Abstract
The bacterial acyltransferases of the SxxK superfamily vary enormously in sequence and function, with conservation of particular amino acid groups and all-α and α/β folds. They occur as independent entities (free-standing polypeptides) and as modules linked to other polypeptides (protein fusions). They can be classified into three groups. The group I SxxK d,d-acyltransferases are ubiquitous in the bacterial world. They invariably bear the motifs SxxK, SxN(D), and KT(S)G. Anchored in the plasma membrane with the bulk of the polypeptide chain exposed on the outer face of it, they are implicated in the synthesis of wall peptidoglycans of the most frequently encountered (4→3) type. They are inactivated by penicillin and other β-lactam antibiotics acting as suicide carbonyl donors in the form of penicillin-binding proteins (PBPs). They are components of a morphogenetic apparatus which, as a whole, controls multiple parameters such as shape and size and allows the bacterial cells to enlarge and duplicate their particular pattern. Class A PBP fusions comprise a glycosyltransferase module fused to an SxxK acyltransferase of class A. Class B PBP fusions comprise a linker, i.e., protein recognition, module fused to an SxxK acyltransferase of class B. They ensure the remodeling of the (4→3) peptidoglycans in a cell cycle-dependent manner. The free-standing PBPs hydrolyze d,d peptide bonds. The group II SxxK acyltransferases frequently have a partially modified bar code, but the SxxK motif is invariant. They react with penicillin in various ways and illustrate the great plasticity of the catalytic centers. The secreted free-standing PBPs, the serine β-lactamases, and the penicillin sensors of several penicillin sensory transducers help the d,d-acyltransferases of group I escape penicillin action. The group III SxxK acyltransferases are indistinguishable from the PBP fusion proteins of group I in motifs and membrane topology, but they resist penicillin. They are referred to as Penr protein fusions. Plausible hypotheses are put forward on the roles that the Penr protein fusions, acting as l,d-acyltransferases, may play in the (3→3) peptidoglycan-synthesizing molecular machines. Shifting the wall peptidoglycan from the (4→3) type to the (3→3) type could help Mycobacterium tuberculosis and Mycobacterium leprae survive by making them penicillin resistant.
INTRODUCTION
The bacterial wall peptidoglycans are covalently closed, net-like polymers (77, 203). The glycan chains are made of alternating β-1,4-linked N-acetylglucosamine and N-acetylmuramic acid residues. The d-lactyl groups of the muramic acid residues are amidated with l-alanyl-γ-d-glutamyl-(l)-diaminoacyl-d-alanine stem tetrapeptides and l-alanyl-γ-d-glutamyl-l-diaminoacid stem tripeptides. The stem peptides can be branched, in which case the ω amino group of the diaminoacid residue is substituted by an additional amino acid residue or a short peptide. Unbranched or branched stem peptides belonging to adjacent glycan strands are covalently linked, resulting in polymeric peptidoglycan.
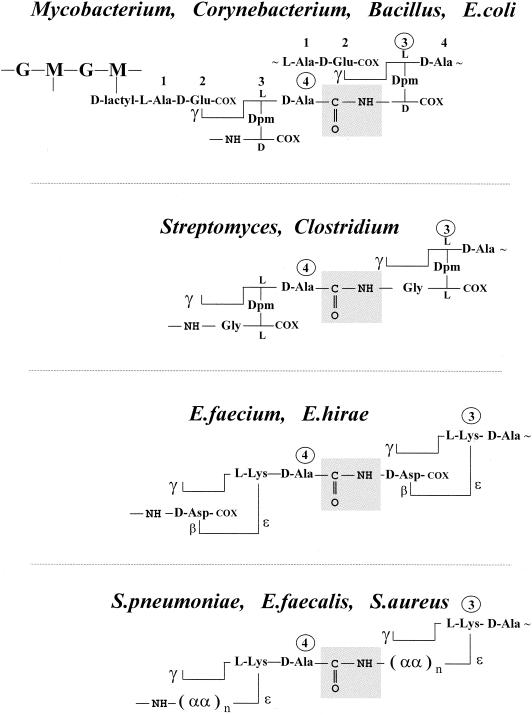
All peptidoglycan-containing bacteria in the exponential phase of growth manufacture a (4→3) peptidoglycan (Fig. 1 ) in a penicillin-susceptible manner. Peptidoglycan crosslinking extends from the carboxy-terminal d-alanine residue at position 4 of a stem tetrapeptide to the lateral amino group at position 3 of another, unbranched or branched, stem peptide. The (4→3) interpeptide linkages or cross-bridges are made by specialized acyltransferases which are immobilized by penicillin in the form of stable, enzymatically inactive penicillin-binding proteins (PBPs) (214). Escherichia coli, the mycobacteria, and leprosy-derived corynebacteria (111) produce unbranched (4→3) peptidoglycans with meso-diaminopimelic acid at position 3 of the stem peptides. Peptidoglycan crosslinking is mediated by direct (d)-alanyl-(d)-meso-diaminopimelic acid interpeptide linkages (Fig. 1). In mycobacteria, however, muramic acid is either acylated or glycolylated (12). The α-carboxylate of d-glutamic acid can be amidated. A glycine residue substitutes for the l-alanine residue at the amino end of the stem peptides in Mycobacterium leprae.
FIG. 1.
(4→3) peptidoglycans. d-Alanyl-diaminoacyl interpeptide linkages and cross-bridges (boxed) between l-Ala-γ-d-Glu-(l)-diaminoacyl-d-alanine stem peptides. The diamino acid residues are meso-diaminopimelic acid (Dpm), l, l-diaminopimelic acid, or l-lysine. The stem peptides are unsubstituted at position 3 in Mycobacterium, Corynebacterium, and Bacillus spp. and E. coli. They are substituted at position 3 by one or several additional amino acid residues (αα) in the other organisms shown. G, N-acetylglucosamine; M, N-acetylmuramic acid (see Fig. 4); COX = COOH or CONH2. In S. pneumoniae, the cross-bridges are Nɛ-(l-alanyl-l-alanyl)- or (l-alanyl-l-seryl)-l-lysine. In S. aureus, the cross-bridges comprise five glycine residues or three glycine and two l-serine residues.
Bacteria also exist which have the dual ability to manufacture a peptidoglycan of the (4→3) type and another peptidoglycan of the (3→3) type (Fig. 2). The (3→3) interpeptide linkages or cross-bridges extend from the α-carbonyl of the diaminoacid residue (l-center) at position 3 of a stem peptide to the lateral amino group at position 3 of another, unbranched or branched, stem peptide.
FIG. 2.
(3→3) peptidoglycans. Diaminoacyl-diaminoacyl interpeptide linkages and cross-bridges (boxed) between l-Ala-γ-d-Glu-(l)-diaminoacyl-d-alanine stem peptides. The stem peptides are unsubstituted at position 3 in Mycobacterium spp. and E. coli. They are substituted at position 3 by Gly in Streptomyces spp. and Clostridium perfringens and by β-d-Asp in E. faecium
The identification in the early 1970s of the occurrence of (3→3) peptidoglycans in Streptomyces albus G, Clostridium perfringens (139), Mycobacterium smegmatis ATCC 21732, and Mycobacterium tuberculosis BCG Pasteur strain (244) came as an exclamation point. Mycobacteria have a highly crosslinked peptidoglycan. About 70% to 80% of the total meso-diaminopimelic acid residues are involved in interpeptide linkages. In Mycobacterium smegmatis grown in Sauton's medium in Roux bottles for 9 days at 37°C, the (4→3) and (3→3) peptidoglycans occur in the proportion of about 2 to 1. The pattern of distribution of the two peptidoglycans is not known, but (3→3)-linked peptide trimers occur in M. smegmatis and M. tuberculosis BCG.
Subsequently, it was found that Escherichia coli manufactures a (3→3) peptidoglycan (Fig. 2) in increasing proportions of total peptidoglycan and becomes increasingly more resistant to β-lactam antibiotics as the generation time increases (227). Penicillin-induced lysis also causes an increased proportion of (3→3) peptidoglycan (127). The contribution of (3→3) peptidoglycan in Aeromonas spp., Acinetobacter acetoaceticus, Agrobacterium tumefaciens, Enterobacter cloacae, Proteus morganii, Pseudomonas aeruginosa, Pseudomonas putida, Salmonella enterica serovar Typhimurium, Vibrio parahaemolyticus, Yersinia enterocolitica, and E. coli grown to late exponential phase varies from 1% to 45% of total peptidoglycan (183, 184). Finally, Enterococcus faecium is also a (3→3) peptidoglycan manufacturer (Fig. 2). A laboratory mutant has been isolated which resists penicillin in the exponential phase of growth, conditions under which it manufactures a wall peptidoglycan of the (3→3) type exclusively (145, 205).
From the foregoing, it follows that, in all likelihood, (3→3) peptidoglycan crosslinking is carried out by acyltransferases that escape penicillin action and that, in particular genetic backgrounds or under specific growth conditions, the penicillin-resistant (3→3) peptidoglycan assembly molecular machine can substitute for the penicillin-susceptible (4→3) peptidoglycan assembly molecular machine. This conclusion raises questions of fundamental and practical importance, related, in particular, to the mycobacterial pathogens.
