not there indeed exists a requirement for two cells to interact early in the pathway to Aβ formation. This can be achieved by preparing cultures of two cell types: the cells of one expressing β-APP but no PS and the other expressing PS but no β-APP. One may then determine whether Aβ is produced in mixed cultures of the two cells in much greater amounts than in various single cell culture controls. If only mixed cocultures of these cell types produced substantial amounts of Aβ, and further if membrane-impermeable specific inhibitors of the β-APP:PS binding, present from the start of the incubation, considerably reduced the production of Aβ, our proposal would be strongly supported. These and related experiments are described in this article.
Results
The Strategy for Producing β-APP-only and PS-only-Expressing Cells. PS-null ES mouse cells, expressing small amounts of endogenous mouse β-APP but no PS were transiently transfected for human β-APP to produce cells expressing β-APP but no PS-1 or PS-2. These were designated β-APP-only cells. Embryonic mouse neurons described in Materials and Methods from β-APP-null mice, expressing only small amounts of endogenous PS-1 and PS-2, were transfected for either human PS-1 or PS-2 to produce cells expressing PS-1 or PS-2, respectively, but no β-APP. These were designated PS-1-only or PS-2-only cells, respectively.
PS-null ES cells were transiently transfected with 15 μg of pcDNA constructs of full-length human β-APP 695, PS-1, PS-2, or vector only by using the lipofectamine (Invitrogen) method. Primary hippocampal neurons from APP-null mouse embryos (embryonic day 18) were transiently transfected with 15 μg of PS-1, PS-2, or vector only by using the identical method. In brief, the lipofectamine-DNA solution was left at room temperature for 30 min, mixed with enough serum-free medium, and added to the cells. Cells were incubated for 5 h at 37°C in a CO2 incubator after which the medium was replenished with serum. Cells were harvested 24 h after transfection.
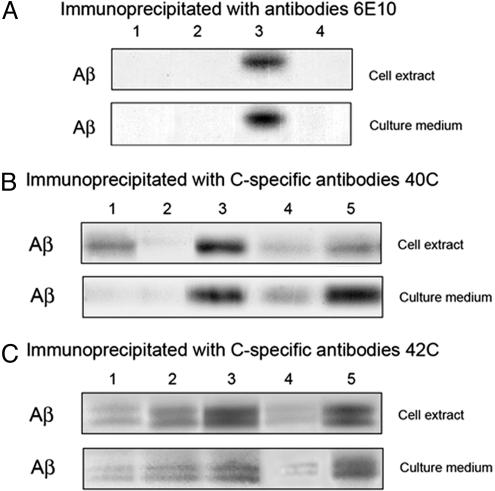
Aβ Production in Cocultures of PS-only-Expressing Neurons with β-APP-only-Expressing ES Cells. Cocultures of the cells were prepared and treated as described in Materials and Methods. Control cocultures in which pcDNA3-transfected neurons were substituted for the PS-1-transfected neurons are shown in Fig. 1A (lane 1), and, for the reverse controls, pcDNA3-transfected ES cells were substituted for the β-APP-transfected ES cells (Fig. 1 A, lane 2). Both controls show little Aβ production. However, the coculture of β-APP-only ES cells with PS-1-only neurons, exhibited substantial amounts of Aβ in both the cell extracts and culture media (Fig. 1 A, lane 3).
Fig. 1.
PS-1:β-APP cocultures. Autoradiographs of immunoprecipitated Aβ separated on Bicene·Tris gels. (A) Mouse ES cells (PS-1-/-; PS-2-/-) that were transfected with human β-APP and expressing β-APP but no PS, cocultured with mouse neurons (β-APP-/-) (N) expressing only transfected human PS-1 (lane 3), and showing the resultant Aβ produced by autoradiography of the gels of both cell extracts and culture media. (Lane 1, control) ES cells transfected with β-APP cocultured with N cells transfected with pcDNA3. (Lane 2, control) ES cells transfected with pcDNA3 cocultured with N cells transfected with PS-1. (Lane 4) Same as lane 3 except that the specific inhibitor N-terminal domain of PS-1 was present throughout the culture period. (Upper) Cell extracts were examined. (Lower) Culture media. These solutions were immunoprecipitated with mAbs 6E10 specific for residues 1-17 of Aβ and then separated by gel electrophoresis as described in Materials and Methods. Note the smaller amount of Aβ produced in the presence of the specific inhibitor (lane 4) than in its absence (lane 3). (B) The same as A except that Abs specific for the COOH-terminal of Aβ 1-40 were used for the immunoprecipitations. Therefore, no Aβ 1-42 is detected. Also, lane 5 shows the lesser inhibitory effect on Aβ production of the nonspecific N-terminal domain of PS-2 than that of the specific inhibitor N-terminal domain of PS-1 (lane 4). (C) The same as B except that Abs specific for the COOH-terminal of Aβ 1-42 were used for the immunoprecipitations. Therefore, no Aβ 1-40 is detected. Lane 4 shows the effect of the specific inhibitor, the N-terminal domain of PS-1, in reducing Aβ production compared with the nonspecific N-2 domain (lane 5).
