Abstract
Activation of T cells by antigen requires adhesive interactions with antigen-presenting cells (APC) in which leukocyte function-associated antigen 1 (LFA-1) and intercellular adhesion molecules (ICAMs) are important. However, it is not well understood what signaling molecules regulate this process and how the modulation of adhesive events influences T-cell activation. Here we show that Rap1 is activated in T cells in an antigen-dependent manner and accumulated at the contact site of T-cell and antigen-loaded APC. Inhibition of Rap1 activation by a dominant-negative Rap1 or SPA-1, a Rap1 GTPase-activating protein, abrogates LFA-1-ICAM-1-mediated adhesive interactions with antigen-pulsed APC and the subsequent T-cell-receptor triggering and interleukin-2 production. Conversely, augmented antigen-dependent Rap1 activation by the expression of wild-type Rap1 enhances these responses but culminates in apoptosis by Fas and FasL. Thus, Rap1 functions as a key regulator of T-cell and APC interactions and modulates T-cell responses from productive activation to activation-induced cell death by regulating the strength of adhesive interactions. Moreover, constitutive Rap1 activation rendered T cells unresponsive with accumulation of p27Kip1. Our study indicates that the activation state of Rap1 has a decisive effect on the T-cell response to antigen.
Critical to the adaptive immune system is T-cell activation, which depends on the interaction of T-cell receptors (TCR) with antigen peptides bound to the major histocompatibility complex (MHC) displayed on the surface of antigen-presenting cells (APC), including dendritic cells, macrophages, and B cells. Rapid progress has been made in dissecting the signal transduction of T-cell activation after TCR engagement in early (tyrosine phosphorylation and calcium mobilization) and late events (cytokine production and cell proliferation) (37). A characteristic feature of T-cell activation is that sustained TCR signaling is required for cytokine production and proliferation (18, 58). However, it is not clear how sustained TCR signaling is achieved. In fact, the TCR has difficulty in recognizing the peptide-MHC complex due to a low affinity and high off rate (9, 40, 60), as well as the limited amount of peptide-MHC complex displayed on APC (10, 21). Leukocyte function-associated antigen 1 (LFA-1) was demonstrated to play pivotal roles in facilitating the functional triggering of the TCR at lower antigen densities on APC through T-cell-APC adhesion. In LFA-1-deficient T cells, 100-fold more antigen was required for T-cell activation (2). Thus, regulated adhesion of T cells with APC through LFA-1 is thought to be a critical step in the generation of a sustained TCR-mediated signal.
Recent observations have revealed that T cells and APC form distinct contact zones, referred to as a supramolecular activation clusters (42) or immunological synapses (13, 14, 61), with a central cluster of the TCR-peptide-MHC complex surrounded by a ring of LFA-1-intracellular adhesion molecule 1 (ICAM-1). The formation of these antigen-specific, spatially segregated contact zones was correlated with T-cell proliferation (20). Real-time imaging analysis revealed sequential events of redistribution of TCR-peptide-MHC complexes and LFA-1-ICAM-1 (20). The initial contact is established between adhesion molecules like LFA-1 on T cells and ICAM-1 on APC (11, 22, 52). These molecules mediate a low-affinity adhesion, and the TCR attempts to engage the specific peptide-MHC complex. Once the TCR is successfully engaged, LFA-1 is converted to a high-affinity state (15) and the T cell stops migrating (11), leading to the formation of immunological synapses (12). Therefore, dynamic changes in the adhesive activity of LFA-1 induced by TCR signaling appear to play an important role in T-cell activation through regulation of interactions with APC.
Avidity modulation of LFA-1, like other integrins, is regulated by so-called inside-out signals (53) triggered by cytokines, chemokines, or antigens. These stimuli are thought to generate intracellular second messengers, leading to alteration of the diffusion, clustering, and/or affinity of LFA-1 (56). We previously demonstrated that protein kinase C, phosphatidylinositol 3-kinase, and Ras/Rho family small GTPases were capable of upregulating the adhesive activity of LFA-1 through distinct effects on conformation and affinity. In particular, the active form of Rap1 induced changes in the conformation and affinity of LFA-1 and caused marked actin cytoskeleton-dependent cell aggregation (30). Rap1-mediated LFA-1 activation was demonstrated by cross-linking of CD31 (46). Furthermore, TCR-mediated LFA-1 activation was reduced by a dominant-negative Rap1 in Jurkat cells (30). Based on these results, we hypothesize that Rap1 regulates T-cell activation by controlling the interaction of T cells with APC through LFA-1.
Here we demonstrate that antigen-dependent activation of Rap1 causes conjugate formation with antigen-loaded APC and subsequent interleukin-2 (IL-2) production. Enhanced T-cell-APC interactions on the expression of wild-type Rap1 led to activation-induced cell death. Nevertheless, persistent Rap1 preactivation rendered T cells unresponsive to antigen with accumulation of p27Kip1. These results indicate that Rap1 is a key regulator of T-cell activation through the dynamics of T-cell-APC interactions.
MATERIALS AND METHODS
Cell culture and transfection.
Jurkat cells and 3A9 hen egg lysozyme (HEL)-specific, I-Ak-restricted T-cell hybridoma (1) (provided by P. M. Allen, Washington University, St. Louis, Mo.), CH27 B-lymphoblastoid cells (39) (provided by D. Gerlier, CNRS-ENS Lyon, France), and A20.2J B cell lymphoma cells (50) (provided by T. Kakiuchi, Toho University School of Medicine) were suspended in RPMI 1640 medium (Gibco-BRL) containing 10% fetal calf serum (Sigma Chemical Co.), with or without 50 μM β-mercaptoethanol. T-cell clones were established from ovalbumin (OVA)-specific, I-Ad-restricted T-cell lines (provided by Y. Ohta, Takeda Chemical Industries, Ltd.) as described elsewhere (44).
Electroporation or retrovirus-mediated transfection with GP+E86 packaging cells was employed to introduce cDNAs into T cells as described earlier (30, 33). Cells were grown for 48 h and selected with appropriate drugs. The selected 3A9 T cells were cloned by limiting dilution, and more than five clones were used for each experiment. An OVA-specific T-cell clone was cocultured with GP+E86 cells producing SPA-1 and Rap1V12.
Plasmids with Rap1 GTPase mutants and Spa-1.
Plasmids with T7 epitope-tagged wild-type and mutant Rap1 were as described elsewhere (30). Flag epitope tag was introduced at the N terminus of Spa-1 (55). The constructs were subcloned in pcDNA3 (Invitrogen) or a retroviral vector, pMX-neo. Wild-type Rap1 cDNA was inserted into the N terminus of enhanced green fluorescent protein (EGFP) (Clontech) to produce Rap1-GFP. Flag-tagged Spa-1 was also conditionally expressed using a Cre/loxP system (Takara). Briefly, flag-tagged Spa-1 subcloned into the pCALN vector (pCALN Spa-1) containing a Cre-mediated switching expression cassette (29) was transfected into Jurkat cells. To induce SPA-1 expressions, the pCALN Spa-1 transfected Jurkat cells were infected with the adenovirus expressing Cre recombinase (AxCANCre), which was produced in 293 cells. A dominant-negative form of H-ras which has a point mutation created by substitution of serine for asparagine at position 17 (H-rasN17) was also subcloned in pcDNA3.
Cell adhesion assays.
For Jurkat cells, human ICAM-1 was purified by immunoaffinity chromatography from cell lysates prepared from JY cells (109), as described earlier (31). Each well of a polystyrene microtiter plate (96-well plate; Limbro-Flow) was coated with 2 μg of the purified ICAM-1 for 90 min and then blocked in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) for 30 min at room temperature. The amount of ICAM-1 used for coating was chosen for maximum cell binding. With this coating, site density was ca. 2,400 sites/mm2 as quantified by using 125I-labeled RR1/1 (2 μCi/mg) at a final concentration of 20 μg/ml. Assays of adhesion with ICAM-1-coated plates were performed as described earlier (32). For inhibition, coated wells or labeled cells were incubated with 20 μg of anti-human ICAM-1 antibody (RR1/1) or anti-human LFA-1 antibody (TS1/22) per ml for 30 min at room temperature before the assay.
For adhesion assays for 3A9 T cells, a mouse ICAM-1-Ig chimera was used, which was prepared essentially as described earlier (41). Briefly, the extracellular region of mouse ICAM-1 (25) was subcloned into the pIG-1, which encoded in frame the Fc portion (hinge, CH2 and CH3 domains) of human immunoglobulin G1 (IgG1) (24). The construct was then transfected into Cos cells by electroporation. The mouse ICAM-1-Ig chimeric proteins were purified from culture supernatants by using protein A-Sepharose 4 Fast Flow (Pharmacia). To coat mICAM-1-Ig, the 96-well plate was precoated with 100 μl of a 10-μg/ml concentration of rabbit anti-human IgG Fc (Cappel) at 4°C overnight. The plate was then rinsed twice with PBS and further incubated with 100 μl of a 0.25-μg/ml concentration of mICAM-1-Ig at room temperature for 2 h, followed by blocking with 1% BSA for 30 min. Adhesion assays were performed as described elsewhere (32). Adhesion without coating with mICAM-1-Ig was <1% of that of the input cells.
T-cell stimulation by APC or antibody cross-linking and IL-2 measurement.
3A9 and the OVA-specific T cells (3 × 104) were cultured with CH27 and A20.2J B cells (3 × 104) with or without 100 μg of HEL and 1 mg of OVA (Sigma) per ml, respectively, in a final volume of 200 μl in a 96-well flat-bottom microtiter plate. For inhibition with antibodies, monoclonal rat anti-mouse LFA-1 (FD441.8 and KBA2) antibodies and anti-mouse ICAM-1 (KAT-1) were included in the culture medium. For antibody stimulation, Jurkat cells (1 × 105) or the T-cell clone (3 × 104) were stimulated with anti-CD3 (OKT3) and CD28 (CD28.2; PharMingen) or anti-CD3 (2C11) and CD28 (37.51; Pharmingen) in a final volume of 100 μl in a 96-well flat-bottom microtiter plate. After 8 to 16 h, the amount of IL-2 in culture supernatants was measured by using the IL-2-dependent cell line CTLL-2 (17). The proliferation of CTLL-2 cells was evaluated with a WST Colorimetric Assay (Wako). IL-2 concentrations were calibrated to a standard curve by using recombinant mouse IL-2 (Genzyme).