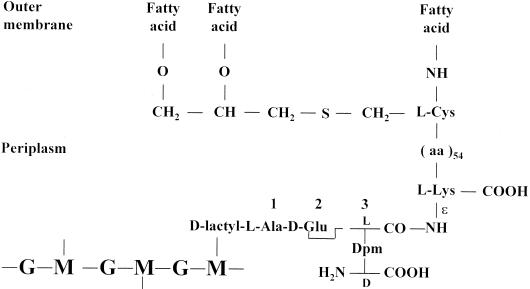
E. coli has a 4.60-Mb genome (24). M. tuberculosis H37Rv has a 4.41-Mb genome (38). Mycobacterium leprae has a 3.27-Mb genome (39) that shows extensive decay and downsizing. M. tuberculosis and M. leprae share about 1,500 genes. As stated above, E. coli and Mycobacterium spp. manufacture similar, unbranched, meso-diaminopimelic acid-containing peptidoglycans of the (4→3) and (3→3) types. The nonpeptidoglycan wall polymers, however, are different. In E. coli, several lipoproteins are linked to the peptidoglycan. The 56-amino-acid residue Braun's lipoprotein (29) contains one fatty acid bound as an amide to the amino group of a cysteine residue and two fatty acids bound as esters to the hydroxyl groups of S-glyceryl cysteine (Fig. 3). The fatty acids are embedded in the outer membrane. Lipoprotein molecules occur both in a free form and in covalent linkage with the underlying peptidoglycan. The linkage is an amide bond between the ɛ-amino group of the l-lysine residue at the carboxy end of the lipoprotein and the carbonyl group at the l-center of the meso-diaminopimelic acid residue at position 3 of a stem tripeptide of the peptidoglycan.
FIG. 3.
Braun's lipoprotein-peptidoglycan complex of E. coli.
In mycobacteria, mycolic acid, arabinogalactan, and peptidoglycan form a covalent complex (30). Mycolic acids are 2-alkyl 3-hydroxy branched-chain fatty acids. They are ester linked to arabinogalactan, which itself is linked to carbon C-6 of muramic acid via phosphodiester bonds. One may also note that a large portion of the coding ability to M. tuberculosis H37Rv is involved in lipid and polyketide metabolism (38) and 8% of the genome is devoted to the production of two families of glycine-rich proteins of repetitive structure (20, 186).
M. tuberculosis and M. leprae have different life styles. M. tuberculosis enters host macrophages at cholesterol-rich domains of the plasma membrane (75). It survives in the macrophage by preventing fusion of the mycobacterial phagosomes with lysosomes (50, 201, 218, 236). It may persist inside macrophages lodged in calcified structures of the lungs and be reactivated decades after initial infection (153). It grows in synthetic media with a generation time of 12 to 24 h. Tuberculosis, once almost vanquished, resurged in the late 1980s because of the emergence of multidrug-resistant strains (25, 250). Often acting in deadly combination with AIDS, it kills more than 2 million people each year. Mycobacterium leprae enters the body via the mucosal linings of the nose or through open wounds. It shows a marked tropism for myelin-producing Schwann cells, which insulate nerves (187, 188). It may be dormant for years until the body's immune system attacks the infected cells, destroying the nerves and degrading soft tissues and even bones. It is unculturable but can be grown in the nine-banded armadillo and the footpad of mice with an estimated generation time of about 2 weeks. Leprosy still flourishes in developing countries, where more than 750,000 people contract the disease each year.
Consistent with its ability to manufacture a (4→3) peptidoglycan, M. tuberculosis H37Ra (ATCC 25177, an attenuated laboratory strain) produces four major PBPs of 94, 82, 52, and 37 kDa (35). The inactivation of the 94-, 82-, and 52-kDa PBPs in cells grown to mid-exponential phase is associated with antibacterial activity. β-Lactamase production (240), more than the relatively low permeability of the cell envelope, is the major determinant of resistance (35, 63, 112, 185). Consequently, the MICs of ampicillin associated with the β-lactamase inactivator sulbactam are ≅0.1 μg ml−1 for M. tuberculosis H37Ra and ≅2 μg ml−1 for M. tuberculosis H37Rv (35). The drug combination is bactericidal to M. tuberculosis in exponential-phase cultures. It is bactericidal to M. tuberculosis multiplying in macrophages (35) and to M. leprae multiplying in mouse footpads (180, 189).
Paradoxically in view of the above data, the β-lactam antibiotics are not effective therapeutic agents for the treatment of tuberculosis and leprosy (101). This lack of efficiency could be due to a shift of peptidoglycan synthesis from the (4→3) type to the (3→3) type. Information that can help apprehend the problem was sought from comparative genomics of bacterial species (E. coli, Enterobacter faecium, Mycobacterium spp., and others) which have the ability to assemble, presumably from the same pool of precursors, peptidoglycans of the (4→3) and (3→3) types. Searches were also made for the positions of genes on the chromosomes because operons are common in prokaryotes and genes that are neighbors tend to be functionally linked.
PREDICTIVE STUDIES
Data concerning unfinished genome sequences were from the Institute for Genomic Research (TIGR) website at http://www.tigr.org. They were produced at TIGR with the support of the National Institute of Allergy and Infectious Diseases for Mycobacterium avium 104, at the Sanger Center/Institut Pasteur/VLA Weibridge with the support of MAFF/Beowulf Genomics for Mycobacterium bovis AF2122/97, at the Sanger Center/World Health Organization/Public Health Laboratory with the support of Beowulf Genomics for Corynebacterium diphtheriae NCTC 13129, and at the Sanger Center/John Innes Center with the support of BBSRC/Beowulf Genomics for Streptomyces coelicolor A3 (2).
Amino acid sequence similarities were searched with the Basic Local Alignment Search Tool Blast programs of the National Center for Biotechnology Information (NCBI) from their website at http://www.ncbi.nlm.nih.gov/Blast (4). Program BlastP compares a query amino acid sequence against a protein database. Program tBlastN compares a query amino acid sequence against a nucleotide sequence database dynamically translated in all six reading frames. E. coli, M. tuberculosis, M. leprae, C. diphtheriae, and S. coelicolor proteins were searched in the nonredundant protein database with BlastP and in the finished and unfinished genome database with tBlastN. The Expect value E of the NCBI's programs allows estimation of how far from the background noise is the similarity between aligned sequences (3). For values of ≤0.01, E becomes equivalent to the probability P that the sequences align by chance (118). P values smaller than 10−3 to 10−4 (depending on the sizes of the databanks) indicate statistically significant similarity.
The P values depend on the number and size of the gaps introduced to optimize the alignments. When the P value is small, equal or close to 0.0, the percentage of identical amino acid residues allows the sequences to be hierarchically classified. Combining the P values, the lengths of the overlapping regions, and the percentages of identities (I) present in the aligned regions gives an estimate of the extent of similarity between the sequences under comparison. Proteins encoded by genes having a common ancestor are homologues (193). Proteins encoded by genes related by duplication within a genome followed by diversification normally evolve new functions (103); they are paralogues. Homologous proteins encoded by genes in different organisms separated by speciation are orthologues (51, 103).
Protein orthologues that are essential and related by small P values and large I values normally retain similar biological functions. This conclusion is especially pertinent when low P values and large I values apply, over the entire sequences, to protein fusions for which the constitutive modules evolved from different protein ancestors. In contrast, little information is obtained when the similarities are restricted to peptide stretches or amino acid groupings. Conserved motifs defining the boundary of a catalytic center give valuable information on the catalytic mechanism, not on the specificity and fate of the reactions catalyzed.
LIPID II PRECURSORS: PEPTIDOGLYCAN ASSEMBLY
Lipid II molecules are the immediate biosynthetic precursors of the wall peptidoglycans (102). A disaccharide peptide exposed on the outer face of the plasma membrane is linked to a C55H89 undecaprenyl carrier via a pyrophosphate bridge involving carbon C-1 of N-acetylmuramic acid. The stem peptide borne by N-acetylmuramic acid is a pentapeptide terminating in d-alanyl-d-alanine. It can be unsubstituted or branched at position 3.
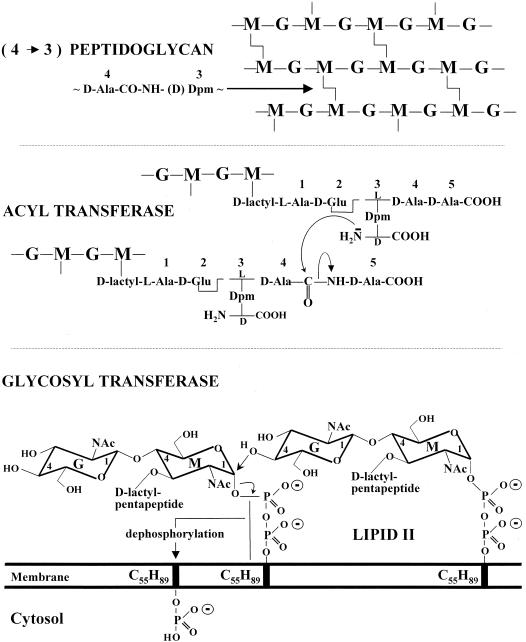
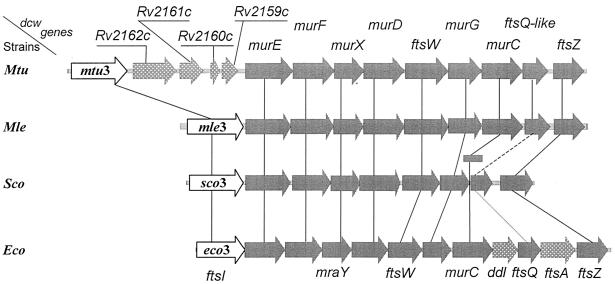
In E. coli, the synthesis of lipid II (Fig. 4) involves an interchange of carriers that are compatible with the environments of the cell (231). UDP-N-acetylglucosamine is converted into UDP-N-acetylglucosamine-enolpyruvate by MurA and from this into UDP-N-acetylmuramic acid by MurB. The Ddl (ATP,ADP + Pi) ligase catalyzes the formation of a d-alanyl-d-alanine dipeptide, and the MurC, MurD, MurE, and MurF (ATP,ADP + Pi) ligases catalyze the formation of UDP-N-acetylmuramoyl pentapeptide by the sequential additions to UDP-N-acetylmuramic acid of l-alanine, d-glutamic acid, meso-diaminopimelic acid, and the preformed d-alanyl-d-alanine dipeptide. Then, MraY transfers the phospho-N-acetylmuramoyl pentapeptide from its uridylic carrier to a membrane-bound C55-isoprenoid alcohol phosphate, giving rise to lipid I. MurG transfers the N-acetylglucosamine from its uridylic carrier to carbon atom C-4 of N-acetylmuramic acid, giving rise to lipid II. Somehow, lipid II flips over the membrane bilayer, and the disaccharide pentapeptide moiety is exposed on the outer face of the membrane (Fig. 4). The genes ddl, murC, murD, murE, murF, murG, and mraY reside in the dcw (or mra) cluster at the 2-min region of the chromosome (Fig. 5).