Similar results to those in Fig. 1 A were obtained if different Aβ-specific Abs were used to immunoprecipitate Aβ from the cell extracts and the culture media. In Fig. 1 A, immunoprecipitation used the mAb 6E10, directed to an epitope within the sequence 1-17 of Aβ; in Fig. 1B, the immunoprecipitating Ab used (40C) was specific for the COOH-terminal residue of Aβ 1-40; and in Fig. 1C, the immunoprecipitating Ab (42C) was specific for the COOH-terminal residue of Aβ 1-42. Therefore, both Aβ 1-40 and Aβ 1-42 were produced by the coculture of PS-1-only cells with β-APP-only cells.
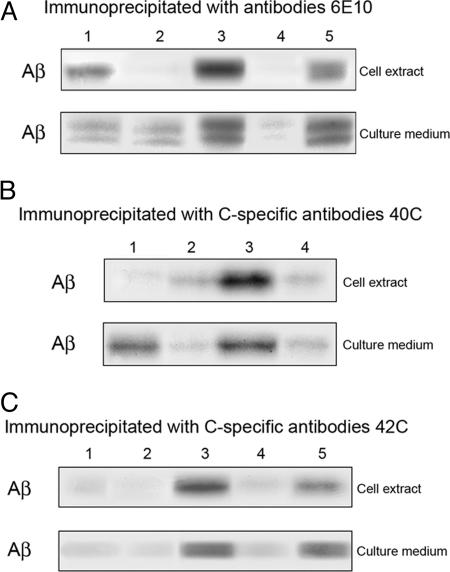
In similar coculture experiments using PS-2-transfected instead of PS-1-transfected-neurons and controls (Fig. 2 A-C compared with Fig. 1 A-C), generally similar results were obtained as with the PS-1 case. The cocultures of cells expressing only PS-2 with cells expressing only β-APP produced (Fig. 2 A, lane 3) significantly more Aβ than appeared in the controls (Fig. 2 A, lanes 1 and 2).
Fig. 2.
PS-2:β-APP cocultures. Autoradiographs of immunoprecipitated Aβ separated on Bicene·Tris gels. (A) Same conditions as Fig. 1 A, except that PS-2 was used instead of PS-1 to transfect the ES cells; in lane 4, the N-2 domain of PS-2 was present during the coculture period to serve as the specific inhibitor of the β-APP:PS-2 binding, whereas, in lane 5, the nonspecific N domain of PS-1 was present. (B) Same conditions as Fig. 1B, except that PS-2 was used instead of PS-1 to transfect the ES cells; in lane 4, the N-2 domain of PS-2 was present during the culture period instead of the N-1 domain of PS-1; the N-1 domain was instead used in lane 5. (C) Same conditions as B, except that Abs 6E10 were used for the immunoprecipitation of Aβ from the cell extracts and the culture media.
Specific Inhibition Experiments. The water-soluble cloned N-terminal domains of PS-1 and PS-2 fused with the FLAG protein (referred to as N-1 and N-2, respectively) have been shown to serve as membrane-impermeable specific inhibitors of the intercellular binding of plasma membrane-bound β-APP on one cell with the membrane-bound PS on another (15, 16). In the current experiments, the addition of excess N-1 at the start of the coculture of PS-1-only neurons with β-APP-only ES cells (Fig. 1 A-C, lane 4) resulted in a substantial decrease in the amount of Aβ produced, measured both in cell extracts and in the culture medium. That this inhibition of Aβ production was specific was affirmed by the absence of inhibition when N-2 was used in this experiment instead of N-1 (Fig. 1C, lane 5). Similar specific inhibition of Aβ production by N-2 in cocultures of PS-2-only neurons in the presence of β-APP-only ES cells (Fig. 2 A-C, lane 4), with no or less inhibition by nonspecific N-1 (Fig. 2 A and C, lane 5), was observed. These specific inhibitions of Aβ production by either N-1 or N-2 are very likely due to the specific reduction of intercellular binding of β-APP with the N-terminal domain of PS. This result is consistent with the 7-TM model of PS (15, 16) for which the N-terminal domain is extracellular but not in the 8-TM model, where it protrudes into the cytoplasm. Because the molecular sizes of N-1 and N-2 are too large to allow them to permeate the intact membrane of a live cell, these specific inhibitory effects of N-1 and N-2 must be acting only on extracellular PS:β-APP bonds.