Pulldown assays.
Pulldown of Rap1-GTP using a glutathione S-transferase (GST)-RalGDS-Ras binding domain (RBD) fusion protein was previously described (16). Pulldown assays of H-ras-GTP and Rac-GTP by using GST-Raf1-RBD and PAK-CD (CRIB domain) fusion proteins were also performed as described earlier (47, 48). Briefly, 107 cells lysed in ice-cold lysis buffer (1% Triton X-100; 50 mM Tris-HCl, pH 7.5; 100 mM NaCl; 10 mM MgCl2; 1 mM phenylmethylsulfonyl fluoride; 1 mM leupeptin; 0.5 mM aprotinin) were incubated with GST-RalGDS-RBD, GST-Raf-RBD, and GST-PAK-CD fusion proteins coupled to glutathione agarose beads for 1 h at 4°C. Beads were washed three times with lysis buffer and subjected to Western blotting by using anti-Rap1A (Transduction Laboratory) or anti-T7 epitope (Novagen), anti-H-ras (Transduction Laboratory), and anti-Rac (Transduction Laboratory) as described previously (30). Western blotting of total cell lysates (5 × 104 cells) was also performed (30).
Conjugation assays.
APC were labeled with the dye PKH-26 (Sigma) and then incubated for 16 h with or without 100 μg of HEL or 1 mg of OVA per ml. T cells were labeled with 0.1 μM 5,6-carboxyfluorescein diacetate (CFSE) (Molecular Probes) for 15 min at 37°C. T cells were incubated with an equal amount of APC (105 cells) for 30 min at 37°C. Nonspecific aggregates were disrupted by vortexing, and the samples were analyzed by using FACS Calibur (Becton Dickinson). For the inhibition of antibodies, T cells were incubated for 30 min at 37°C with or without 20 μg of anti-LFA-1 antibody (FD441.8) per ml. The percent conjugates, defined as the number of live-gated, double-positive events in the upper right quadrant divided by the total number of live-gated events, was determined for each sample (49).
TCR downregulation.
3A9 T-cell hybridoma (105) were mixed with antigen-pulsed CH27 B cells (105) and incubated at 37°C for 1 or 3 h. Cells were harvested and stained with biotinylated 2C11 (Pharmingen), followed by treatment with streptavidin-fluorescein isothiocyanate (FITC) (Pharmingen). To gate out the B cells, CH27 B cells were labeled with the dye PKH-26. The percentage of downregulation of TCR-CD3 was determined from the median values by using 3A9 T cells mixed with unpulsed APC as a reference.
Immunostaining.
3A9T cells expressing T7-tagged Rap1V12 were mixed with CH27 B cells for 30 min at 37°C. Cellular conjugates were bound to poly-l-lysine-coated coverslips and then fixed for 15 min with 3% formaldehyde. Cells were permealized with 0.2% Triton X-100 for 5 min, followed by blocking with 10% goat serum for 20 min, and then stained with anti-T7 monoclonal antibody (1/1,000 dilution with 10% goat serum) (Novagen) and detected with Alexa Fluor 488 goat anti-mouse IgG (1/400 dilution with 10% goat serum) (Molecular Probes). The cells were inspected with a confocal laser microscope (LSM510; Zeiss).
Apoptosis and cell cycle analysis.
Cell viability was assessed by the addition of 10 μg of propidium iodide (PI; Sigma) per ml and immediate analysis by flow cytometry. Chromosomal DNA was isolated by using a DNA purification kit (Qiagen, Inc.) according to the manufacturer's instructions and analyzed in a 1.5% agarose gel for DNA fragmentation. To elucidate whether apoptosis was mediated by Fas/FasL, 10 μg of of chimeric Fas-Fc or TNFR1-Fc fusion protein (28, 59) (provided by S.-K. Jung, Kyoto University Institute of Virus Research) was added per ml to the culture of T cells and APC, as described above. For cell cycle analysis, cells were permeabilized by adding 0.1% Triton X-100, incubated with 0.2 mg of RNase A (Sigma) per ml, and stained with 50 μg of PI/ml. The DNA content of the cells was analyzed by flow cytometry. Anti-mouse FasL (MFL1; PharMingen) was used for flow cytometric analysis. Goat polyclonal anti-p27Kip1 (Santa Cruz) was used to detect p27Kip1 by Western blotting. Mouse monoclonal antiphosphorylated ERK1 and ERK2 (Santa Cruz) and rabbit anti-ERK1 and ERK2 antibodies (Santa Cruz) were used to determine the phosphorylation status of ERKs in Western blotting analyses.
RESULTS
Rap1 mediates TCR-stimulated adhesion to ICAM-1 via LFA-1 in Jurkat cells.
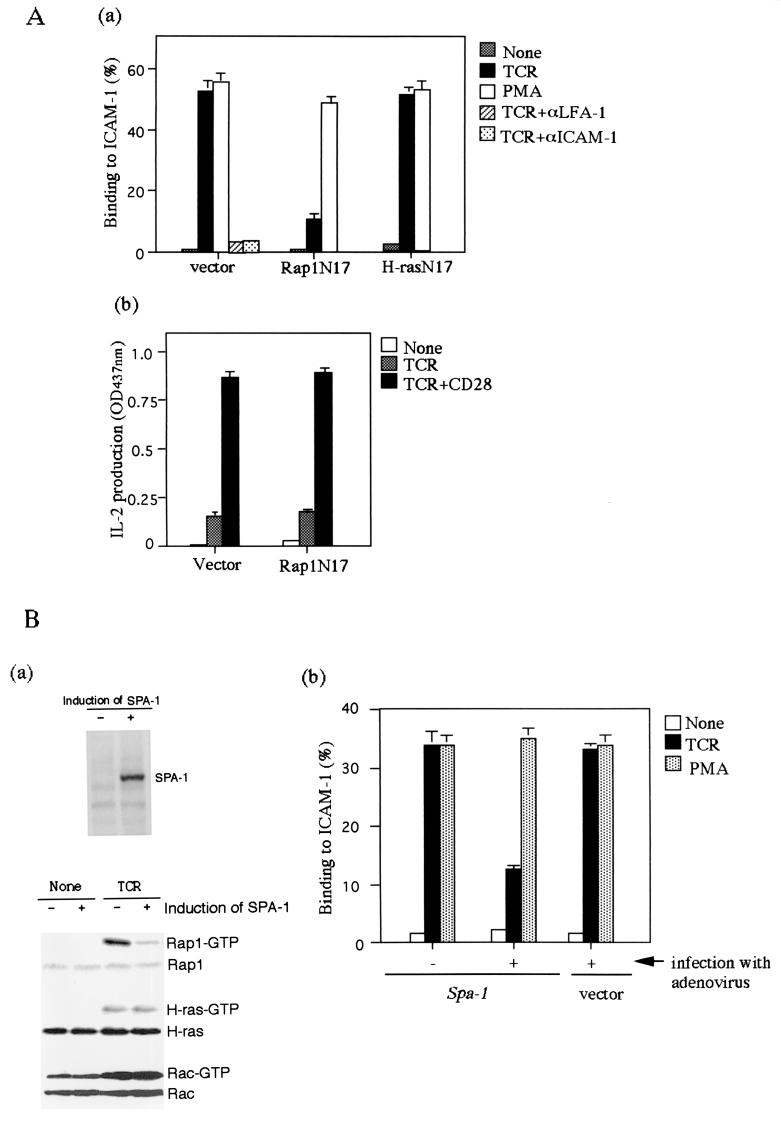
To examine the relative contributions of Rap1 in adhesion and activation of T cells, we examined the effect of a dominant-negative Rap1 (Rap1N17) in adhesion to ICAM-1 and IL-2 production of Jurkat cells. With antibody cross-linking of the TCR complex for 30 min, Jurkat cells adhered to ICAM-1, which was inhibited by blocking antibodies to LFA-1 (TS1/22) or ICAM-1 (RR1/1) (Fig. 1Aa). Activation of Rap1 occurred within minutes and lasted as long as 30 min (Fig. 1Ba and data not shown). The expression of Rap1N17, but not H-rasN17, almost completely blocked the TCR-dependent increase in adhesion, whereas Rap1N17 failed to inhibit PMA-induced LFA-1-ICAM-1 adhesion (Fig. 1Aa). On the other hand, the expression of Rap1N17 did not affect IL-2 production by the TCR or TCR-CD28 stimulation (Fig. 1Ab), suggesting that Rap1 functions mainly in the inside-out signaling through the TCR.
FIG. 1.
TCR-induced adhesion of Jurkat cells to ICAM-1 is inhibited by Rap1N17 or SPA-1. (Aa) Adhesion of Jurkat cells transfected with vector alone, Rap1N17, and H-rasN17. Jurkat cells were stimulated with or without 1 μg of OKT3 (TCR) or 10 ng of PMA per ml for 30 min at 37°C in ICAM-1-coated plates, as described in Materials and Methods. The level of adhesion to BSA was <1%. Average and standard errors of triplicate experiments are shown. (Ab) IL-2 production upon stimulation with TCR-CD28 antibody cross-linking. Rap1N17 or pcDNA3 (vector) stable transfectants were stimulated by cross-linking of the TCR complex with OKT3 (TCR) or OKT3 and CD28.2 (TCR+CD28) for 16 h, and the supernatants were harvested for IL-2 measurement. An optical density at 437 nm of 1 was equal to 0.15 ng of recombinant mouse IL-2/ml. (Ba) The upper panel shows the induction of SPA-1 detected with anti-flag antibody. SPA-1 was uninduced (−) or induced (+) by infection with adenovirus expressing cre recombinase for 2 days. The lower panel shows the inhibition of TCR-mediated Rap1 activation by the induction of SPA-1 expression. Jurkat cells uninduced (−) or induced (+) to express SPA-1 were stimulated with OKT3 (TCR) for 10 min and lysed, and GTP-bound Rap1, H-ras, and Rac were detected with pull-down assays by using immobilized GST fusion proteins of RalGDS-RBD, Raf-RBD, and PAK-CD. Western blots of total cell lysates are also shown. (Bb) Adhesion of Jurkat cells uninduced (−) or induced (+) to express SPA-1 with OKT3 (TCR) or PMA. Jurkat cells transfected with Spa-1 or empty vector were infected with adenovirus carrying cre recombinase. Infected cells were stimulated with or without 1 μg of OKT3 (TCR) or 10 ng of PMA per ml for 30 min at 37°C in ICAM-1-coated plates, as described in Materials and Methods. Average and standard errors of triplicate experiments are shown. (Ca) Decrease in TCR-dependent Rap1 activation by costimulation with antibody cross-linking of CD28. Jurkat cells were stimulated with OKT3 in the absence (TCR) or presence of CD28.2 (TCR/CD28) for 10 min and lysed, and GTP-bound Rap1, H-ras, and Rac were measured as described in Fig. 1Ba. (Cb) TCR-induced adhesion of Jurkat cells was reduced by costimulation with CD28. Jurkat cells were unstimulated or stimulated with OKT3 (TCR), CD28.2 (CD28), or TS2/18 (CD2) alone or in combination for 30 min at 37°C in ICAM-1-coated plates. Average and standard errors of triplicate experiments are shown.