FIG. 4.
Lipid II precursor (bottom) and polymeric (4→3) peptidoglycan (top) of E. coli. Reactions catalyzed by the glycosyl- and acyltransferases.
FIG. 5.
Organization of the division cell wall (dcw) gene clusters of M. tuberculosis (Mtu), M. leprae (Mle), Streptomyces coelicolor (Sco), and E. coli (Eco). All the coding sequences are on the same DNA strands. Open arrows, mtu3, mle3, sco3, and eco3 (synonymous to ftsI), coding for cell septation SxxK PBP fusions. Shaded arrows, with fts standing for the filamentous phenotype of thermosensitive E. coli mutants, ftsW, ftsQ, ftsA, and ftsZ code for cell septation-related, non-penicillin-binding proteins. Genes murE, murF, murX (synonymous to mraY), murD, murG, and murC code for enzymes of the lipid II-synthesizing pathway. In S. coelicolor, murC (shaded rectangle) is not in the dcw cluster. Stippled grey arrows, genes of a dcw cluster having no homologues in the other clusters. In M. tuberculosis, the genes inserted between mtu3 and murE probably code for a glycine-rich protein of repetitive structure (Rv2162c), a protein of the lincomycin-synthesizing pathway (Rv2161c), and proteins of unknown function (Rv2160c and Rv2159c). A gene homologous to E. coli ddl (coding for the ligase which catalyzes the formation of d-alanyl-d-alanine) is located elsewhere in the chromosomes of M. tuberculosis, M. leprae, and S. coelicolor. E. coli ftsA codes for a cell division, actin-like ATPase. Solid, dashed, and dotted lines connecting the dcw genes indicate the extents, in decreasing order, of similarity between homologous genes. The E. coli cell septation genes mraZ, mraW, and ftsL (not shown) are upstream from eco3.
The stem pentapeptides can be modified at different stages of the biosynthesis of lipid II (234). Amidation of the α-carboxylate of d-glutamic acid (88) and the carboxylate at the d-center of meso-diaminopimelic acid probably takes place at the level of the UDP-N-acetylmuramoyl pentapeptide precursors. In many gram-positive bacteria, the diaminoacid residue at position 3 of the stem pentapeptide is l-lysine. Addition of one or several amino acid residues to the ɛ-amino group of the l-lysine residue, resulting in the formation of branched-stem pentapeptides, probably occurs at the level of lipid II while it is still oriented toward the cytosol.
In Staphylococcus aureus, there are four glycyl tRNAs. One is involved in protein synthesis. The three others seem to be used exclusively for peptidoglycan synthesis. Lipid II precursor molecules with one, three, and five glycine residues attached to the ɛ-amino group of the l-lysine residue are formed by the sequential actions of the glycine-adding enzymes FemX (also called FemhB), FemA, and FemB (128, 197). Loss of FemX is lethal. In some strains, the FemAB-like Lif and Epr incorporate an l-serine residue instead of a glycine residue within the branches at positions 3 and 5 (60). In Streptococcus pneumoniae, the Nɛ-(l-alanyl-l-alanyl)-l-lysine and Nɛ-(l-alanyl-l-seryl)-l-lysine branches are synthesized by MurM and MurN, respectively (64, 242), with MurM being responsible for the addition of the first amino acid residue to the ɛ-amino group of l-lysine.
Vancomycin-resistant enterococci are programmed to produce lipid II precursor molecules which terminate in d-alanyl-d-lactate (8, 136). Five enzymes are necessary and sufficient. The Zn2+-dependent VanX dipeptidase hydrolyzes the d-alanyl-d-alanine dipeptide produced by the Ddl ligase. VanH and VanA act sequentially to synthesize the depsipeptide d-alanyl-d-lactate, which is incorporated in lipid II rather than the dipeptide d-alanyl-d-alanine. The production of VanHAX is controlled by a two-component regulatory system that involves the transmembrane sensor kinase VanS and the transcription factor VanR, which becomes active when phosphorylated by VanS (8).
The conversion of the disaccharide peptide (depsipeptide) moiety of lipid II into polymeric (4→3) peptidoglycan requires the sequential actions of two transferases (Fig. 4). A glycosyltransferase catalyzes attack of carbon C-1 of N-acetylmuramic acid of a lipid II molecule by the acceptor nucleophile OH of carbon C-4 of N-acetylglucosamine of another lipid II molecule (or at the nonreducing end of a growing glycan chain; not shown). At each transfer, the delivery of a disaccharide peptide unit generates an undecaprenyl pyrophosphate which is dephosphorylated. The C55-isoprenoid alcohol phosphate turns over the membrane bilayer so that the phosphate group faces the cytosol, allowing a new cycle to start.
In turn, the required d,d-acyltransferase (Fig. 4 and reaction I in Fig. 6) catalyzes attack of the carbonyl of the d-alanine residue at position 4 of a pentapeptide (depsipeptide) borne by a glycan chain, by the lateral amino group at position 3 of a peptide borne by another glycan chain. The reaction proceeds via the transitory formation of an N-acyl-d-alanyl-enzyme intermediate in which the d-alanine residue is linked as an ester to a serine residue at the catalytic center (69, 70, 78). The carbonyl donor involved in enzyme acylation is invariably a d-alanyl-d-alanine(-d-lactic acid) sequence. In contrast, the acceptor involved in enzyme deacylation can be the amino group of a glycine residue, the ɛ-amino group of an l-lysine residue, or an amino group borne by an l- or a d-configured carbon atom (Fig. 1).
FIG. 6.
Acyl transfer reactions. Formation of (4→3) peptidoglycan interpeptide linkages by penicillin-susceptible d,d-N-acyl-d-alanyl-d-alanine transpeptidases (reaction I). Formation of (3→3) peptidoglycan interpeptide linkages by penicillin-resistant l,d-N-acyl-l-diaminoacyl-d-dipeptidyl transpeptidases (reaction II) and l,d-N-acyl-l-diaminoacyl-d-alanine transpeptidases (reaction III). Hydrolysis of stem pentapeptides into stem tetrapeptides by penicillin-resistant d,d-N-acyl-d-alanyl-d-alanine carboxypeptidases (reaction IIIa).
Water can also serve as an acceptor. Stem pentapeptides are hydrolyzed into stem tetrapeptides, preventing further (4→3) peptidoglycan crosslinking. The β-lactam antibiotics act as suicide carbonyl donors of the d,d-acyltransferases (224). Rupture of the β-lactam amide bond produces a serine ester-linked acyl (penicilloyl, cephalosporoyl,…) enzyme, which is a dead end because the bulky acyl moiety is a steric hindrance to the approach of any attacking nucleophile. The catalytic center turns over very slowly, once or less per hour (71, 72), and the inactivated d,d-acyltransferases are detectable as PBPs (214).
No one knows exactly how the (3→3) peptidoglycans are made in a penicillin-resistant manner. The (3→3) interpeptide linkages or cross-bridges could be made through the action of an l,d-acyltransferase that cleaves the bond between position 3 and position 4 of pentapeptide carbonyl donors, with release of the dipeptide d-alanyl-d-alanine (reaction II in Fig. 6). Alternatively, they could be made through the sequential actions of a d,d-carboxypeptidase catalyzing reaction IIIa (Fig. 6) and an l,d-acyltransferase that cleaves the bond between position 3 and position 4 of tetrapeptide carbonyl donors (reaction III in Fig. 6). Reactions IIIa and III each cause the release of a single d-alanine residue.
SxxK ACYLTRANSFERASE SUPERFAMILY
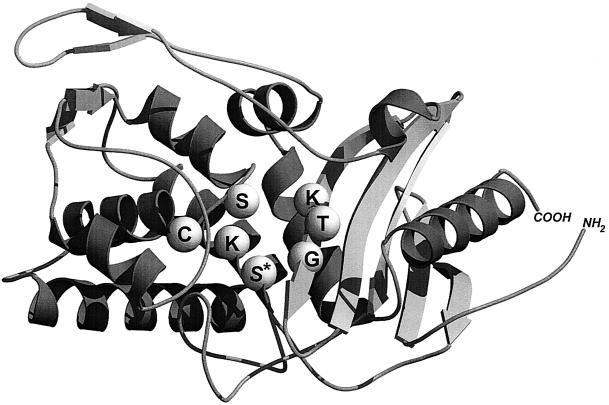
The serine acyltransferases implicated in wall peptidoglycan assembly are members of a superfamily of SxxK serine enzymes. The term superfamily is used to include proteins with no statistically significant sequence similarities but with similar structures in the classical sequence-based families (169). With x denoting a variable amino acid residue, the acyltransferases of this superfamily have a specific bar code in the form of three motifs, SxxK, SxN (or analogue), and KTG (or analogue). The motifs occur at equivalent places and with roughly the same spacing along the polypeptide chains. In the three-dimensional structures, they are brought close to each other at the immediate boundary of the catalytic center between an all-α domain and an α/β domain, the five-stranded β-sheet of which is covered by α-helices (79, 123, 124).