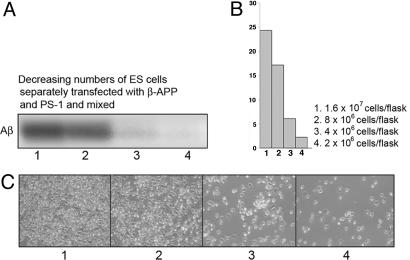
Cocultures of Mainly PS-Expressing ES Cells and only β-APP-Expressing ES Cells at Different Cell Densities. If an early specific cell-cell interaction is required for the production of Aβ, a prediction is that mixtures containing PS-only and β-APP-only cells cocultured at different total cell densities should result in decreasing Aβ production of the same numbers of cells with decreasing cell density. This would be expected because of the expected decrease in the required cell-cell contacts with dilution of the plated cells. On the other hand, if the processing of β-APP to Aβ involved entirely single cells, dilution of the cocultures should not change the Aβ production in the same numbers of cells.
Cocultures of equal numbers of β-APP-only-expressing ES cells and mainly human PS-1-expressing (along with a small amount of endogenous mouse APP) ES cells were used in these experiments, the latter instead of PS-1-only-expressing neurons because replating transfected neurons at different densities was not an option.
The mainly PS-expressing ES cells were prepared by transfection of the ES cells with the cDNA for PS-1 by the procedures described in Materials and Methods. The cocultures were plated at different cell densities ranging from 1.6 × 107 total cells per flask to 2 × 106 total cells per flask. The amount of Aβ produced by the same numbers of cells decreased steadily with the cell density on the plate (Fig. 3A, lanes 1-4; Fig. 3B, plot of density scans of bands in Fig. 3A), consistent with the requirement for a cell-cell interaction to generate the Aβ. Light microscopic observations of these plated cells at different cell densities, shown in Fig. 3C, indicate a steady increase in empty areas on the plates with decreasing cell density, resulting in a steady decrease in cell-cell contacts with cell dilution. These results are consistent with our proposal that a cell-cell contact is required for Aβ production.
Fig. 3.
Determination of Aβ from PS-1:β-APP cocultures plated at decreasing densities. ES cells transfected with β-APP were cocultured with ES cells transfected with PS-1, at the four different total cell densities shown in A, and equivalent amounts (100 μg of protein) of each cell extract were immunoprecipitated with Abs 6E10 and electrophoresed, and the gels were autora-diographed and densitometrically scanned (B, bar graphs). The cell cultures at each density were also plated out and photographed (C). The decreases in the amount of Aβ produced per cell with decreasing cell density (A and B) was reflected in the decreased densities of cell-cell contacts in the photographs (C).
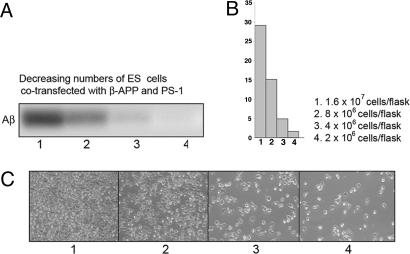
ES Cells Cotransfected with both β-APP and PS-1 Cultured at Different Cell Densities. Previous experiments by others on Aβ production in cultured cells have used cells expressing both β-APP and PS (usually cotransfected). Implicit in the design of such experiments has been the assumption that all of the events in the processing of β-APP to Aβ involve the β-APP and the β- and γ-secretases (the latter requiring PS) that are initially present in the same single cell. If this assumption is correct, then upon culturing at different cell densities, the same numbers of cotransfected cells should produce the same amount of Aβ. Onthe other hand, at sufficiently large cell densities, cells that coexpress both surface β-APP and surface PS might interact with one another via the β-APP on one cotransfected cell engaging the PS on another, thus generating a situation similar to that in cocultures of cells expressing only β-APP with cells expressing only PS. If cells cotransfected with β-APP and PS underwent such cell-cell interactions and produced Aβ only if they did, then dilutions of the cotransfected cells should show decreasing Aβ formation by the same numbers of cells. This indeed occurs as shown in Fig. 4 A and B. Furthermore, microscopic analysis of these cultures at the several cell densities (Fig. 4C) yielded results very similar to those with cocultures of singly expressing cells at the same total cell density (compare Fig. 4C with Fig. 3C). These results therefore provide further support for the proposal that the cotransfected cells underwent early cell-cell interaction to an extent similar to that of mixtures of the singly expressing cells at the same total cell densities.