To confirm that Rap1 is required for TCR-dependent adhesion, we employed a Cre/loxP expression system for SPA-1, a Rap1-specific GTPase activating protein (36), to inhibit Rap1 activation in Jurkat cells. Adenovirus infection alone did not affect LFA-1-ICAM-1 adhesion (Fig. 1Bb). Expression of SPA-1, induced by infection with adenovirus carrying cre recombinase, reduced the TCR-dependent activation of Rap1 by 82% without affecting the activation of H-ras and Rac (Fig. 1Ba). Concomitantly, the induction of SPA-1 expression inhibited TCR-stimulated adhesion to ICAM-1 by 69%, without decreasing phorbol myristate acetate (PMA)-stimulated adhesion (Fig. 1Bb), a finding consistent with the results obtained with Rap1N17, indicating that Rap1 activation is required for TCR-induced LFA-1-ICAM-1 adhesion.
It was reported that coengagement of CD28 reduces TCR-dependent activation of Rap1, which was suggested to be due to Rap1 GAP activation (7, 45). We next examined how TCR-mediated Rap1 activation and LFA-1-ICAM-1 adhesion are modulated by T-cell costimulatory molecules such as CD2 and CD28. Costimulation with cross-linking of antibody to CD28 reduced TCR-dependent activation of Rap1 by 64%, whereas it had little effect against the activation of H-ras and Rac (Fig. 1Ca). Concomitantly, costimulation with CD28 decreased TCR-stimulated adhesion to ICAM-1 by 52% (Fig. 1Cb), although it promoted IL-2 production (Fig. 1Ab). Costimulation with cross-linking of antibody to CD2, which did not inhibit the TCR-dependent Rap1 activation (data not shown), had no effect on the adhesion (Fig. 1Cb). Taken together, these results show a clear correlation of Rap1 activation with the LFA-1-ICAM-1 adhesion and suggest that CD28 play a specific role to modulate LFA-1-ICAM-1 adhesion through Rap1.
Antigen-dependent activation and redistribution of Rap1 at the contact site.
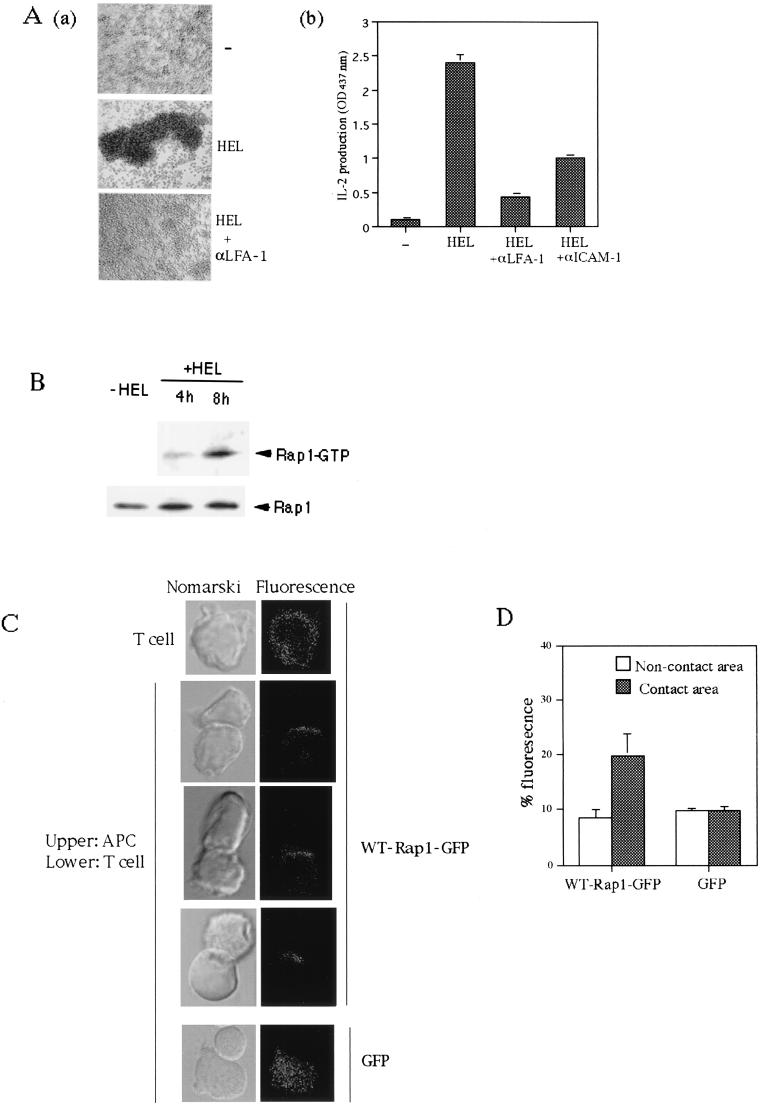
To examine whether TCR-mediated activation of Rap1 regulates adhesive interaction between T cells and APC and subsequent IL-2 production, we employed 3A9, a HEL-specific I-Ak-restricted T-cell hybridoma, and I-Ak-bearing CH27 B cells as APC. 3A9 T cells and CH27 B cells formed large multicellular aggregates in the presence of the HEL antigen, and this process was prevented by anti-LFA-1 or anti-ICAM-1 antibodies (Fig. 2Aa and data not shown). 3A9 T cells produced IL-2 16 h after culture with CH27 B cells in the presence of antigen, which was markedly inhibited by the antibodies to LFA-1 and ICAM-1 (Fig. 2Ab). Thus, unlike the case of anti-TCR cross-linking, the IL-2 production of T cells in response to the specific antigen presented by APC is dependent on the LFA-1-ICAM-1 interaction.
FIG. 2.
Cellular aggregation of T cells and APC via LFA-1-ICAM-1 accompanies activation and accumulation of Rap1 at the contact site. (Aa) The formation of large T-cell-APC clusters was dependent on LFA-1. HEL-specific I-Ak-restricted 3A9 T-cell hybridoma and I-Ak-bearing CH27 B cells were incubated in the absence (−) or presence of HEL (100 μg/ml) for 16 h with or without 20μg of anti-LFA-1 antibody/ml. Original magnification, ca. ×100. (Ab) LFA-1-ICAM-1-dependent IL-2 production. 3A9 T cells and CH27 B cells were cultured as described above with HEL and antibodies as indicated, and the supernatants were harvested for IL-2 measurement. An optical density at 437 nm of 1 was equal to 0.2 ng of recombinant mouse IL-2/ml. (B) Antigen-dependent activation of Rap1 in T cells. 3A9 T cells expressing T7-tagged WT-Rap1 were cultured with CH27 B cells without (−HEL) or with (+HEL) antigen for 4 and 8 h. GTP-bound Rap1 was analyzed as in Fig. 1B with pull-down of GST-RalGDS-RBD. Bound Rap1 (upper panels) and total Rap1 (lower panels) were detected by Western blotting with anti-T7 antibody. (C) Redistribution of Rap1 at the contact site. 3A9 T cells expressing WT-Rap1-GFP fusion protein or GFP protein were mixed with antigen-pulsed CH27 B cells for 30 min at 37°C. Conjugates were bound to poly-l-lysine-coated coverslips, fixed for 5 min with 3% formaldehyde, and observed under a confocal microscope (LSM510; Zeiss). Three representative confocal images of conjugates for WT-Rap1-GFP are shown. The left panels are Nomarski views of the same conjugates shown for GFP in the right panels. (D) Quantification of redistributed Rap1-GFP. The conjugates of WT-Rap1-GFP or GFP-expressing T cells with APC in panel C were imaged in 0.3-μm steps through the entire cell volume. The data shown represent the integral of the fluorescence intensity of the manually defined contact site or noncontact site over the total value of the cell. The manually defined contact or noncontact sites represent 10% of the cell volume. The relative enrichment (the fluorescence per unit volume at the contact site divided by the fluorescence per unit volume of the entire cell) of Rap1-GFP or of GFP is 2.01 ± 0.59 or 0.98 ± 0.12, respectively. Error bars represent the standard deviation.
To measure Rap1 activation in this system, we introduced T7-tagged wild-type Rap1 into 3A9 T cells. Activation of Rap1 in 3A9 T cells was detected 8 h after culture with CH27 B cells in the presence of antigen, at which time cellular aggregates started to form (Fig. 2B). The level of Rap1-GTP was 3 to 5% of the total Rap1. The same results were obtained by using the antibody to endogenous Rap1 (see Fig. 5A).