The serine residue of the invariant motif SxxK occupies a central position in the catalytic center and is central to the enzyme acylation and deacylation steps, which, mechanistically, are similar to the proteolytic reactions of the trypsin protein family. The mature SxxK acyltransferase of Streptomyces sp. strain K15 is 262 amino acid residues long. It is one of the smallest members of the superfamily (Fig. 7) (68). Diverging evolution gave rise to a constellation of SxxK acyltransferases with various amino acid sequences. Fusion to other polypeptide chains resulted in a combinatorial system of structural modules that contributed to a massive increase in functional diversity.
FIG. 7.
Basic polypeptide fold of the acyltransferases of the SxxK superfamily and spatial disposition of the three catalytic center-defining amino acid groupings. The structure shown is that of the 262-amino-acid dd-transpeptidase-PBP of Streptomyces sp. strain K15. The SxxK acyltransferases comprise an all-α domain (left side) and an α/β domain (right side). The invariant motif 1, S*xxK, where S* is the essential serine nucleophile, forms the amino-terminal turn of helix α2 of the all-α domain. Motif 2, most often SxN or SxD (here SxC), is on a loop connecting two helices of the all-α domain. Motif 3, most often KTG or KSG, is on strand β3 of the α/β domain. The structure was built with Molscript (130) and Raster3D (155). Illustration courtesy of Paulette Charlier, Eveline Fonzé, Michaël Delmarcelle, and André Piette, Center for Protein Engineering.
The acyltransferases of the SxxK superfamily are a paradigm of catalytic versatility. They fall into three main groups. The d,d-acyltransferases-PBPs, implicated in (4→3) peptidoglycan synthesis, are of group I. Acyltransferases endowed with diverse functions unrelated to peptidoglycan biochemistry are of group II. Penicillin-resistant acyltransferases that could act as l,d-peptidases in (3→3) peptidoglycan synthesis are of group III. The ensuing sections provide critical coverage of mature topics and speculations on emerging topics. The identifiers of the SxxK acyltransferases are shown in Table 1. They combine the name of the producing bacterial species as a three-letter prefix flanked by a number or additional letters that specify the protein under consideration (e.g., Eco1a, protein 1a of E. coli).
TABLE 1.
Prefixes identifying bacterial species
| Prefix | Species |
|---|---|
| Aae | Aquifex aeolicus |
| Ana | Anabaena sp. strain PCC7120 |
| Bbu | Borrelia burgdorferi |
| Bsu | Bacillus subtilis |
| Cdi | Corynebacterium diphtheriae |
| Eco | Escherichia coli |
| Efam | Enterococcus faecium |
| Efas | Enterococcus faecalis |
| Ehi | Enterococcus hirae |
| Hin | Haemophilus influenzae |
| Hpy | Helicobacter pylori |
| Mle | Mycobacterium leprae |
| Msm | Mycobacterium smegmatis |
| Mtu | Mycobacterium tuberculosis |
| Nme | Neisseria meningitidis |
| Pae | Pseudomonas aeruginosa |
| Sau | Staphylococcus aureus |
| Scl | Streptomyces clavuligerus |
| Sco | Streptomyces coelicolor |
| Sgr | Streptomyces griseus |
| Sor | Streptococcus oralis |
| Spn | Streptococcus pneumoniae |
| Ssc | Staphylococcus sciuri |
| Spy | Streptococcus pyogenes |
| Sth | Streptococcus thermophilus |
| Syn | Synechocystis sp. strain PCC6203 |
STRUCTURE-ACTIVITY RELATIONSHIPS OF β-LACTAM ANTIBIOTICS
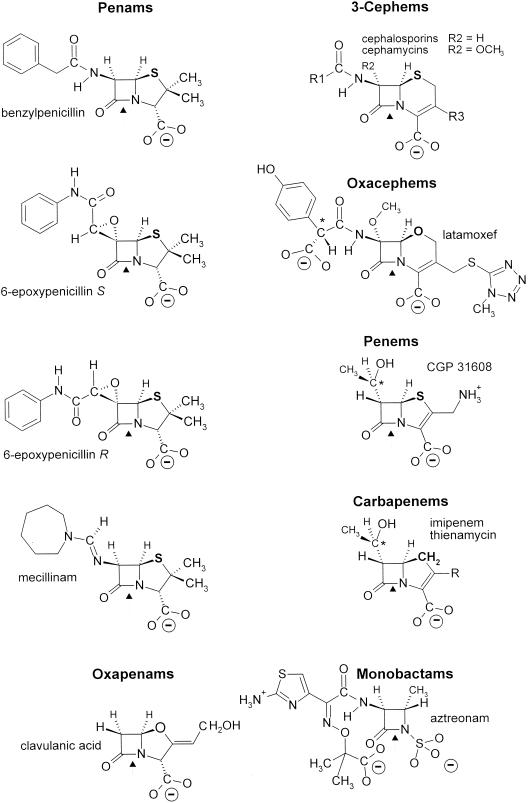
The SxxK d,d-acyltransferases of group I are the target proteins of the β-lactam antibiotics. These compounds are a large group of molecules of which the common structural feature is the presence of a β-lactam (azetidinone) ring (Fig. 8). Currently, they are regarded as a molecular mimic of N-acyl-d-alanyl-d-alanine (224), explaining why they are suicide carbonyl donors of the d,d-acyltransferases-PBPs implicated in (4→3) peptidoglycan synthesis (Fig. 4 and reaction I in Fig. 6).
FIG. 8.
β-Lactam antibiotic family. Only 6-epoxypenicillin S is active. *, d-configured carbon atom. (“Mecillinam” is another name for amdinocillin.)
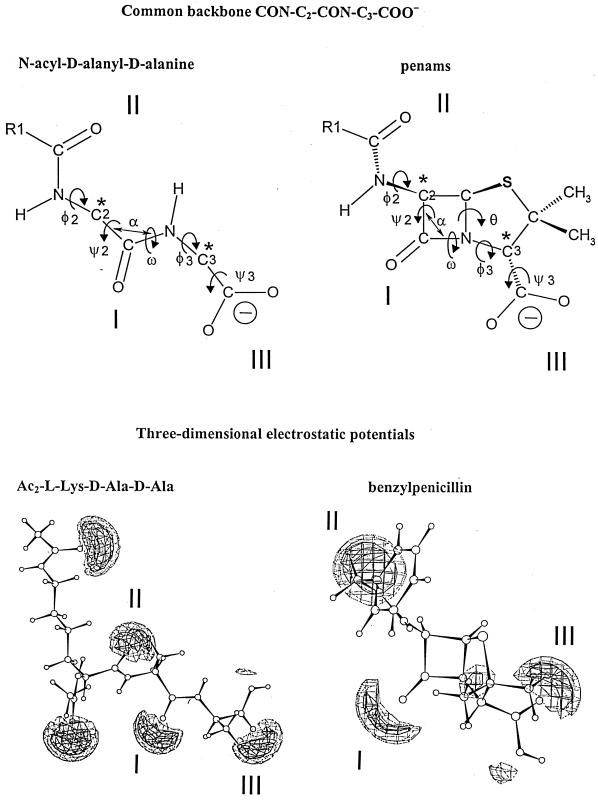
The penams, 3-cephems, and N-acyl-d-alanyl-d-alanine-terminated peptides (in the extended conformation) have a common backbone, CON-C2-CON-C3-COO− (Fig. 9, upper part). Carbon atoms C-2 and C-3 (marked by asterisks) have a d-configuration. They are the pivots that connect the central CON amide plane at position I to the CON amide plane at position II (through the rotation angles ψ2 and φ2) and the carboxylate at position III (through the rotation angles φ3 and ψ3). The scissile CON bonds at position I, however, are far from being isosteric. The nitrogen atom is planar in the peptides. It is pyramidal in the azetidinones. The bond angle α is 117° in the peptides and 90° in the azetidinones. The rotation angle ω is 180° in the peptides, 155° in the 3-cephems, and 135° in the penams.
FIG. 9.
Common backbone of extended N-acyl-d-alanyl-d-alanine peptides and penams (top) and ab initio electrostatic potentials of bisacetyl-l-lysyl-d-alanyl-d-alanine and benzylpenicillin (bottom) optimized at level AM1 (the terminal carboxylates are protonated). The electrostatic negative wells I, II, and II are shown at levels of −40 kcal (solid contours) and −30 kcal (dotted contours). They are coplanar. The negative wells generated by the acetyl substituents of the α- and ɛ-amino groups of the l-lysine residue of bisacetyl-l-lysyl-d-alanyl-d-alanine are above and below the plane, respectively. The negative well of small amplitude seen between well I and well III of benzylpenicillin is due to the pair of free electrons of the nitrogen atom of the azetidinone ring. Illustration courtesy of Georges Dive, Center for Protein Engineering.
Rather than being related to similarities between linked atoms, structural analogy between the most stable d-alanyl-d-alanine-terminated peptide, penam, and 3-cephem conformers relies on the spatial disposition of the electrostatic negative wells created by the carbonyl CO at position I, the carbonyl CO at position II, and the carboxylate COO− at position III (80, 131, 132). These three groupings are almost coplanar, and the spanning distances between the oxygen atoms at position I and position II (≅5 Å) and between the oxygen atom at position I and the carbon atom of the carboxylate at position III (3.5 to 4.5 Å) are roughly similar. Figure 9 (lower part) shows the three-dimensional electrostatic potentials of the optimized benzylpenicillin and extended bisacetyl-l-lysyl-d-alanyl-d-alanine molecules. The two additional negative wells seen in the peptide are due to the acetyl groupings that substitute the α- and ɛ-amino groups of the l-lysine residue.