Fig. 4.
Determination of Aβ from PS-1:β-APP cotransfected cultures plated at decreasing densities. Same as Fig. 3, except that ES cells cotransfected with both β-APP and PS-1 were cultured at different cell densities. Note that the amount of Aβ produced per cell at different densities (B, bar graphs), and the photographs of the cells at different densities (C), were very similar for these cultures of cotransfected cells to those obtained with the cocultures of mixed, single protein-expressing cells (Fig. 3).
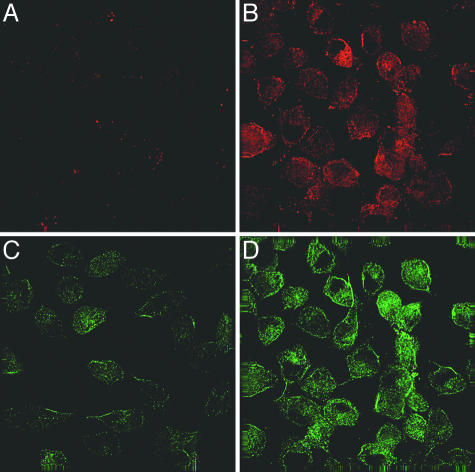
Expression of PS and β-APP. The immunofluorescence data in Fig. 5 show that the untransfected ES (PS-1-/-/PS-2-/-) double-null cells expressed negligible amounts of β-APP (Fig. 5A) and no PS-1 (Fig. 5C), but that the same cells cotransfected with both proteins coexpressed both β-APP (Fig. 5B) and PS-1 (Fig. 5D) robustly. The singly transfected cells expressed only the protein with which they were transfected (not shown). The results in Fig. 5 B and D indicate that a large fraction (>80%) of the cells were cotransfected by the procedures used.
Fig. 5.
Immunofluorescence deconvolution micrographs of ES cells cotransfected with β-APP and PS-1. Double immunofluorescence labeling for PS-1 (C and D) and for β-APP (A and B) on untransfected ES (PS-1-/-/PS-2-/-) cells (A and C) and on the same cells that were first cotransfected with both PS-1 and β-APP (B and D, respectively) (in lane 3, a small amount of endogenous mouse β-APP is observed). Note that the cotransfected cells, with few exceptions, express both PS-1 (B) and β-APP (D).
Discussion
The results presented provide strong support for our hypothesis (10) that the production of Aβ from β-APP occurs in substantial amounts via an initial specific cell-cell interaction, involving the binding of β-APP molecules on one cell surface to PS molecules on another (Figs. 1, 2, 3). The specificity of this interaction is demonstrated by the marked inhibition of Aβ formation by the presence in the culture medium of the specific N-terminal domain of the particular PS used (Fig. 2 A-C). The Aβ produced appears in both the cell extracts and the culture media and contains both Aβ 1-40 (Fig. 1B) and Aβ 1-42 (Fig. 1C).
This cell-cell adhesion induced by the specific binding of two membrane-bound proteins on opposing cells is probably first used in vivo to transmit two-way signals into both cells (17). We have shown that a transient protein tyrosine phosphorylation occurs within a few minutes of mixing β-APP-expressing cells with PS-expressing cells, which is specific for these two cell surface molecules (18). Also, it has been shown that PS-1 and PS-2 are both 7-TM helical integral membrane proteins that in nonidentical ways bind and activate heterotrimeric G proteins (N.N.D., unpublished data). Therefore, there is plausibility to the suggestion that a two-way signaling process follows shortly after the intercellular binding of β-APP and PS. The downstream events following these proposed two-way signaling processes may have important effects on normal cell physiology and the further development of the cells involved (17).
Whether the subsequent production of Aβ requires that such early two-way signaling processes take place is not yet known. It is conceivable that the two-way signaling processes are the normally significant ones resulting from the specific cell-cell interaction by influencing normal cell fates and development, whereas the production of Aβ is independent, perhaps part of the normal pathway of β-APP turnover, in this case with unfortunate consequences for specific neurons in the brain in AD.