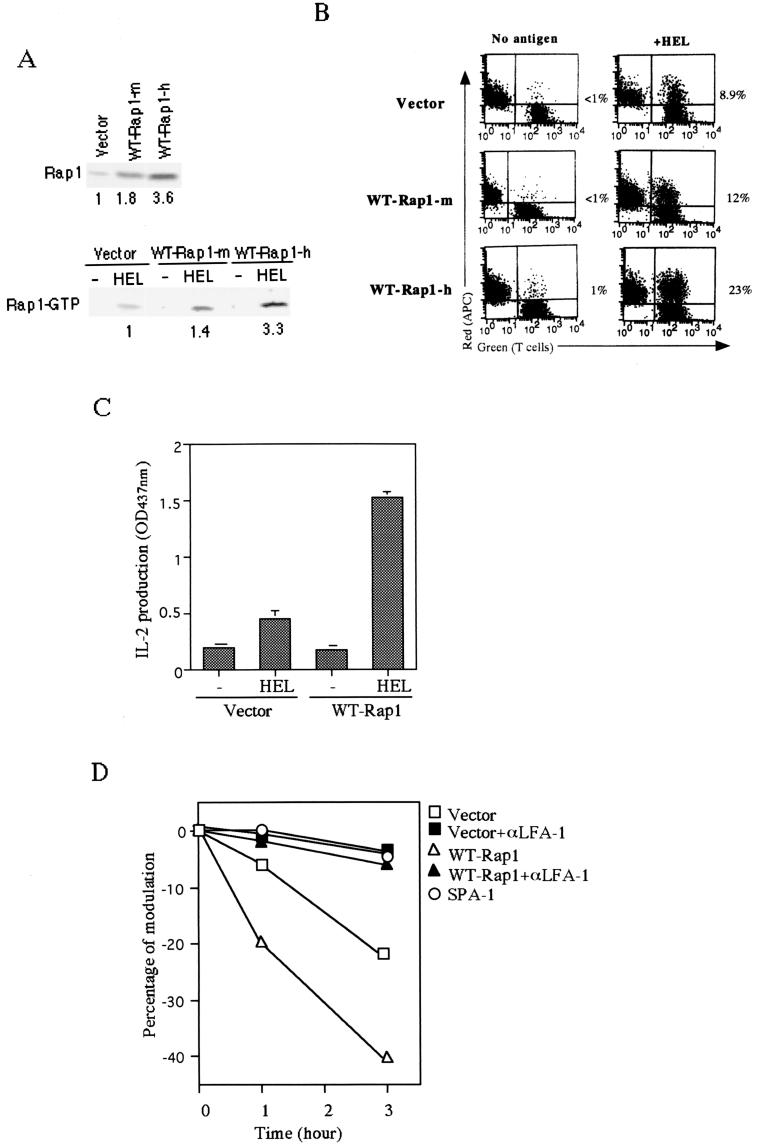
FIG. 5.
Enhanced T-cell-APC interaction by overexpression of WT-Rap1 promoted IL-2 production and TCR downregulation. (A) The upper panel shows the increased expression levels of Rap1 in WT-Rap1 transfectants. The 3A9 T cells transfected with vector alone and the representative clones expressing a modest level (WT-Rap1-m) and a high level (WT-Rap1-h) of Rap1 are shown. The levels of Rap1 expression were detected by Western blotting with monoclonal anti-Rap1 antibody. The lower panel shows the enhanced antigen-dependent activation of Rap1 in transfectants. The transfectants were cultured with CH27 B cells without (−HEL) or with antigen (+HEL) for 8 h. GTP-bound Rap1 was analyzed as in Fig. 1B with pull-down of GST-Ra1GDS-RBD. Bound Rap1 was detected by Western blotting with anti-Rap1 antibody. The relative levels of Rap1 quantitated by an LAS1000 apparatus (Fuji) are shown under each blot. (B) Expression of WT-Rap1 enhanced conjugate formation between 3A9 T cells and CH27 B cells in the presence of antigen. Transfectants were incubated with nonpulsed (No antigen) or antigen-pulsed (+HEL) CH27 B cells as APC and analyzed for conjugate formation as described in Fig. 3B. A representative set of two-dimensional plots of T cells (green) versus APC (red) is shown. The number in each plot is the percentage of conjugates. (C) Promotion of IL-2 production by expression of WT-Rap1. 3A9 T cells transfected with vector alone, as well as WT-Rap1-expressing cells, were incubated with APC for 8 h as described in Fig. 2A, and the supernatants were harvested for IL-2 measurement. An optical density at 437 nm of 1 was equal to 0.35 ng of recombinant mouse IL-2/ml. The average and the standard error of two representative experiments performed in triplicate with three WT-Rap1-expressing clones are shown. (D) Increased TCR downregulation after antigen stimulation in WT-Rap1 transfectants. 3A9 T cells transfected with vector alone or WT-Rap1 were stimulated with antigen-pulsed CH27 B cells in the presence (▪ and ▴) or absence (□ and ▵) of anti-LFA-1 antibody. SPA-1-expressing 3A9 T cells were also stimulated with antigen-pulsed CH27 B cells (○). TCR expression levels were assessed 1 and 3 h later by flow cytometry as described in Materials and Methods.
To examine the subcellular distribution of Rap1 upon interaction with APC, we introduced a Rap1-GFP fusion protein into 3A9 T cells. As shown in Fig. 2C, Rap1-GFP, which was distributed diffusely in the cytoplasm in unstimulated 3A9 T cells, was accumulated at the site of contact with antigen-loaded CH27 B cells. The redistribution was specific for Rap1, since GFP alone remained to distribute equally throughout the cytoplasm. The amount of Rap1-GFP that was redistributed to the T-cell-APC interface in response to antigen stimulation was quantified (Fig. 2D). The amount of Rap1-GFP in the cytoplasmic region of the T-cell-APC contact increased by approximately 10%, whereas the level of GFP did not change in response to antigen stimulation (Fig. 2D). The amount of translocated Rap1 is in agreement with that of Rap1 activation determined biochemically, considering the sensitivity of the assay used.
Essential requirement of Rap1 activation for the direct interaction between T cells and antigen-loaded APC mediated by LFA-1-ICAM-1.
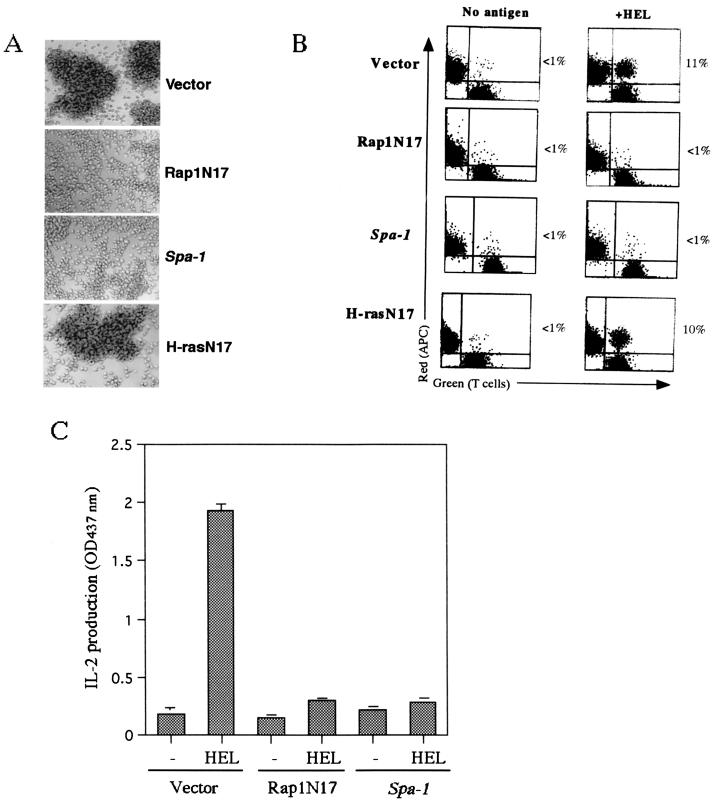
We then examined the requirement of Rap1 activation for the T-cell-APC interaction and subsequent IL-2 production. We introduced empty vector, Rap1N17 and Spa-1 into 3A9 T cells. H-RasN17 was also used as a control. We isolated more than five stable clones for each construct and examined them in the experiments described below. Expressions of Rap1N17 and SPA-1, but not of H-RasN17, inhibited large aggregate formation with CH27 B cells in the presence of antigen (Fig. 3A).The inhibition of adhesive interactions by Rap1N17 or SPA-1 was quantified by the conjugation assay as described in Materials and Methods. Conjugates of 3A9 T cells and CH27 B cells were formed in an antigen-dependent manner (Fig. 3B), and the process was inhibited by anti-LFA-1 or ICAM-1 antibodies (data not shown). 3A9 T cells expressing Rap1N17 or SPA-1 formed few conjugates when incubated with antigen-pulsed CH27 B cells (Fig. 3B). However, H-rasN17 did not affect the LFA-1-ICAM-1-mediated interaction between T cells and APC (Fig. 3). The prevention of the antigen-specific T-cell-APC interactions by Rap1N17 or SPA-1 resulted in complete inhibition of IL-2 production (Fig. 3C).
FIG. 3.
Inhibition of LFA-1-ICAM-1-dependent T-cell-APC interaction and IL-2 production by Rap1N17 or SPA-1. (A) Inhibition of cell aggregation by Rap1N17 and SPA-1. 3A9 T cells were transfected with empty vector, or genes encoding Rap1N17, Spa-1, or H-rasN17, and more than five stable clones for each construct were isolated for the experiment. The result is representative of five independent clones, which gave similar results. They were cultured with CH27 B cells in the presence of antigen, as described in Fig. 2A. Original magnification, ×100. (B) Conjugate formation of 3A9 T cells (green) and CH27 B cells as APC (red) loaded with (+HEL) or without (No antigen) antigen. T cells were mixed with an equal number of APC and incubated for 30 min at 37°C. Nonspecific aggregates were disrupted by vortexing, and the samples were analyzed by flow cytometry. A representative set of two-dimensional plots is shown. The number in each plot is the percentage of conjugates. (C) Inhibition of IL-2 production by Rap1N17 and SPA-1. 3A9 T cells transfected with the vector alone, Rap1N17, or Spa-1 were incubated with CH27 B cells as described in Fig. 2A, and the supernatants were harvested for IL-2 measurement. An optical density at 437 nm of 1 was equal to 0.25 ng of recombinant mouse IL-2/ml. Bars represent the average and standard error of two representative experiments performed in triplicate with each of five clones.