As a result of the coplanarity of the negative wells at positions I, II, and III, the α-face of the azetidinone ring of the β-lactam antibiotics is well exposed, facilitating the attack of the electrophilic center at position I by the serine nucleophile of the SxxK d,d-acyltransferases. The backbone CON-C2-CON-C3-COO− is modified into CON-C2-CON-SO3− in the monobactam aztreonam (Fig. 8). It is modified into C=N-C2-CON-C3-COO− in the penam amdinocillin. The carbon atom C-2 of the carbapenem imipenem has an l-configuration. In spite of these structural variations, the disposition of the sulfamate in aztreonam is roughly comparable to that of the carboxylate at position III in the bicyclic penams and 3-cephems. The Π environment of the CH=N amidino bond of amdinocillin generates a negative well at position II, albeit of reduced amplitude. Because the rotation angle θ (Fig. 9, upper part) is less open in the carbapenems than in the penams and 3-cephems, the carboxylate at position III of imipenem is moved upward, ensuring coplanarity with the oxygen atom at position I and the alcohol oxygen atom at position II. Of the two 6-epoxypenicillin isomers (Fig. 8), the side chain of which, at position II, has a frozen conformation due to the epoxy cycle, the S isomer is active, but the R isomer is not.
Depending on the (noncyclic, monocyclic, or bicyclic) framework and conformation of the backbone and the presence of bulky, ionized, and electron-withdrawing side chains, the electrostatic negative wells at positions II and III around the electrophilic center at position I of the carbonyl donors can vary in shape and strength, be displaced along the reference plane, be better expressed in other sections of the molecules, be fused, or be masked. In turn, the SxxK acyltransferases are large bodies studded with positively and negatively charged magnets. Upon binding of a carbonyl donor to the catalytic center, a dense hydrogen bonding network is formed, and a multimembered ring that is both enzyme and ligand specific is created, in which the scissile CO-N amide bond at position I of the carbonyl donor is connected to the catalytic serine γOH of motif SxxK of the acyltransferase by one or several water molecules and the side chains of several amino acid residues (53, 54, 80). This multimembered ring is utilized as a motorway, allowing the proton of the serine γOH to be transferred via a transition state to the nitrogen atom of the scissile bond, resulting in enzyme acylation. For the reaction to reach completion, the serine ester-linked acyl enzyme must adopt a conformation that allows entry of an amino group or a water molecule and formation of a new multimembered ring that performs enzyme deacylation.
Potential energy hypersurfaces best portray the geometric rearrangements, electronic redistributions, and free energy barriers that occur along the reaction pathways (80). Depending on the freedom of the water molecules, the ease with which the ligands undergo deformation, and the ease with which the enzyme backbone undergoes relaxation, numerous possible routes for proton transfer exist. The energetically most favorable route dictates the specificity of the SxxK acyltransferases and the completeness (protein binding, protein acylation, protein acylation, and deacylation) and productiveness of the catalyzed reactions.
The d,d-acyltransferases-PBPs of group I perform multiple functions related to (4→3) peptidoglycan assembly (see below). Changes in protein structure, with conservation of the overall fold, could go as far as a change from d,d specificity to l,d specificity (see the chapter on SxxK acyltransferases of group III).
GROUP I SxxK ACYLTRANSFERASES: IMPLICATED IN (4→3) PEPTIDOGLYCAN BIOCHEMISTRY
The SxxK d,d-acyltransferases-PBPs of group I occur as independent entities referred to as free-standing PBPs. They also occur as modules of PBP fusions. A d,d-acyltransferase module of class A or class B is fused, in a class-dependent manner, to polypeptides having their own bar codes and three-dimensional structures (78, 84). Traces of similarity between the free-standing PBPs and the acyltransferase modules of class A or B are generally limited to the three active-site defining motifs. Similarity between the acyltransferase modules of class A and class B is marginal (P ≥ 1 × 10−5).
The PBPs are bound to the plasma membrane, with the bulk of the polypeptide chains exposed on the outer face. The PBP fusions are synthesized with an amino-terminal hydrophobic segment that functions as both a signal sequence for secretion and a stop transfer signal that serves as a membrane anchor. Despite a great divergence in the amino acid sequences, the bar codes SxxK, SxN, and KTG of the free-standing PBPs and the acyltransferase modules of the PBP fusions are conserved except that, occasionally, SxD substitutes for SxN and KSG substitutes for KTG.
The core of an SxxK acyltransferase is defined as the sequence starting about 70 amino acid residues upstream from the SxxK motif and terminating about 70 amino acid residues downstream from the KT(S)G motif. Adducts may occur as inserts and/or as carboxy-terminal extensions. The free-standing PBP5 of E. coli (45) and the class B PBP fusion Spn2x of Streptococcus pneumoniae (86, 173) are of known structure. They each have carboxy-terminal extensions. That of E. coli PBP5 is made of β-structures that form a loose β-barrel; that of Snp2x is made of two α/β/β/β domains.
Class A PBP Fusions: Nascent (4→3) Peptidoglycan
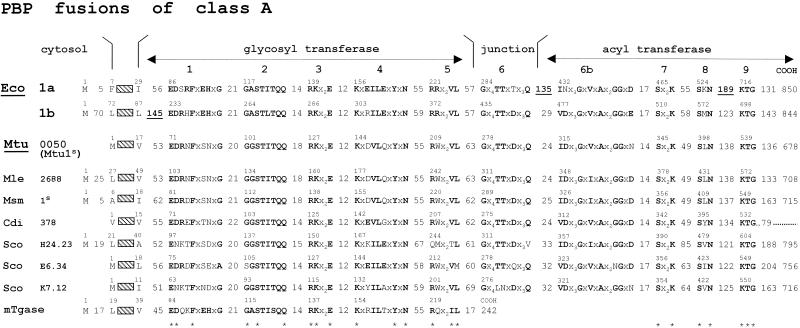
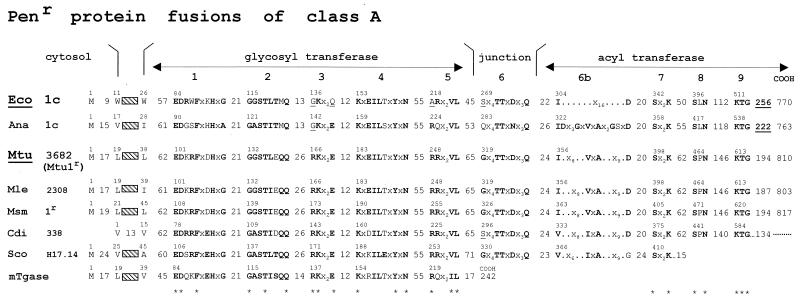
The class A PBP fusions consist of a penicillin-binding acyltransferase module of class A linked to the carboxy end of a glycosyltransferase module, itself linked to the carboxy end of the membrane anchor (Fig. 10). The full bar code comprises motifs 1 to 5 of the glycosyltransferase module, motif 6 of the intermodule junction, and motifs 7 to 9 of the acyltransferase module (84). By combining a glycosyltransferase and an acyltransferase in a single polypeptide chain, class A PBP fusions catalyze the polymerization of the disaccharide pentapeptide units borne by lipid II precursor molecules into nascent (4→3) peptidoglycans (Fig. 4) (162, 222, 232, 235).
FIG. 10.
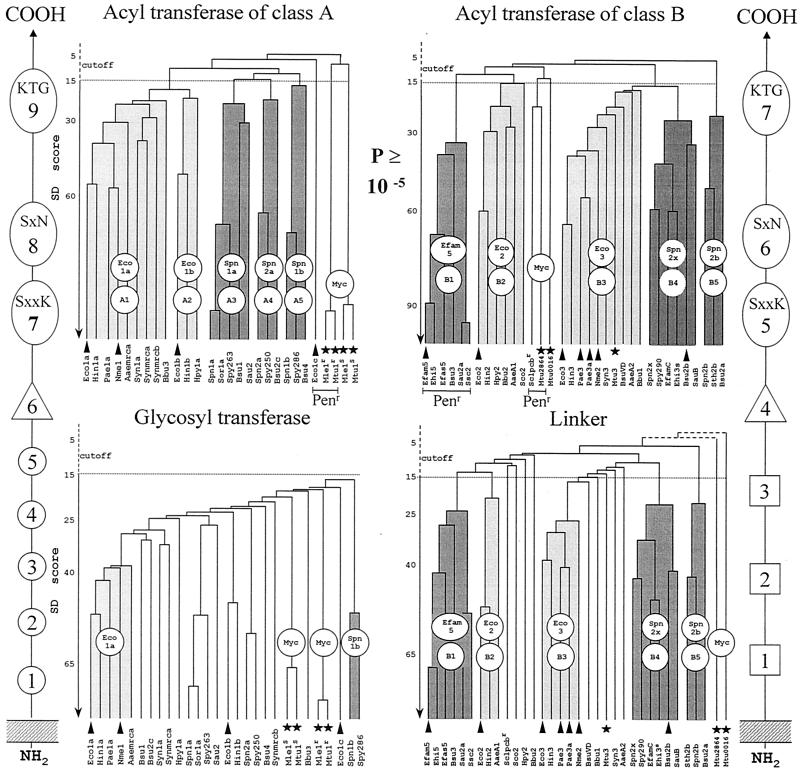
Hierarchical distribution of the SxxK PBP fusions and Penr protein fusions of classes A and B. The occurrence of class-specific motifs 1 to 9 (class A) and 1 to 7 (class B) along the polypeptide chains is shown. Adapted from reference 84. Scores (vertical axis of the dendrograms) are the standard deviation values above that expected from a run of 100 randomized pairs of sequences with the same amino acid composition as the two sequences under comparison. The protein identifiers (bottom of the dendrograms) are defined in Table 1. The clusters are labeled by two circles, one of which defines a particular subclass (A1 to A5 and B1 to B5) and the other the prototypic protein. Solid arrowheads help identify proteins that are discussed in the text. Solid stars help identify the mycobacterial proteins. The Penr acyltransferase modules are underlined.