We have not yet experimentally studied the detailed downstream events after the specific intercellular β-APP:PS binding, which lead to the eventual production of Aβ. If we continue to use the mechanisms of the pre-R7-R8 neuron interaction in the ommatidium of the fly eye (8, 9) as rough analogs of the mechanisms involved in Aβ production from β-APP, we suggest that after the binding of a sufficient number of β-APP molecules on one cell surface to PS molecules on the other, there occurs over the next 30 min or so a “mutual capping” (11), or coclustering, of β-APP in one cell membrane with its bound PS molecules in the other, leading to a double-membrane “patch” where the two cells adhere to one another (8, 9). Such patches may then be endocytosed into the β-APP-expressing cell as double-membrane vesicles containing intact β-APP and PS molecules in their membranes (8, 9). These vesicles subsequently wind up inside large lysosome-like multivesicular bodies, where various degradative enzymes are present. We propose that, inside these multivesicular bodies, the internalized β-APP is proteolyzed by β-secretase and γ-secretase to form Aβ, which is then either transferred to the cytoplasm of the β-APP-expressing cell or transported to the cell exterior.
Our proposed mechanism for β-APP proteolysis to Aβ in the lysosomal compartment can explain the so-called “spatial paradox” (23); there is none.
Although experiments to test this proposed downstream pathway to Aβ production have not been done yet, there are at least three sets of observations by others that are consistent with our proposal. One is that surface-labeled β-APP is in part internalized without cleavage in neuronal cell cultures and in a pathway that targets them to the lysosomal compartment, and that this surface β-APP is also used to produce Aβ (19, 20), as our proposal predicts. The second observation is that PS-1, nicastrin, and a lysosomal associated protein (LAMP-1) are all colocalized on lysosomal bodies by immunoelectron microscopy (21). The third is that the isolated β-secretase enzyme, that liberates the NH2 terminus of Aβ from β-APP, has an unusual pH optimum of its proteolytic activity of 4.5 (22), which is compatible with the localization of its activity to an acidic lysosomal-like compartment.
Whether an initial β-APP:PS-mediated cell-cell interaction is obligatory for any and all Aβ production or whether there are also additional pathways to produce Aβ, e.g., via the usual secretory pathway (12, 13), is not yet clear. We have, however, provided evidence, by dilution experiments with cells that coexpress both β-APP and PS, that Aβ production decreases with decreasing cell density (Fig. 4) in a manner that is quantitatively closely similar to the cocultures of singly expressing cells (Fig. 3). This suggests that in the cotransfected cultures, most, if not all, of the Aβ production also involved an obligatory cell-cell interaction.
Materials and Methods
Primary Neuron Culture from APP-/- Mice. Mice rendered null for β-APP-/- (24), denoted as β-APP-null mice, were obtained from Merck. Primary hippocampal neurons from these mice were cultured from embryos at E18 according to established protocols (25). Cells were plated on 25-cm2 tissue culture flasks pretreated with 2 mg/ml laminin (BD Biosciences) and grown in Neurobasal medium (GIBCO) supplemented with B27 complement mixture (GIBCO) and 0.2M l-glutamine for 4-5 days. The cultures were then grown in the presence of 2 μM Ara-C and used within 10 days.
ES (PS-1-/-/PS-2-/-) Cell Culture. ES (PS-1-/-/PS-2-/-) cells, null for both PS-1 and PS-2 (referred to as PS-null cells), were a kind gift from D. Donoviel and A. Bernstein and were cultured according to published protocols (26).
Abs. Aβ-specific mAb 6E10 raised to residues 1-17 of Aβ were purchased from Senetek (Napa, CA). Polyclonal Ab specific for the C-terminal residue of Aβ 1-40 (Ab40C) or of Aβ 1-42 (Ab42C), recognizing either Aβ 1-40 or Aβ 1-42, respectively, were purchased from Biosource.