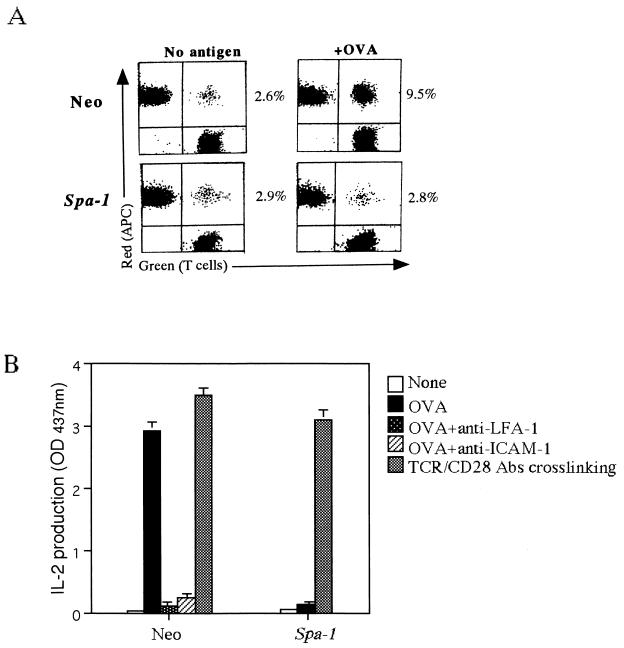
Similar experiments were performed with an OVA-specific H-2d-restricted T-cell clone, established from spleen cells of BALB/c mice immunized with OVA. As with 3A9 T cells, the T-cell clone formed conjugates with A20.2J B cells as APC in the presence of OVA and produced IL-2, which was also entirely dependent on LFA-1-ICAM-1 (Fig. 4A and B and data not shown). Expression of SPA-1 by retrovirus almost completely suppressed the increase in T-cell-APC conjugates induced by antigen stimulation, as well as the subsequent IL-2 production and proliferation (Fig. 4A and B and data not shown). The control infection with retrovirus carrying the neomycin gene only did not affect the LFA-1-ICAM-1 interaction and IL-2 production (Fig. 4).In contrast, the stimulation of TCR and CD28 by antibody cross-linking induced IL-2 production in the SPA-1-expressing T-cell clone to a degree comparable to that of the control cells (Fig. 4B). Taken together, these results indicate that Rap1 is not directly involved in the signaling pathways downstream of TCR leading to the IL-2 production but is required for the initiation of T-cell activation per se by induction of adhesive interaction with APC via LFA-1-ICAM-1.
FIG. 4.
Inhibition of conjugate formation of the OVA-specific T-cell clone and APC (A) and IL-2 production by SPA-1 (B). (A) Conjugate formation of T-cell clone (green) transfected with the neomycin gene (Neo) or Spa-1 and A20.2J B cells as APC (red) loaded with (+OVA) or without (No antigen) 1 mg of OVA per ml was analyzed as in Fig. 3B. The number in each plot is the percentage of conjugates. (B) IL-2 production. T-cell clones transfected with the neomycin gene (Neo) or Spa-1 were incubated with A20.2J B cells in the absence (None) or presence of antigen for 16 h with or without anti-LFA-1 or anti-ICAM-1 antibodies. T-cell clones were also stimulated with anti-CD3ɛ (2C11) and CD28 (37.51) for 16 h (TCR/CD28). The supernatants were harvested, and IL-2 concentrations were measured. An optical density at 437 nm of 1 was equal to 0.13 ng of recombinant mouse IL-2/ml. The average with the standard error of two representative experiments in triplicate is shown.
Overexpression of wild-type Rap1 results in enhanced T-cell activation followed by activation-induced cell death.
We then examined the effects of increased Rap1 activation on T-cell response to antigen-pulsed APC by overexpressing wild-type Rap1 (WT-Rap1) in 3A9 T cells. Representative 3A9 T-cell clones with modest (1.8-fold) and high (3.6-fold) levels of WT-Rap1 were isolated (Fig. 5A)and subjected to antigen stimulation. The activation status of Rap1 in cells expressing modest and high levels of WT-Rap1 was augmented 1.4- and 3.3-fold, respectively (Fig. 5A). Conjugate formation with antigen-pulsed CH27 B cells was increased from 8.9% with cells transfected with the vector alone to 12 and 23%, respectively, with cells expressing modest and high levels of WT-Rap1 (Fig. 5B), a result which was in parallel with the activation levels of Rap1. Conjugate formations were barely induced without antigen (Fig. 5B). IL-2 production was accelerated ∼3-fold in 3A9 T cells expressing high levels of WT-Rap1 at 8 h after antigen stimulation compared with the cells transfected with vector alone (Fig. 5C), while the increase in cells with modest levels of WT-Rap1 was only marginal (not shown). Thus, Rap1 activation levels were in good correlation with conjugate formations with APC, whereas augmented IL-2 production appeared to require a >2-fold increase in conjugate formations.
We also examined the TCR downregulation after the interaction with APC, since the extent of TCR downregulation was reported to correlate to the level of T-cell activation (3, 23, 27, 57). TCR downregulation was reported to be due to specific degradation of ligated TCR after internalization (38). 3A9 T cells transfected with vector alone (control), wild-type Rap1, or Spa-1 were incubated with antigen-pulsed CH27 B cells, and the expression levels of the TCRs were assessed 1 and 3 h later. Significant TCR downregulation occurred in the control cells, which was totally inhibited by either anti-LFA-1 antibody or SPA-1 (Fig. 5D). Because SPA-1 interfered with the LFA-1-ICAM-1 interaction between T cells and APC (Fig. 4), these results indicated that the TCR downregulation was indeed dependent on the LFA-1-ICAM-1 interactions. On the other hand, the extent of downregulation of TCR in the 3A9 cells expressing high levels of wild-type Rap1 was accelerated ca. 3.2-fold at 1 h and ca. 1.8-fold at 3 h after culture with antigen-pulsed APC (Fig. 5D). These results support the notion that the level of TCR-mediated Rap1 activation affects the extent of functional TCR triggering via T-cell-APC interaction.
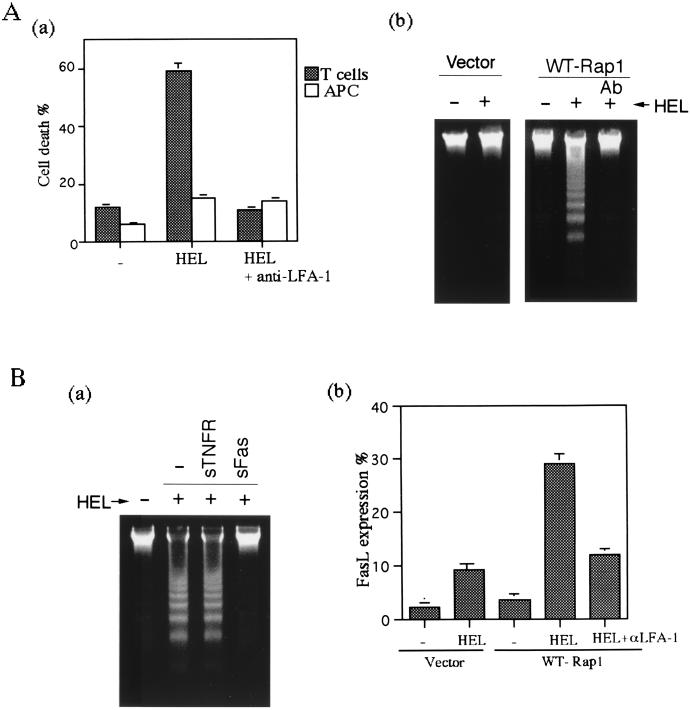
When observed 16 h after stimulation, however, cultures of cells with high levels of WT-Rap1 were found to contain many dead cells. PI staining experiments with differentially labeled cells showed that as much as 60% of the 3A9 T cells, but few CH27 B cells, were dead (Fig. 6Aa).Cell death was due to apoptosis, since DNA fragmentation was detected in the culture of the Rap1-transfectant with antigen-loaded CH27 B cells (Fig. 6Ab). Inhibition of the conjugate formation with anti-LFA-1 antibody completely blocked apoptosis (Fig. 6A), suggesting that the cell death was a consequence of the excess adhesive interaction of T cells overexpressing the WT-Rap1 with APC. The cell death was mediated by Fas/FasL, because soluble Fas, but not TNFR, totally blocked the DNA fragmentation (Fig. 6Ba). FasL expression was upregulated in the Rap1 transfectant cultured with antigen-loaded CH27 B cells threefold more than that in control transfectant cells and was inhibited by anti-LFA-1 antibody (Fig. 6Bb). Taken together, these results indicated that increased antigen-specific Rap1 activation, which led to the enhanced T-cell-APC interaction, ultimately resulted in the activation-induced cell death (AICD).
FIG. 6.
Enhancing T-cell-APC interaction resulted in activation-induced cell death. (A) Apoptosis of WT-Rap1-expressing 3A9T cells. (Aa) WT-Rap1-expressing 3A9 T cells (▩) were cultured with CH27 B cells (□) loaded with (HEL) or without (−) 100 μg HEL per ml for 16 h and then stained with PI and analyzed by flow cytometry. Anti-LFA-1 antibody was included as indicated. CH27 B cells were labeled with CFSE before culture to distinguish them from 3A9 T cells. The average and standard error of the percent PI positive for each population of three experiments are shown. (Ab) 3A9 T cells transfected with vector alone or with WT-Rap1 were cultured with CH27 B cells in the absence (−) or presence (+) of HEL antigen for 16 h. Anti-LFA-1 antibody (Ab) was included as indicated. Chromosomal DNA was extracted and analyzed by a 1.5% agarose electrophoresis to detect DNA fragmentation. (B) Apoptosis mediated by Fas/FasL. (Ba) WT-Rap1-expressing 3A9 T cells were cultured with CH27 B cells with (+) or without (−) HEL in the absence (−) or presence of soluble Fas-Fc (sFas) and TNFR1-Fc (sTNFR) chimeric proteins. DNA fragmentation was detected as described in Fig. 6Ab. (Bb) Upregulation of FasL expression in 3A9 T cells. 3A9T cells transfected with vector alone or with WT-Rap1 were cultured with APC in the presence or absence (−) of antigen. Anti-LFA-1 antibody was included as indicated. Cells harvested after 8 h were stained with anti-FasL antibody, and FITC-labeled anti-mouse immunoglobulin and then analyzed by flow cytometry. To gate out the APC, CH27 B cells were labeled with PKH-26 before culture. The data are shown for 3A9 T cells. CH27 B cells did not express FasL. The percentage of positive 3A9 T cells stained with the secondary antibody alone in each condition was <1%. The average and standard errors of two experiments are shown.