The structure of the glycan backbones of the wall peptidoglycans is invariant. Consistently, the glycosyltransferase modules of the class A PBP fusions form a continuum of sequences (Fig. 10), indicating steady divergence and functional conservation. In contrast, the structures of the peptidoglycan interpeptide linkages and cross-bridges are variable (Fig. 1 and 2). Consistently, the acyltransferase modules of class A PBP fusions cluster into several subclasses (Fig. 10), indicating functional diversification. Modules of the same subclass are related by P values smaller than ≅1 × 10−30. Subclasses A1 and A2 are typical of gram-negative bacteria. Subclasses A3, A4, and A5 are typical of gram-positive bacteria.
E. coli produces two class A PBP fusions, Eco1a and Eco1b (Fig. 11). The Eco1a-encoding gene, at the 75-min region of the chromosome, and the Eco1b-encoding gene, at the 4-min region, are not linked to particular operons. An insert occurs downstream from the membrane anchor in Eco1b. Inserts occur downstream from the intermodule junction and between motifs 8 and 9 in Eco1a. Eco1b occurs in three forms, each of which is functional and encoded by the same gene (219). The α form, shown in Fig. 11, has a cytosolic amino-terminal tail which is 70 amino acid residues long. The glycosyltransferase modules of Eco1a and Eco1b are related by a P value of 4 × 10−33 (identity [I], 35%). The acyltransferase modules of Eco1a and Eco1b are distantly related by a P value of 2 × 10−15 (identity, 27%) for overlaps 190 amino acid residues long. They belong to distinct subclasses. Eco1a (subclass A1) and Eco1b (subclass B2) are produced in large quantities, amounting together to several thousand molecules per cell. In in vitro assays in the absence of preformed peptidoglycan primer, they each catalyze the conversion of the disaccharide peptide moiety of lipid II into polymeric (4→3) peptidoglycan.
FIG. 11.
Class A PBP fusions. Bar code, motifs 1 to 9 and inserts (underlined). For protein identifiers, see Table 1. mTgase, free-standing transglycosylase of E. coli.
Lysozyme cleaves glycosidic bonds with net retention of configuration of the anomeric center via a covalent glycosyl-enzyme intermediate that involves Asp52 (239). The glycosyltransferase of class A PBP fusions make new glycosidic bonds with inversion of configuration at carbon C-1, from the α-configuration in lipid II to the β-configuration in the peptidoglycan (Fig. 4). The essential Glu233 of Eco1b, at the amino end of motif 1 of the glycosyltransferase module (Fig. 11), is involved in proton donation to the oxygen atom of the scissile phosphoester bond, resulting in the formation of a muramic oxocarbonium intermediate (222). Asp234 of motif 1 and Glu290 of motif 3 could be responsible for the activation of the 4-OH of the nucleophile N-acetylglucosamine and the attachment on the β-face of N-acetylmuramic acid.
Crosslinking between peptide-substituted glycan chains is an acylation-deacylation reaction that is strictly dependent on the serine residue of motif SxxK of the acyltransferase module. It is aided by amino acid residues of the other catalytic center-defining motifs and one or several catalytic water molecules. In in vitro assays with lipid II, Eco1b transfers the carbonyl of the d-alanine residue at position 4 of stem pentapeptides to water (reaction products, tetrapeptide monomers) and to the d-amino group of the meso-diaminopimelic acid residue at position 3 of pentapeptide and tetrapeptide monomers [reaction products: (4→3)-linked tetrapeptide-pentapeptide and tetrapeptide-tetrapeptide dimers]. Peptide oligomers larger than dimers are not produced in detectable amounts, indicating that, in vitro, the stem pentapeptide of the tetrapeptide-pentapeptide dimers is not used as a carbonyl donor for further crosslinking.
The acyltransferase of Eco1b is inert on exogenous N-acyl-d-alanyl-d-alanine-terminated peptides (222). However, it catalyzes hydrolysis and aminolysis of ester and thiolester analogues, indicating that the d-alanyl-d-alanine-cleaving activity is glycosyltransferase dependent. Conversely, Eco1b, the acyltransferase module of which is inactivated by penicillin, catalyzes glycan chain elongation, indicating that the glycosyltransferase module is acyltransferase independent.
Loss of Eco1a and Eco1b is fatal, but loss of either Eco1a or Eco1b is tolerated, indicating that Eco1a and Eco1b can substitute for each other (119, 220, 252). PBP fusions of subclass A2 are dispensable in some gram-negative bacteria. The coccus-shaped Neisseria meningitidis, the genome sequence of which is known (223), produces a single class A PBP fusion of subclass A1 (199). Consistently, the cluster formed by the acyltransferase modules of subclass A1 (to which Eco1a belongs) is more populated, i.e., comprises more sequences, than the cluster formed by the acyltransferase modules of subclass A2 (to which Eco1b belongs) (Fig. 10). It remains possible that Eco1a and Eco1b cause distinct, subtle traits in peptidoglycan crosslinking in E. coli that are difficult to detect (34).
Class B PBP Fusions: Morphogenetic Apparatus
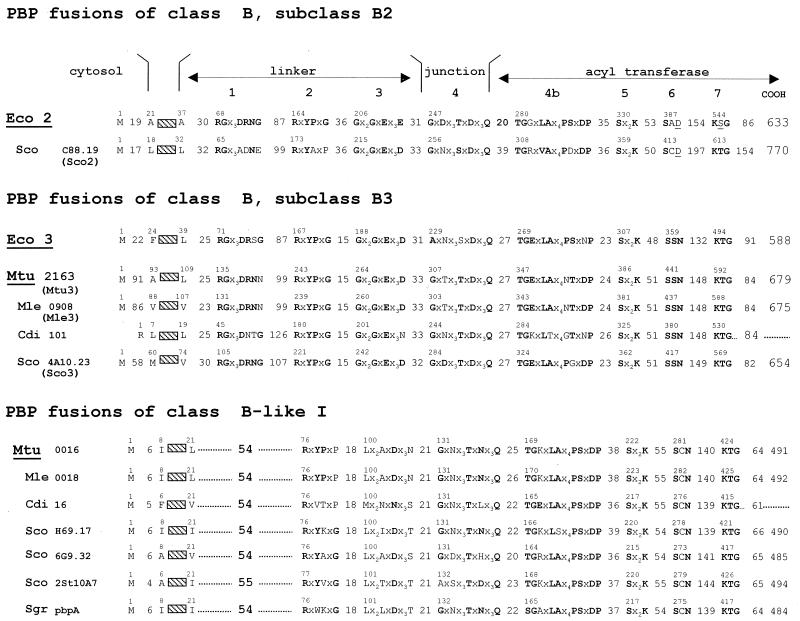
The class B PBP fusions are components of the morphogenetic apparatus that are dynamic in abundance and composition. They control wall expansion, ensure cell shape maintenance, and direct and carry out septum formation and cell division (55, 146, 163). The class B PBP fusions consist of a penicillin-binding acyltransferase module of class B linked to the carboxy end of a module which does not have the bar code of a transglycosylase and is itself linked to the carboxy end of a membrane anchor (Fig. 10). The non-penicillin-binding module mediates protein-protein interactions critical for peptidoglycan assembly in a cell cycle-dependent manner. For this reason, it is referred to as a linker (or protein recognition) module. The full bar code of the class B PBP fusions comprises motifs 1 to 3 of the linker module, motif 4 of the intermodule junction, and motifs 5 to 7 of the acyltransferase module.
The acyltransferase and linker modules diverged in concert (Fig. 10). In gram-negative bacteria, an acyltransferase module of subclass B2 or B3 is fused to a linker module of subclass B2 or B3, respectively. In gram-positive bacteria, an acyltransferase module of subclass B4 or B5 is fused to a linker module of subclass B4 or B5, respectively. E. coli produces two class B PBP fusions, Eco2 of subclass B2 and Eco3 of subclass B3 (Fig. 10 and 12). The 588-amino-acid Eco3 is processed into M1-V577 Eco3 (161).
FIG. 12.
Class B PBP fusions. Bar code, motifs 1 to 7. For protein identifiers, see Table 1.
The cell shape apparatus of E. coli comprises at least six proteins encoded by genes located at the 14-min and the 71-min regions of the chromosome. Genes of the 14-min cluster code for the PBP fusion Eco2, RodA, and the free-standing PBP Eco5. RodA is an integral membrane protein (108). It belongs to a large protein family called SEDS for shape, elongation, division, and sporulation (100) or SFR for SpoVE, FtsW, and RodA (32). Eco2, RodA, and ribosomal activities are coordinated by a chain of relaying elements, one of which is regulated by the alarmone ppGpp (115, 191). Genes of the 71-min cluster code for MreB, MreC, and MreD (241). MreB and actin, a central component of the eukaryotic cytoskeleton, are very similar in three dimensions (229). MreB-like proteins are widely distributed among rod-shaped, filamentous and helical bacteria, suggesting that an MreB cytoskeleton is important to generate a nonspherical shape.
The cell septation apparatus of E. coli, known as the divisome, is a Lego-like interlocking of a set of at least 10 proteins that assemble into a septal ring at midcell. With Fts standing for temperature-sensitive filamentous phenotype, the PBP fusion Eco3 (or FtsI), FtsW, FtsQ, FtsA, FtsZ are encoded by genes of the 2-min dcw cluster, which also contains several lipid II synthetase-encoding genes (Fig. 5) (162, 237). FtsK, ZipA, FtsN, and YgbQ are encoded by genes occurring at different places on the chromosome. Like Eco3, FtsL, YgbQ, FtsQ, and FtsN are bitopic membrane proteins with a short cytoplasmic amino tail and a relatively large periplasmic domain (31, 36).