Coculture Experiments. PS-double null ES cells or β-APP-null primary neurons derived from β-APP mice were plated at 1 × 107 cells per 25-cm2 flask and transfected with appropriate cDNAs as detailed above. Five hours after transfection, the PS-null ES cells transfected with β-APP were detached by mild trypsinization, washed two times with methionine (met)-free culture medium containing heat-inactivated, dialyzed FCS (10% vol/vol), and resuspended in this medium at 0.33 × 107 cells/ml. These cells (1 × 107 cells per 3 ml of met-free medium) were added to 1 × 107 PS-1-, PS-2-, or pcDNA3 vector only-transfected primary neurons from the β-APP-null mice in 3 ml of met-free medium (total volume was 6 ml). The cell densities used ensured that essentially all of the cells when plated would be in contact with another cell (see Results). The [35S]met [66 μCi/ml; 1,175 Ci/mmol, NEN (1 Ci = 37 GBq)] was added, and the cultures were incubated for 24 h. The medium was then removed, and cells were harvested by scraping. A protease inhibitor mix was added to the medium before freezing on dry ice. Extraction buffer (100 μl; 50 mM Tris, pH 8.0/150 mM NaCl/0.5% Nonidet-P40) containing protease inhibitors [1 mM 4-(2-aminoethyl) benzene sulfonyl fluoride hydrochloride/1 μg/ml antipain/0.1 μg/ml pepstatin A/0.1 μg/ml leupeptin] was added to the cell pellet, and the samples were quick-frozen on dry ice.
Preparation of Whole Cell Extracts. Cell-pellets were sonicated with three bursts of 20 s each on ice and proteins determined according to Lowry et al. (27).
Immunoprecipitations. Cell extract (100 μg) or 1 ml of conditioned medium were subjected to immunoprecipitation in an end-over-end rotator at 4°C overnight with 2 μg of Aβ-specific mAbs 6E10, or C-terminal-specific polyclonal Abs, 40C or 42C. Protein G Sepharose (40 μl of slurry) (Amersham Pharmacia) was then added and allowed to mix end-over-end for 1 h at room temperature. The antigen-Ab-protein G Sepharose complex was washed once with each of the following: buffer 1 (10 mM Tris·HCl, pH 7.4/1 mM EDTA, pH 8/0.65 M NaCl/1% Nonidet P-40), buffer 2 (10 mM Tris·HCl, pH 7.4/1 mM EDTA, pH 8/0.75% Nonidet P-40) and buffer 3 (10 mM Tris·HCl, pH 7.4/1 mM EDTA, pH 8/0.1% Nonidet P-40). The washed complex was then boiled for 10 min in bicene-Tris sample buffer (28) and subjected to SDS/PAGE on Bicene-Tris gels.
Bicene-Tris Gel Analysis. Bicene-Tris gels (15% T/5% C) with 8 M urea were cast and run according to Klafki et al. (28). The gels were then fixed for 30 min with 5% glutaraldehyde in 0.4 M sodium borate/phosphate buffer and stained for 1 h with 0.1% Coomassie blue G250 in methanol-acetic acid. After destaining, the gels were prepared for autoradiography.
Autoradiography. The destained gels were treated with ethanol (30%) and glycerol (5%) for 30 min, impregnated with Amplify (Amersham Pharmacia) for 30 min, dried under vacuum at 80°C, and exposed to X-Omat Film at -70°C for 4-5 days.
Coculture Experiments at Lower Cell Densities. For these experiments, PS-transfected ES-null cells were resuspended in met-free medium at the lower density of 0.1 × 107 cells per ml. β-APP-transfected ES-null cells were similarly resuspended in met-free medium at 0.1 × 107 cells per ml. Equal volumes of cell suspensions were mixed for 5 min and immediately plated on 75-mm flasks to give final total cell numbers that ranged from 1.6 × 107 cells in 16 ml down to 0.2 × 107 cells in 2 ml. The [35S]met (66 μCi/ml) was added, and cultures were incubated for 24 h. The cells were harvested and processed as described above. The numbers of total cells per diluted coculture, and the amounts of each culture taken for analysis of Aβ ensured that, if all Aβ production were carried out by single cells, the amount of Aβ on the bicene gels would be the same, but if two or more cells were required, the amounts of Aβ on the gels would decrease with dilution of the cultures. The same applies to the cotransfected cultures.
Cotransfection Experiments. Cotransfection experiments entailed cotransfecting ES-PS double null cells with both β-APP and PS simultaneously and then plating them, as for cocultures, at the higher densities or at several lower densities to reduce cell-cell contact. As with the singly transfected cells, 5 h after transfection, heat-inactivated, dialyzed FCS was added to 10%, and the cells were rinsed with met-free medium containing heat-inactivated dialyzed FCS and replated in met-free medium containing heat-inactivated, dialyzed FCS in the range of 1.6 × 107 cells in 16 ml of medium per flask, down to 0.2 × 107 cells in 2 ml of medium. The 66 μCi [35S]met/ml was added, and the cultures were incubated at 37°C for 0-24 h and further processed as described above.