Constitutive and high levels of Rap1 activation suppresses IL-2 production and induces cell cycle arrest with accumulation of p27Kip1.
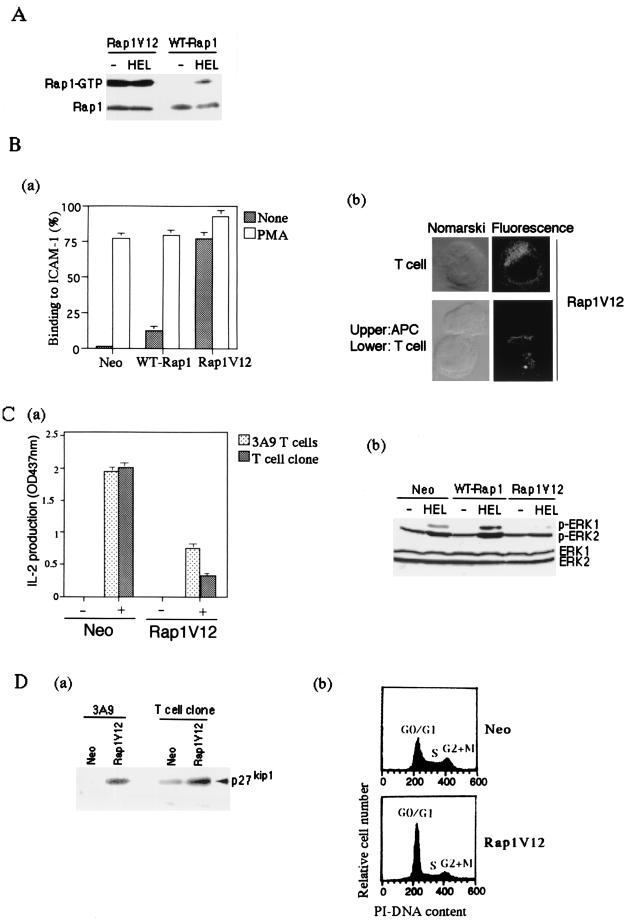
We finally examined the effect of high-level preactivation of Rap1 on T-cell action by APC. For this purpose, we examined the effects of either coexpressions of WT-Rap1 and C3G-F, a membrane-anchored form of C3G, a Rap1-specific guanine nucleotide exchange factor (19, 55), or a constitutively active form, Rap1V12. While coexpressions of WT-Rap1 and C3G-F led to only modest increases of Rap1-GTP, Rap1V12 expressions were found to be much more potent when it was introduced into 3A9 T cells and the OVA-specific T cell clone by retrovirus. The level of Rap1 activation in nonstimulated Rap1V12 transfectants was more than 10 times higher than that of WT-Rap1 transfectants stimulated with antigen-loaded APC, which caused AICD (Fig. 7A). The Rap1V12 transfectants showed a marked increase in LFA-1-ICAM-1-mediated adhesion (Fig. 7Ba) and underwent spontaneous aggregation with APC in the absence of antigen (data not shown). Rap1V12 was recruited to the contact site with unloaded APC (Fig. 7Bb), whereas in unconjugated cells it tended to be more concentrated around the perinuclear-Golgi complex region. Nonetheless, IL-2 production was severely reduced in Rap1V12 transfectants of 3A9 T cells, as well as in the OVA-specific T-cell clone, when cultured with appropriate APC and antigens (Fig. 7Ca). This was not due to apoptosis, since cell viability was not affected by culture with APC (data not shown). Therefore, we examined activation of extracellular signal-regulated kinases (ERKs) and the expression levels of p27Kip1, a cyclin-CDK inhibitor, because they were reported to be involved in induction of anergic states (5, 6). The phosphorylation of ERKs in Rap1V12 transfectants stimulated with antigen-pulsed APC was severely reduced compared to that of the cells infected with retrovirus carrying the neomycin-resistant gene alone (Fig. 7Cb), which is in agreement with previous studies showing that overexpression of the constitutively active Rap1 inhibited ERK and Raf-1 kinase activities (5, 7). In contrast, the phosphorylation levels of ERKs in WT-Rap1-expressing cells were rather augmented (Fig. 7Cb), and T-cell activation was accelerated, as shown in Fig. 5 and 6, showing that antigen-dependent activation of endogenous Rap1 does not inhibit the activation of ERKs. Interestingly, p27Kip1 accumulated in both Rap1V12-expressing 3A9 and OVA-specific T cells (Fig. 7Da). Concomitantly, the growth of transfectants was noticeably retarded with an increase in the cell population at the G0/G1 phase (Fig. 7Db). Thus, persistent preactivation of Rap1 resulted in the accumulation of p27Kip1 and rendered T cells unresponsive to antigen stimulation.
FIG. 7.
Suppression of IL-2 production and accumulation of p27Kip1 by Rap1V12. (A) Level of Rap1-GTP in Rap1V12- and WT-Rap1-expressing cells. 3A9 T cells expressing T7-tagged Rap1V12 and WT-Rap1 were cultured with CH27 B cells without (−HEL) or with antigen (+HEL) for 8 h. GTP-bound Rap1 was analyzed as in Fig. 1B with pull-down of GST-RalGDS-RBD. Bound Rap1 (upper) and total Rap1 (lower) were detected by Western blotting with anti-T7 antibody. (B) Rap1V12 increased LFA-1-ICAM-1-mediated adhesion of 3A9 T cells and accumulated at the contact site with APC in the absence of antigen. (Ba) Adhesion of 3A9 T cells transfected with vector alone, WT-Rap1, and Rap1V12. T cells were stimulated with or without 10 ng of PMA per ml for 30 min at 37°C in mouse ICAM-1-Ig-coated plates and analyzed as described in Materials and Methods. The average and standard errors of triplicate experiments are shown. (Bb) Cellular localization of Rap1V12. 3A9 T cells expressing T7-tagged Rap1V12 were mixed with CH27 B cells for 30 min at 37°C and immunostained with anti-T7 antibody and Alexa Fluor 488 goat anti-mouse IgG (Molecular Probes). Representative confocal images of Rap1V12 in T cells either unconjugated or conjugated with CH27 B cells are shown. The left panels are Nomarski views of the same cells shown for Rap1V12 in the right panels. Cells transfected with vector alone did not show any significant fluorescent signals under the same conditions. (C) Persistent and marked preactivation of Rap1 rendered T cells unresponsive to antigen. (Ca) Decreased IL-2 production in Rap1V12-expressing 3A9 T cells and OVA-specific T-cell clone. 3A9 T cells and the OVA-specific T-cell clone transfected with vector alone (Neo) or Rap1V12 were cultured with appropriate APC, as in Fig. 3 and 4, with (+) or without (−) antigen for 16 h. IL-2 concentrations of the supernatants were measured. An optical density at 437 nm of 1 was equal to 0.23 ng of recombinant mouse IL-2/ml. Bars represent the average and standard error of two representative experiments performed in triplicate. (Cb) Antigen-induced phosphorylation of ERKs in 3A9 T cells expressing Rap1V12 or WT-Rap1. 3A9 T cells transfected with the neomycin genes (Neo), WT-Rap1, and Rap1V12 were cultured for 1 h with CH27 B cells which were preloaded without (−HEL) or with antigen (+HEL) for 16 h and fixed with 1% paraformaldehyde. The phosphorylation levels of ERKs were examined by Western blotting with antiphosphorylated ERK1 (pERK1)- and ERK2 (pERK2)-specific antibodies (upper panel). The expression levels of total ERK1 and ERK2 are shown (lower panel). (D) Accumulation of p27Kip1 and cell cycle arrest in Rap1V12-expressing T cells. (Da) Increased expression levels of p27Kip1 in Rap1V12-expressing 3A9 T cells and OVA-specific T-cell clone were detected by Western blotting with anti-p27Kip1 antibody. (Db) Increased cell population in the G0/G1 phase caused by Rap1V12 expression. The OVA-specific T-cell clones transfected with vector alone (Neo) or Rap1V12 were stained with PI and analyzed by flow cytometry.
DISCUSSION
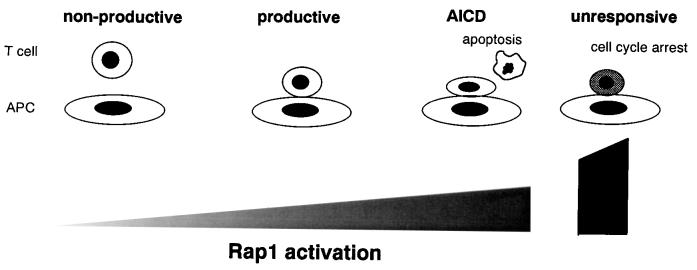
In this study we investigated the function of Rap1 in the regulation of T-cell-APC interactions and T-cell activation. We demonstrated that Rap1 activation was the major inside-out signal for LFA-1 triggered by the TCR and is a critical step for adhesive interactions of T cells with APC and the subsequent production of IL-2 in a system with two different antigen-specific T cells. However, augmentation of antigen-dependent Rap1 activation not only accelerated TCR triggering and IL-2 production but caused AICD by enhancing T-cell-APC interactions. Moreover, marked and prolonged Rap1 preactivation severely reduced IL-2 production with accumulation of p27Kip1, a phenotype reminiscent of anergic states. Thus, modulation of Rap1 activity in T cells produces a spectrum of immunological responses from nonproductive to AICD and to unresponsive states (Fig. 8). These results indicate that precise control of Rap1 activation is crucial for full T-cell activation.
FIG. 8.
Spectrum of immunological responses modulated by Rap1. It can be seen that low levels of Rap1 activation do not induce T-cell-APC adhesive interactions via LFA-1-ICAM-1, resulting in a nonproductive T-cell response. Appropriate Rap1 activation does induce stable T-cell-APC association, leading to full T-cell activation. Overactivated Rap1 enhances T-cell-APC interactions and precipitates T cells into AICD. Extremely accumulated active Rap1 causes cell cycle arrest and renders T cells unresponsive to antigen stimulation.