FtsL and YgbQ have a leucine-like zipper motif in the periplasmic domain. They belong to a family of proteins that exhibit a great propensity to form coiled-coil structures (31). FtsW is a homologue of RodA (28). By analogy with FtsW of S. pneumoniae (76), the E. coli FtsW probably comprises 10 transmembrane segments, with a large extracytoplasmic loop extending between segments 7 and 8. FtsA and the eukaryotic actin are members of the same ATPase domain protein superfamily (to which Hsp70 also belongs) (237). FtsZ is a GTPase homologue of tubulin, the building block of the eukaryotic cell division microtubules (97, 142, 143).
FtsZ arrives first at mid-cell; it serves as scaffold holding the other proteins of the divisome together, and it provides the driving force for cytokinesis (2). FtsA and ZipA localize to the septum independently but in an FtsZ-dependent manner. The other proteins then assemble in a sequential dependency order as follows: FtsK, FtsQ, FtsL, YgbQ, FtsW, Eco3, and FtsN. It is likely that class A PBP fusions are also components of the divisome (233). The class A PBP fusion Bsu1 (subclass A3) of Bacillus subtilis is localized at the division septum in vegetative cells (175).
The cell shape PBP fusion Eco2 of subclass B2 and the cell septation PBP fusion Eco3 of subclass B3 are produced in small amounts: a few tens and about 200 molecules per cell, respectively. Their acyltransferase modules are distantly related by a P value of 6 × 10−15 (identity, 29%). Loss of Eco2 results in a block of cell division, transformation of the E. coli cells into spherical bodies, and cell death. Loss of Eco3 causes a block of cell septation, formation of multigenomic filaments, and cell death. Loss of either Eco2 or Eco3 is fatal (211). PBP fusions of subclass B2, however, are dispensable in some gram-negative bacteria. Neisseria meningitidis possesses a single PBP fusion of class B that belongs to subclass B3 (255). This PBP fusion may fulfil functions comparable to those of Eco2 and Eco3 in E. coli (14). Consistently, the clusters formed by the linker and acyltransferase modules of the PBP fusions of subclass B3 are more populated than the clusters formed by the corresponding modules of the PBP fusions of subclass B2 (Fig. 10).
Both Eco2 and Eco3 are implicated in the exponential phase of growth of E. coli cells (19), conditions under which the peptidoglycan precursors are incorporated all over the lateral wall in a diffuse way (49). Newly formed (4→3) peptidoglycan is mixed with existing (4→3) peptidoglycan except at the time of cell septation. At this stage, peptidoglycan synthesis is strictly localized to the septum in an Eco3-dependent manner (27). In the course of the hierarchical assembly of the divisome, FtsZ, FtsQ, FtsL, and YgbQ are required for the septal localization of FtsW, and FtsW is essential for the subsequent recruitment of Eco3 (154).
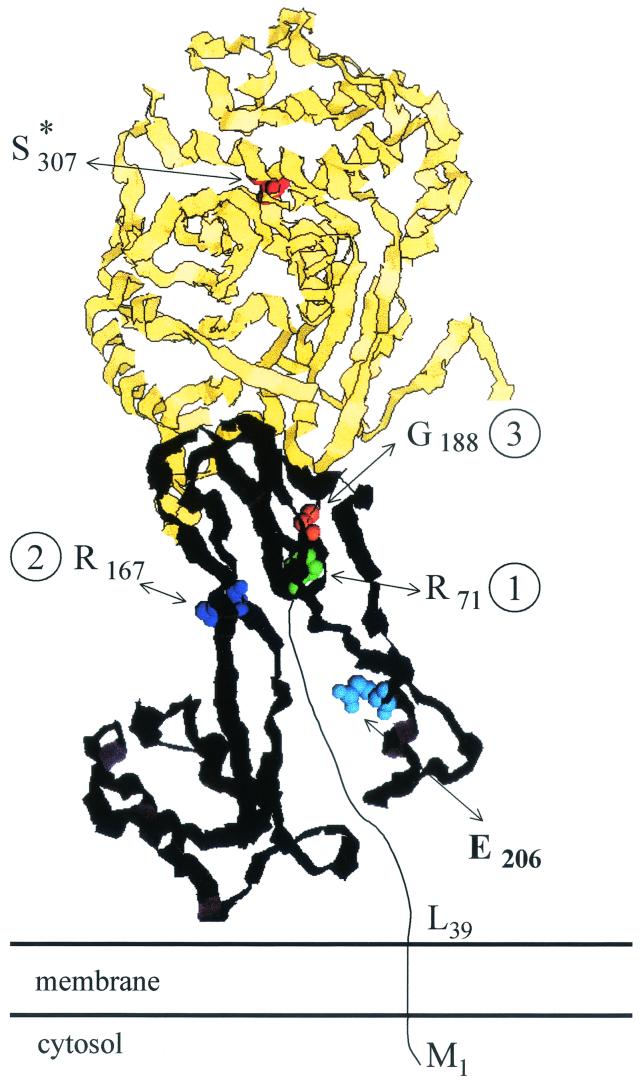
Consistently, the linker module of Eco3 appears to be designed in such a way that the acyltransferase module is positioned, in an active form, within the divisome where it needs to be (148) (Fig. 13 ). The membrane anchor (243) and the segment upstream from motif 1 of the linker module (148) have the information ensuring that Eco3 localizes at the cell septation site. Motifs 1, 2, and 3 and other peptide segments which form the core of the linker module have the information ensuring that Eco3 folds correctly and that the acyltransferase catalytic center adopts the active conformation. The Glu206-Val217 peptide segment at the surface of the linker module has the information ensuring that Eco3 fulfils its cell septation activity within the fully complemented divisome by interacting with other components of the morphogenetic apparatus.
FIG. 13.
Schematic structure of the membrane-bound SxxK subclass B3 PBP fusion protein Eco3 of E. coli. Spatial disposition along the polypeptide chain of the essential S*307 of the SxxK motif of the acyltransferase module (see Fig. 7) and of motifs 1, 2, and 3 (identified by the residue at the amino side of the sequences) and segment E206 to V217 of the linker module. The acyltransferase module is in yellow, with S*307 of the SxxK motif in red. The linker module is in black with motif 1 (R71 to G79) in green, motif 2 (R167 to G172) in dark blue, motif 3 (G188 to D197) in orange, and segment E206 to V217 in light blue. Motifs 1, 2, and 3 and other peptide segments form the core of the linker module in interaction with a noncatalytic groove of the acyltransferase module. The peptide segment M1 to R71 is of unknown structure. Adapted from reference 148. Illustration courtesy of Robert Brasseur, Faculté Universitaire des Sciences Agronomiques, Gembloux.
In in vitro assays, Eco3, which lacks glycosyltransferase activity, is inert on lipid II. As observed with the PBP fusion Eco1b of class A, Eco3 is inert on N-acyl-d-alanyl-d-alanine-terminated peptides, but it catalyzes the hydrolysis and aminolysis of thiolester analogues of the peptides (1), indicating that in vivo, Eco3 identifies N-acyl-d-alanyl-d-alanine sequences as carbonyl donors. As derived from studies carried out both in vivo and with ether-permeabilized cells (179), the acceptor of the Eco3-catalyzed transfer reaction could be the lateral amino group at position 3 of tripeptide-derived precursors, resulting in the formation of (4→3)-linked tetrapeptide-tripeptide dimers. The required stem tripeptides could be brought into the periplasm in the form of incomplete (disaccharide tripeptide) lipid II molecules lacking the d-alanyl-d-alanine dipeptide normally incorporated by the ligase MurF.
E. coli produces, in the cytosol, penicillin-resistant peptidases that hydrolyze the bond between meso-diaminopimelic acid (l-center) and d-alanine at positions 3 and 4 of the stem peptides. An l,d-endopeptidase (85) and an l,d-carboxypeptidase I (156) act on the nucleotides UDP-N-acetylmuramoyl-(d-alanyl-d-alanine-terminated) pentapeptide and UDP-N-acetylmuramoyl-(d-alanine-terminated) tetrapeptide, respectively. The l,d carboxypeptidase LdcA (221, 228) is made without signal peptide. Loss of LdcA causes cell lysis, and the effect is restricted to the onset of the stationary phase. LdcA does not belong to the SxxK peptidase family. The required stem tripeptides can also be produced, in the periplasm, by the action of the l,d-carboxypeptidase II (17). LdcII acts on stem tetrapeptides of nascent peptidoglycan at the time of cell division. It may be present in nondividing cells, but then its activity is masked. LdcII has not been characterized biochemically.
Eco2 is essential to multiplying rod-shaped E. coli cells. It may be also important in the stationary phase (23). In rounded E. coli cells, in which Eco2 is impaired, the incorporation of the peptidoglycan precursors is a zonal process (49). There is no mixing of new peptidoglycan and old peptidoglycan, and the wall polymer cannot undergo remodeling. In vitro assays that would help identify the reaction that the acyltransferase module of Eco2 performs in vivo have not been developed. Eco2 binds benzylpenicillin and ampicillin with high affinity, suggesting that the acyltransferase module is targeted to N-acyl-d-alanyl-d-alanine sequences. However, Eco2 is also very susceptible to the nonclassical penam amdinocillin, the carbapenem thienamycin, and the oxapenem clavulanic acid (213) (Fig. 8).
The linker modules of Eco2 and Eco3 have the same conserved motifs 1, 2, and 3. They probably adopt the same fold. As a corollary, peptide segments other than the conserved motifs must serve as recognition sites specifying the morphogenetic apparatus to which Eco2 and Eco3 attach. Cell division and viability in the absence of Eco2 but in the presence of Eco3 are restored by increasing the pool of ppGpp or the level of FtsQAZ (115, 238). Hence Eco2, but not Eco3, is dispensable in particular genetic backgrounds. It is likely that the morphogenetic apparatus is not composed of fixed structures and proteins can belong to several morphogenetic apparatuses simultaneously or at different times.