Specific Inhibition of Cell-Cell Interaction. The N-terminal domains of PS-1 and PS-2 were expressed as FLAG fusion proteins in bacteria and affinity was purified (16). In inhibition experiments, 20 μg of purified protein of the N-terminal domain of either PS-1 or PS-2 fused to FLAG was added to the β-APP:PS cocultures at the time of mixing the transfected cells, and another 10 μg was added after 12 h.
Light Microscopy. Light micrographs of the culture plates of the cocultured singly transfected cells and of the cotransfected cells at the several plated densities were captured with a Nikon digital D 50 camera.
Immunofluorescence Microscopy. Primary Abs. Primary rat anti-PS-1 mAb no. 1563 was purchased from Chemicon. It was raised to a fusion protein antigen containing part of the N-terminal of human PS-1 (residues 21-80) fused to GST. The mouse mAb β-APP no. 348 raised to the exoplasmic domain was purchased from Boehringer Ingelheim.
Secondary Abs. FITC-conjugated affinity pure donkey anti-mouse IgG and tetramethylrhodamine B isothiocyanate-conjugated affinity pure goat anti-rat IgG secondary Abs were purchased from Jackson ImmunoResearch.
ES-null cells were plated on coverslips and cotransfected with pcDNA3 constructs of both β-APP and PS-1 as described (16). Twenty-four hours after transfection, the cells were fixed with 4% paraformaldehyde in PBS for 10 min. After washing with PBS, cells were double immunolabeled with primary mAbs to PS-1 and β-APP as described, followed by the appropriately labeled secondary Abs. After washing with PBS, the coverslips were mounted onto slides in the presence of mounting medium (Vector Laboratories). The immunolabeled cells were examined with a DeltaVision deconvolution microscope system (Applied Precision, Issaquah, WA) by using a Nikon TE-200 inverted epifluorescence microscope as described (16).
Acknowledgments
We thank Drs. Fumitoshi Irie and Yu Yamaguchi of the Burnham Institute (La Jolla, CA) for their help and training in primary neuron culture; Ms. Michelle Williams of BioMedical Design (San Diego) for help with figures; and Dr. James Feramisco and Mr. Steve Mullen for help with confocal microscopy. This work was supported by National Institutes of Health Grants 5R01 NS27580, 5R01 AG17858, and 5R01 NS044768 (to N.N.D.) and microscopy core grant P30 NS047101.
Author contributions: N.N.D. and S.J.S. designed research; N.N.D. and D.M. performed research; N.N.D., D.M., and S.J.S. analyzed data; and N.N.D. and S.J.S. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: β-APP, β-amyloid precursor protein; PS, presenilin; Aβ, β-amyloid; AD, Alzheimer's disease; TM, transmembrane; met, methionine.
References
- 1.Glenner, G. & Wong, C. (1984) Biochem. Biophys. Res. Commun. 120, 885-890. [DOI] [PubMed] [Google Scholar]
- 2.Kang, J., Lemaire, H.-G., Unterbeck, A., Salbaum, J. M., Masters, C. L., Grzeschik, K.-H., Multhaup, G., Beyreuther, K. & Müller-Hill, B. (1987) Nature 325, 733-736. [DOI] [PubMed] [Google Scholar]
- 3.Goldgaber, D., Lerman, M. I., McBride, O. W., Saffioti, U. & Gajdusek, D. C. (1987) Science 235, 877-880. [DOI] [PubMed] [Google Scholar]
- 4.Tanzi, R. E., Gusella, J. F., Watkins, P. C., Bruns, G. A. P., St. George-Hyslop, P., Van Keuren, M. L., Patterson, D., Pagan, S., Kurnit, D. M. & Neve, R. L. (1987) Science 235, 880-884. [DOI] [PubMed] [Google Scholar]