LFA-1-deficient T cells require 100-fold more antigen for proliferation and lytic activity (2), demonstrating in vivo that LFA-1-mediated T-cell-APC interactions are critical for the serial engagement of TCR molecules on a limited number of antigen peptide-MHC complexes for the generation of a signaling threshold necessary for T-cell activation. However, the regulatory mechanism of the T-cell-APC adhesive interactions has not been clear. Our study demonstrates that Rap1 plays a critical role in the T-cell-APC interactions by regulating the adhesiveness of LFA-1 of T cells to ICAM-1 on APC. Suppression of Rap1 activation by the dominant-negative Rap1 and Spa-1 inhibited the antigen-specific T-APC conjugate formations and subsequent IL-2 production. It should be noted that the dominant-negative Rap1 and Spa-1 did not affect IL-2 production stimulated by antibody cross-linking of TCR and CD28, indicating that TCR-mediated Rap1 activation functions mainly as a specific inside-out signal for LFA-1 and is not involved directly in the signaling pathways leading to IL-2 production. Activation of Rap1 and the redistribution of T-cell-APC contact sites suggest that Rap1 play a role in the formation of immunological synapse. Further study is needed to clarify the effects of Rap1 on the redistribution of LFA-1 and T-cell molecules and cytoskeletal rearrangement.
We showed by introduction of the WT-Rap1 that the extent of Rap1 activation was in a good correlation with that of T-cell-APC conjugate formations. The increased conjugate formation accelerated TCR engagement ca. 2.6-fold, as evidenced by TCR downregulation, which in turn augmented TCR signaling as shown by ERK and IL-2 production. However, increased conjugate formation resulted in apoptosis due to upregulation of FasL. This response is similar to what is referred to as AICD of T cells, in which overactivated T cells undergo Fas/FasL-mediated apoptosis (28, 34). Increased TCR signaling could be strong enough to induce FasL expressions through the promotion of T-cell-APC interactions, although the possibility is not ruled out that enhanced Fas/FasL interaction resulting from increased LFA-1-ICAM-1 adhesion promoted apoptosis (59). Taken together, these results demonstrate that Rap1 affects the intensity of T-cell activation through the regulation of the strength of T-cell-APC interaction.
On the other hand, the constitutive Rap1 activation differently affects the mode of T-cell activation, which directly inhibited TCR signaling pathways to IL-2 production. We showed that the overexpression of Rap1V12 in T cells not only prevented the antigen-dependent activation of ERKs but also led to constitutive accumulation of p27Kip1. The phenotype of Rap1V12-expressing T cells is similar to that of anergic T cells induced by antigen stimulation without costimulation of CD28 (5, 6). The level of Rap1-GTP in nonstimulated Rap1V12-expressing cells with the anergic phenotype was >10-fold greater than that of antigen-stimulated cells with WT-Rap1, which showed AICD. Since p27Kip1, a key negative regulator of the G1-to-S-phase transition, associates with c-jun coactivator JAB1 in the cytoplasm (54), the accumulation of p27Kip1 may cause the cytoplasmic translocation of JAB1 and result in defective transactivation of AP-1 and IL-2 transcription (6). Although the molecular mechanisms remain unclear, this is the first report that Rap1 is able to induce the accumulation of p27Kip1.
Our study shows that Rap1 activation is not only essential for the activation of specific T cells via intimate interactions with antigen-loaded APC but that its extent and duration profoundly affect the subsequent course of the T-cell response. Therefore, regulatory mechanisms for Rap1 activation and inactivation are critically important. We show here that the costimulation with CD28 decreased both Rap1 activity and the adhesion of T cells to ICAM-1. This result is in agreement with recent reports that CD28-mediated signaling induces Rap1 GAP activity and suppresses the TCR-mediated activation of Rap1 (7, 45). Our results imply that CD28 has a novel modulatory role in the adhesive interactions of T-APC by fine-tuning Rap1 activation to generate productive immunological responses without precipitating into apoptosis and anergy. It will interesting to examine in the future whether CD28-induced Rap1 GAP activity is mediated by SPA-1 and whether other T-cell costimulatory molecules influence Rap1 activity and T-cell-APC interactions. Our study provides valuable experimental systems and information with which we can reevaluate the roles of costimulatory molecules.
In addition to the adhesion only effect of LFA-1, engaged integrins transmit signals (outside-in signals) to modulate cell growth, differentiation, and functions (26), including IL-2 production (43, 51). Although most of the molecules involved in outside-in signaling have been described in other receptor systems, a recent study identified a unique role for JAB1, a c-jun coactivator protein which was associated with the cytoplasmic region of the β2 integrin and promoted IL-2 transcription by stabilizing the AP-1 complex upon engaging LFA-1 (4). Our study fell short of dissecting the dual role of LFA-1 in the T-cell-APC system. It will be interesting to examine whether Rap1 also plays a role in the signaling from engaged LFA-1.
This study is the first to provide a direct link between the role of Rap1 in the inside-out signaling from the TCR and T-cell activation through the regulation of adhesive interactions of T cells and APC. Modulation of Rap1 activity in T cells produces a spectrum of immunological responses from productive activation to AICD and to anergy (Fig. 8). Our study predicts a unique signal pathway downstream of Rap1, which is not apparently ascribed to the antagonistic effect on H-ras (8, 35). The identification of downstream effector molecules will be of prime importance to elucidate the molecular mechanisms of Rap1 in the regulation of integrins in T-cell-APC interactions, as well as in integrin-mediated cell adhesion and migration in general.
Acknowledgments
We thank P. M. Allen for the 3A9 T-cell hybridoma line, D. Gerlier for CH27 B-lymphoblastoid cells, T. Kakiuchi for A20.2J B-cell lymphoma cells, Y. Ohta for OVA-specific I-Ad-restricted T-cell lines, and S.-K. Jung for chimeric Fas-Fc or TNFR1-Fc fusion proteins. We are grateful to T. Katagiri for helpful discussions.
This work was supported in part by a grant-in aid from the Ministry of Education, Science, Sports, and Culture of Japan and the Wehara Memorial Foundation.
REFERENCES
- 1.Allen, P. M., and E. R. Unanue. 1984. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J. Immunol. 132:1077-1079. [PubMed] [Google Scholar]
- 2.Bachmann, M. F., K. Mckall-Faienza, R. Schmits, D. Bouchard, J. Beach, D. E. Speiser, T. W. Mak, and P. S. Ohashi. 1997. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity 7:549-557. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann, M. F., A. Oxenius, D. E. Speiser, S. Mariathasan, H. Hengartner, R. M. Zinkernagel, and P. S. Ohashi. 1997. Peptide induced TCR-down regulation on naive T cell predicts agonist/partial agonist properties and strictly correlates with T cell activation. Eur. J. Immunol. 27:2195-2203. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi, E., S. Denti, A. Granata, G. Bossi, J. Geginat, A. Villa, L. Rogge, and R. Pardi. 2000. Integrin LFA-1 interacts with the transcriptional co-activator JAB1 to modulate Ap-1 activity. Nature 404:617-621. [DOI] [PubMed] [Google Scholar]
- 5.Boussiotis, V. A., G. J. Freeman, A. Berezovskaya, D. L. Barber, and L. M. Nadler. 1997. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science 278:124-128. [DOI] [PubMed] [Google Scholar]
- 6.Boussiotis, V. A., G. J. Freeman, P. A. Taylor, A. Berezovskaya, I. Grass, B. R. Blazar, and L. M. Nadler. 2000. p27kip1 functions as an anergy factor inhibiting interleukin 2 transcription and clonal expansion of alloreactive human and mouse helper T lymphocytes. Nat. Med. 6:290-297. [DOI] [PubMed] [Google Scholar]
- 7.Carey, K. D., T. J. Dillon, J. M. Schmitt, A. M. Baird, A. D. Holdorf, D. B. Straus, A. S. Shaw, and P. J. S. Stork. 2000. CD28 and the tyrosine kinase lck stimulate mitogen-activated protein kinase activity in T cells via inhibition of the small G protein Rap1. Mol. Cell. Biol. 20:8409-8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook, S. J., B. Rubinfeld, I. Albert, and F. McCormick. 1993. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 12:3475-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corr, M., A. E. Slanetz, L. F. Boyd, M. T. Jelonek, S. Khilko, B. K. Al-Ramadi, Y. S. Kim, S. E. Maher, A. L. M. Bothwell, and D. H. Margulies. 1994. T cell receptor-MHC class I peptide interactions: affinity, kinetics, and specificity. Science 265:946-949. [DOI] [PubMed] [Google Scholar]
- 10.Demotz, S., H. M. Grey, and A. Sette. 1990. The minimal number of classII MHC-antigen complexes needed for T cell activation. Science 249:1028-1030. [DOI] [PubMed] [Google Scholar]
- 11.Dustin, M. L., S. K. Bromley, Z. Kan, D. A. Peterson, and E. R. Unanue. 1997. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc. Natl. Acad. Sci. USA 94:3909-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dustin, M. L., and J. A. Cooper. 2000. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 1:23-29. [DOI] [PubMed] [Google Scholar]
- 13.Dustin, M. L., M. W. Olszowy, A. D. Holdorf, J. Li, S. Bromley, N. Desal, P. Widder, F. Rosenberger, P. A. van den Merwe, P. M. Allen, and A. S. Shaw. 1998. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 94:667-677. [DOI] [PubMed] [Google Scholar]
- 14.Dustin, M. L., and A. S. Shaw. 1999. Costimulation: building an immunological synapse. Science 29:649-650. [DOI] [PubMed] [Google Scholar]
- 15.Dustin, M. L., and T. A. Springer. 1989. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature 341:619-624. [DOI] [PubMed] [Google Scholar]
- 16.Franke, B., J.-W. N. Akkerman, and J. L. Boss. 1997. Rapid Ca2+-mediated activation of Rap1 in human platelets. EMBO J. 16:252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gieni, R. S., Y. Li, and K. T. HayGlass. 1995. Comparison of [3H]thymidine incorporation with MTT- and MTS-based bioassays for human and murine IL-2 and IL-4 analysis. J. Immunol. Methods 187:85-93. [DOI] [PubMed] [Google Scholar]
- 18.Goldsmith, M. A., and A. Weiss. 1988. Early signal transduction by the antigen receptor without commitment to T cell activation. Science 240:1029-1031. [DOI] [PubMed] [Google Scholar]
- 19.Gotoh, T., S. Hattori, S. Nakamura, H. Kitayama, M. Noda, Y. Takai, K. Kaibuchi, H. Matsui, O. Hatase, H. Takahashi, T. Kurata, and M. Matsuda. 1995. Identification of Rap1 as a target for the Crk SH3 domain-binding nucleotide-releasing factor C3G. Mol. Cell. Biol. 15:6746-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grakoui, A., S. Bromley, C. Sumen, M. Davis, A. Shaw, P. Allen, and M. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science 285:221-227. [DOI] [PubMed] [Google Scholar]
- 21.Harding, C. V., and E. R. Unanue. 1990. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature 346:574-576. [DOI] [PubMed] [Google Scholar]
- 22.Hart, D. N. 1997. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90:3245-3287. [PubMed] [Google Scholar]
- 23.Hemmer, B., I. Stefanova, M. Vergelli, R. N. Germain, and R. Martin. 1998. Relationships among TCR ligand potency, thresholds for effector function elicitation, and the quality of early signaling events in human T cells. J. Immunol. 160:5807-5814. [PubMed] [Google Scholar]
- 24.Holness, C. L., P. A. Bates, A. J. Littier, C. D. Buckley, A. McDowall, D. Bossy, N. Hogg, and D. L. Simmons. 1995. Analysis of the binding site on intercellular adhesion molecule 3 for the leukocyte integrin lymphocyte function-associated antigen1. J. Biol. Chem. 270:877-884. [DOI] [PubMed] [Google Scholar]
- 25.Horley, K. J., C. Carpenito, B. Baker, and F. Takei. 1989. Molecular cloning of murine intercellular adhesion molecule (ICAM-1). EMBO J. 8:2889-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howe, A., A. E. Aplin, S. K. Alaharisk, and R. L. Juliano. 1998. Integrin signaling and cell growth control. Curr. Opin. Cell Biol. 10:220-231. [DOI] [PubMed] [Google Scholar]
- 27.Itoh, Y., B. Hemmer, R. Martin, and R. N. Gemain. 1999. Serial TCR engagement and down-modulation by peptide:MHC molecule ligands: relationship to the quality of individual TCR signaling events. J. Immunol. 162:2073-2080. [PubMed] [Google Scholar]
- 28.Ju, S. T., D. J. Panka, H. Cui, R. Ettinger, M. el-Khatlb, D. H. Sherr, B. Z. Stanger, and A. Marshak-Rothstein. 1995. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 373:444-448. [DOI] [PubMed] [Google Scholar]
- 29.Kanegae, Y., G. Lee, Y. Sato, M. Tanaka, M. Nakai, T. Sakaki, S. Sugano, and I. Saito. 1995. Efficient gene activation in mammalian cells by using recombinant adenovirus expressing site-specific Cre recombinase. Nucleic Acids Res. 23:3816-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katagiri, K., M. Hattori, N. Minato, S. Irie, K. Takatsu, and T. Kinashi. 2000. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol. Cell. Biol. 20:1956-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katagiri, K., T. Kinashi, S. Irie, and T. Katagiri. 1996. Differential regulation of leukocyte function-associated antigen-1/intercellular adhesion molecules-1-dependent adhesion and aggregation in HL-60 cells. Blood 87:4276-4285. [PubMed] [Google Scholar]
- 32.Kinashi, T., J. A. Escobedo, L. T. Williams, K. Takatsu, and T. A. Springer. 1995. Receptor tyrosine kinase stimulates cell-matrix adhesion by phosphatidylinositol 3 kinase and phospholipase C-γ1 pathways. Blood 86:2086-2090. [PubMed] [Google Scholar]
- 33.Kinashi, T., and T. A. Springer. 1994. Steel factor and c-kit regulate cell-matrix adhesion. Blood 83:1033-1038. [PubMed] [Google Scholar]
- 34.Kishimoto, H., and J. Sprent. 1999. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of naive murine CD4+ T cells. J. Immunol. 163:1817-1826. [PubMed] [Google Scholar]
- 35.Kitayama, H., Y. Sugimoto, T. Matsuzaki, Y. Ikawa, and M. Noda. 1989. A ras-related gene with transformation suppressor activity. Cell 56:77-84. [DOI] [PubMed] [Google Scholar]
- 36.Kurachi, H., Y. Wada, N. Tsukamoto, M. Maeda, H. Kubota, M. Hattori, K. Iwai, and N. Minato. 1997. Human SPA-1 gene product selectively expressed in lymphoid tissues is a specific GTPase-activating protein for Rap1 and Rap2. J. Biol. Chem. 272:28081-28088. [DOI] [PubMed] [Google Scholar]
- 37.Lanzavecchia, A., G. Lezzi, and A. Viola. 1999. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell 96:1-4. [DOI] [PubMed] [Google Scholar]
- 38.Liu, H., M. Rhodes, D. L. Wiest, and D. A. A. Vignali. 2000. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity 13:665-675. [DOI] [PubMed] [Google Scholar]
- 39.Lombard-Platlet, S., P. Bertolino, H. Deng, D. Gerlier, and C. Rabourdin-Combe. 1993. Inhibition of chloroquine of the class II major histocompatibility complex-restricted presentation of endogenous antigens varies according to the cellular origin of the antigen-presenting cells, the nature of the T-cell epitope, and the responding T cell. Immunology 80:566-573. [PMC free article] [PubMed] [Google Scholar]
- 40.Matsui, K., J. J. Boniface, P. A. Reay, H. Schild, B. Fazekas-de-St-Groth, and M. M. Davis. 1991. Low-affinity interaction of peptide-MHC complexes with T cell receptors. Science 254:1788-1791. [DOI] [PubMed] [Google Scholar]
- 41.Moll, T., and D. Vestweber. 1999. Construction and purification of adhesion molecule immunoglobulin chimeric proteins. Methods Mol. Biol. 96:77-84. [DOI] [PubMed] [Google Scholar]
- 42.Monks, C. R. F., B. A. Freiberg, H. Kupfer, N. Sciaky, and A. Kupfer. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395:82-86. [DOI] [PubMed] [Google Scholar]
- 43.Ni, H. T., M. J. Deeths, D. L. Mueller, and M. F. Mescher. 1999. Signaling pathways activated by leukocyte function-associated Ag-1-dependent costimulation. J. Immunol. 162:5183-5189. [PubMed] [Google Scholar]
- 44.Ohta, Y., M. Yamane, T. Sohda, and H. Makino. 1997. TAK-603 selectively suppresses Th1-type cytokine production and inhibits the progression of adjuvant arthritis. Immunology 92:75-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reedquist, K. A., and J. L. Bos. 1998. Costimulation through CD28 suppresses T cell receptor-dependent activation of the Ras-like small GTPase Rap1 in human T lymphocytes. J. Biol. Chem. 273:4944-4949. [DOI] [PubMed] [Google Scholar]
- 46.Reedquist, K. A., E. Ross, E. A. Koop, R. M. F. Wolthuis, F. J. T. Zwartkruis, Y. van Kooyk, M. Salmon, C. D. Buckley, and J. L. Bos. 2000. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J. Cell Biol. 148:1151-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rooij, J. d., and J. L. Bos. 1997. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene 14:623-625. [DOI] [PubMed] [Google Scholar]
- 48.Sander, E. E., S. van Delft, J. P. T. Klooster, T. Reid, R. A. van den Kammen, F. Michiels, and J. G. Collard. 1998. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol. 143:1385-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaefer, B. C., M. F. Ware, P. Marrack, G. R. Fanger, J. W. Kappler, G. L. Johnson, and C. R. F. Monks. 1999. Live cell fluorescence imaging of T cell MEKK2: redistribution and activation in response to antigen stimulation of the T cell receptor. Immunity 11:411-421. [DOI] [PubMed] [Google Scholar]
- 50.Seo, A., F. Ishikawa, H. Nakano, H. Nakazaki, K. Kobayashi, and T. Kakiuchi. 1999. Enhancement of B7-1(CD80) expression on B-lymphoma cells by irradiation. Immunology 96:642-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seventer, G. A. V., Y. Shimizu, K. J. Horgan, and S. Shaw. 1990. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J. Immunol. 144:4579-4586. [PubMed] [Google Scholar]
- 52.Shaw, A. S., and M. L. Dustin. 1997. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity 6:361-369. [DOI] [PubMed] [Google Scholar]
- 53.Springer, T. A., M. L. Dustin, T. K. Kishimoto, and S. D. Marlin. 1987. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu. Rev. Immunol. 5:223-252. [DOI] [PubMed] [Google Scholar]
- 54.Tomoda, K., Y. Kubota, and J. Kato. 1999. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature 398:160-165. [DOI] [PubMed] [Google Scholar]
- 55.Tsukamoto, N., M. Hattori, H. Yang, J. L. Bos, and N. Minato. 1999. Spa-1 negatively regulates cell adhesion through GAP activity for Rap1. J. Biol. Chem. 274:18463-18469. [DOI] [PubMed] [Google Scholar]
- 56.van Kooyk, Y., and C. G. Figdor. 2000. Avidity regulation of integrins: the driving force in leukocyte adhesion. Curr. Opin. Cell Biol. 12:542-547. [DOI] [PubMed] [Google Scholar]
- 57.Viola, A., and A. Lanzavecchia. 1996. T cell activation determined by T cell receptor number and tunable thresholds. Science 273:104-106. [DOI] [PubMed] [Google Scholar]
- 58.Wacholtz, M. C., and P. E. Lipsky. 1993. Anti-CD3-stimulated Ca2+ signal in individual human peripheral T cells. Activation correlates with a sustained increase in intracellular calcium. J. Immunol. 150:5338-5349. [PubMed] [Google Scholar]
- 59.Wang, J., and M. J. Lenardo. 1997. Essential lymphocyte function associated 1 (LFA-1): intercellular adhesion molecule interactions for T cell-mediated B cell apoptosis by Fas/APO-1/CD95. J. Exp. Med. 186:1171-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber, S., A. Traunecker, F. Oliveri, W. Gerhard, and K. Karjalainen. 1992. Specific low-affinity recognition of major histo-compatibility complex plus peptide by soluble T-cell receptor. Nature 56:793-796. [DOI] [PubMed] [Google Scholar]
- 61.Wulfing, C., M. D. Sjaastad, and M. M. Davis. 1998. Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc. Natl. Acad. Sci. USA 95:6302-6307. [DOI] [PMC free article] [PubMed] [Google Scholar]