- 5.Sherrington, R., Rogaev, E. I., Liang, Y., Rogaeva, E. A., Levesque, G., Ikeda, M., Chi, H., Lin, C., Li, G., Holman, K., et al. (1995) Nature 375, 754-760. [DOI] [PubMed] [Google Scholar]
- 6.Levy-Lehad, E., Wijsman, E. M., Nemens, E., Anderson, L., Goddard, K. A. B., Weber, J. L., Bird, T. D. & Schellenberg, G. D. (1995) Science 269, 970-973. [DOI] [PubMed] [Google Scholar]
- 7.Rogaev, E. I., Sherrington, R., Rogaeva, E. A., Levesque, G., Ikeda, M., Liang, Y., Chi, H., Lin, C., Holman, K., Tsuda, T., et al. (1995) Nature 376, 775-778. [DOI] [PubMed] [Google Scholar]
- 8.Krämer, H., Cagan, R. L. & Zipursky, S. L. (1991) Nature 352, 207-212. [DOI] [PubMed] [Google Scholar]
- 9.Cagan, R. L., Krämer, H., Hart, A. C. & Zipursky, S. L. (1992) Cell 69, 393-399. [DOI] [PubMed] [Google Scholar]
- 10.Dewji, N. N. & Singer, S. J. (1996) Science 271, 159-160. [DOI] [PubMed] [Google Scholar]
- 11.Singer, S. J. (1992) Science 255, 1671-1677. [DOI] [PubMed] [Google Scholar]
- 12.Busciglio, J., Gabuzda, D. H., Matsudara, P. & Yankner, B. A. (1993) Proc. Natl. Acad. Sci. USA 90, 2092-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoji, M., Golde, T. E., Ghiso, J., Cheung, T. T., Estus, S., Shaffer, L. M., Cai, X.-D., McKay, D. M., Tintner, R., Frangione, B., et al. (1992) Science 258, 126-129. [DOI] [PubMed] [Google Scholar]
- 14.Sisodia, S. S. & St. George-Hyslop, P. H. (2002) Nat. Rev. Neurosci. 3, 281-290. [DOI] [PubMed] [Google Scholar]
- 15.Dewji, N. N. & Singer, S. J. (1997) Proc. Natl. Acad. Sci. USA 94, 14025-14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dewji, N. N., Valdez, D. & Singer, S. J. (2004) Proc. Natl. Acad. Sci. USA 101, 1057-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasquale, E. B. (2005) Nat. Rev. Mol. Cell. Biol. 6, 462-475. [DOI] [PubMed] [Google Scholar]
- 18.Dewji, N. N. & Singer, S. J. (1998) Proc. Natl. Acad. Sci. USA 95, 15055-15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koo, E. H., Squazzo, S. L., Selkoe, D. J. & Koo, C. H. (1996) J. Cell Sci. 109, 991-998. [DOI] [PubMed] [Google Scholar]
- 20.Marquez-Sterling, N. R., Lo, A. C. Y., Sisodia, S. S. & Koo. E. H. (1997) J. Neurosci. 17, 140-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasternak, S. H., Bagshaw, R. D., Guiral, M., Zhang, S., Ackerley, C. A., Pak, B. J., Callahan, J. W. & Mahuran, D. J. (2003) J. Biol. Chem. 278, 26687-26694. [DOI] [PubMed] [Google Scholar]
- 22.Vassar, R., Bennett, B. D., Babu-Khan, S., Kahn, S., Mendiaz, E. A., Denis, P., Teplow, D. B., Ross, S., Amarante, P., Loeloff, R., et al. (1999) Science 286, 735-741. [DOI] [PubMed] [Google Scholar]
- 23.Wolf, M. S. & Haass, C. (2001) J. Biol. Chem. 276, 5413-5416. [DOI] [PubMed] [Google Scholar]
- 24.Zheng, H., Jiang, M., Trumbauer, M. E., Hopkins, R., Sirinathsinghji, D. J., Stevens, K. A., Conner, M. W., Slunt, H. H., Sisodia, S. S., Chen, H. Y., & Van de Ploeg, L. H. (1996) Ann. N.Y. Acad. Sci. 777, 421-426. [DOI] [PubMed] [Google Scholar]
- 25.Fedoroff, S. (2001) in Protocols for Neural Cell Culture, eds. Fedoroff, S. & Richardson, A. (Humana, Totowa, NJ), 3rd Ed.
- 26.Donoviel, D. B., Hadjantonakis, A. K., Ideda, M., Zheng, M., St. George-Hyslop, P. H. & Bernstein, A. (1999) Genes Dev. 13, 2801-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. (1951) J. Biol. Chem. 193, 265-275. [PubMed] [Google Scholar]
- 28.Klafki, H. W., Wiltfang, J. & Staufenbiel, M. (1996) Anal. Biochem. 237, 24-29. [DOI] [PubMed] [Google Scholar]