Free-Standing PBPs: Auxiliary Cell Cycle Proteins
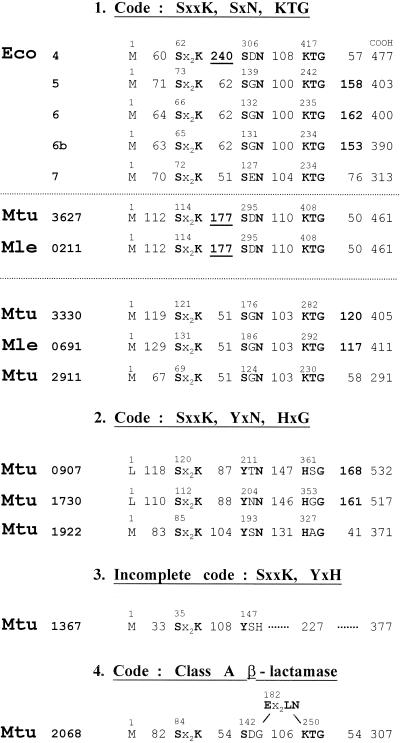
Free-standing PBPs are autonomous folding and catalysis entities. They utilize water as the acceptor nucleophile for the deacylation of the serine ester-linked N-acyl-d-alanyl enzyme intermediate. They are implicated, one way or another, in cell morphogenesis. E. coli produces multiple free-standing PBPs (Fig. 14). Eco5, Eco6, and Eco6b (13) have carboxy-terminal membrane-anchoring domains, and the bulks of the polypeptide chains are exposed in the periplasm. These d-alanyl-d-alanine-cleaving carboxypeptidases, together with other factors, control the balance between different peptidoglycan precursors and help determine whether E. coli cells will elongate or divide (19). The similarities between the pair Eco5-Eco6 (P, 10−141; identity, 60%) and the pair Eco5-Eco6b (P, 10−104; identity, 47%) are great, indicating that Eco5, Eco6, and Eco6b arose by gene duplication with a high level of sequence conservation. Eco5 and Eco6, however, are not functionally redundant. Loss of Eco5 but not of Eco6 suppresses the block in cell division caused by a single base change in the gene encoding FtsK, a protein that performs a septation function and a chromosome partition function (18). Overproduction of Eco5 (147) but not of Eco6 (230) results in growth of wild-type E. coli as spherical cells.
FIG. 14.
Occurrence of catalytic center-defining motifs along the sequences of free-standing SxxK polypeptides of E. coli, M. tuberculosis, and M. leprae. Polypeptides of group 1 bear the bar codes SxxK, SxN, and KTG. They are auxiliary cell cycle PBPs in E. coli. Inserts are underlined. The M. tuberculosis polypeptides of groups 2 and 3 have no equivalent in E. coli. Their bar codes are modified or incomplete. It is likely that they are not implicated in wall peptidoglycan metabolism. The M. tuberculosis β-lactamase of class A (group 4) has a class-specific Ex2LN motif. E. coli can produce two β-lactamases, one of which (plasmid coded) is of class A (Fig. 15).
The free-standing PBPs Eco4 (129) and Eco7 (198) hydrolyze d-alanyl-(d)-meso-diaminopimelic acid interpeptide linkages made by transpeptidation. Eco7 is peculiar. It is released from whole cells by osmotic shock. It hydrolyzes the peptidoglycan sacculus but is inert on isolated disaccharide peptide dimers. It is inactivated by low concentrations of penems with an l,d-configured scissile bond (Fig. 8), and its inactivation causes lysis of nonmultiplying cells. Eco7, Eco5, Eco6, and Eco6b belong to the S11 family in the database MEROPS (190). Eco4 belongs to the S13 family. One may note here that E. coli also produces a penicillin-resistant d-alanyl-(d)-meso-diaminopimelic acid-cleaving peptidase, MepA (120). MepA is secreted in the periplasm. Surprisingly, a fivefold superproduction does not modify the overall extent of peptidoglycan crosslinking. MepA does not belong to the SxxK peptidase family.
E. coli produces two additional chromosome-encoded free-standing SxxK acyltransferases, AmpC and AmpH (99). They are similar in sequence. AmpC is a β-lactamase (see Group II SxxK Acyltransferases below) or a PBP, depending on the structure of the β-lactam antibiotic with which it reacts (74). AmpH is a high-affinity PBP for benzylpenicillin and cephalosporin C (99).
The free-standing PBPs are nonessential auxiliary cell cycle proteins. A triple deletion of Eco4, Eco5, and Eco6 has no obvious effects apart from a slightly longer generation time and slightly altered cell morphology (59). The genes encoding Eco4, Eco5, Eco6, Eco6b, Eco7, AmpC, and AmpH have been deleted in every possible combination; all the deletions give rise to viable E. coli cells. Some of them, however, cause significant morphological aberrations (48, 164, 251).
E. coli PBP Fusions as Targets for β-Lactam Antibiotics
To kill bacteria, a β-lactam antibiotic must inactivate the acyltransferase module of one of several PBP fusions at therapeutically achievable concentrations in time periods shorter than the generation time of the bacterium. Currently, 50% and 90% inhibitory concentration (IC50 and IC90) values, i.e., the concentrations (in micrograms of antibiotic per milliliter) at which a β-lactam compound inhibits the PBPs by 50% or 90%, respectively, after a given time of incubation with isolated membranes, are used to estimate the inactivating efficiency of the drug. IC values are not intrinsic parameters of the interaction. With K (molarity) denoting the dissociation constant of the protein-β-lactam Michaelis complex and k+2 (per second) denoting the first-order rate constant of protein acylation, the second-order rate constant k+2/K expresses the inactivating efficiency of a β-lactam antibiotic if the value of the rate of breakdown of the acyl enzyme is small.
Under the experimental conditions defined in reference 82, k+2/K is equal to −lnX/([β-lactam] · t), where X is the percentage of the target protein left intact, [β-lactam] is the molar drug concentration, and t is the incubation time in seconds. Benzylpenicillin, at 1 μg ml−1 (i.e., 3.0 × 10−6 M) concentration, inactivates a target protein by 50% in 600 s if the k+2/K value is ≅400 M−1 s−1. At the same concentration and in the same time period, benzylpenicillin inactivates another target protein by 95% if the k+2/K value is 1,600 M−1s−1.
Table 2 gives the k+2/K values of the interactions between β-lactam antibiotics and the isolated free-standing PBP Eco4* (194), the His-tagged (Met46-Asn844) PBP fusion Eco1b* (222), the OmpA signal peptide-transported (Gly57-Val577) PBP fusion Eco3* (1), and the membrane-bound PBPs of E. coli, as calculated from published IC50 values (41). Binding of a β-lactam antibiotic to isolated membranes is a competition between multiple target proteins with various affinities for the drug. Traces of a β-lactamase can interfere with the assays. Hence, the k+2/K values of the isolated Eco4*, Eco1b*, and Eco3* are considerably larger than those derived from the corresponding IC50 values, yet conversion of the IC50 values into k+2/K values allows the β-lactams to be roughly classified in the order of their acylating potencies.
TABLE 2.
Second-order rate constant k+2/K values of acylation by β-lactam antibiotics of the membrane-bound PBPs of E. coli and M. tuberculosis RvH37Ra, the water-soluble Met46-Asn844 Eco1b* and Gly57-Val577 Eco3*, and purified Eco4*
| β-Lactam |
k+2/K (M−1 s−1)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
E. coli PBPs
|
M. tuberculosis PBPs
|
|||||||||||||
| 1a | 1b | 1b* | 2 | 3 | 3* | 4 | 4* | 5 | 6 | 94 kDa | 82 kDa | 52 kDa | 37 kDa | |
| Cephaloridine | 2,000 | 200 | 350 | 10 | 65 | 30 | <2 | <2 | 600 | 200 | 400 | <5 | ||
| Cefsulodin | 1,300 | 130 | <2 | <2 | <2 | <2 | <2 | |||||||
| Mecillinam | <2 | <2 | <2,000 | <2 | <2 | <2 | <2 | |||||||
| Mezlocillin | 400 | 80 | 700 | 27,000 | 290,000 | <30 | <30 | <30 | ||||||
| Cefoperazone | 1,500 | 500 | 1,800 | 830 | 15,000 | 80,000 | <15 | <15 | <15 | 30 | 20 | 200 | <5 | |
| Cefotaxime | 10,500 | 800 | 2,600 | 100 | <10,500 | 80,000 | 20 | <10 | <10 | |||||
| Cefuroxime | 4,000 | 300 | 700 | 40 | 6,000 | 66,000 | 3 | <2 | 3 | |||||
| Cephalothin | >1,800 | 30 | 10 | 440 | 7 | 4 | 4 | 80 | 30 | 300 | <5 | |||
| Ampicillin | 300 | 100 | 130 | 500 | 450 | 5,000 | 200 | 3 | 40 | 2,000 | 300 | 250 | <5 | |
| Benzylpenicillin | 800 | 100 | 600 | 480 | 430 | 4,000 | 385 | >100,000 | 16 | 20 | ≅600 | ≅600 | ≅600 | ≅30 |
| Carbenicillin | 200 | 100 | 120 | 100 | 200 | 1,500 | 120 | 3 | 3 | |||||
| Cefoxitin | 5,000 | 150 | 900 | <2 | 90 | 1,000 | 75 | 800 | 550 | 1,200 | 600 | 100 | 800 | |
| Imipenem | 6,000 | 2,000 | 400 | 250 | ||||||||||
| Clavulanate | 230 | 90 | 10 | 10 | ||||||||||
| Sulbactam | 330 | 150 | <3 | <3 | ||||||||||
E. coli is killed via cell spheroplasting and lysis as a result of the selective inactivation of both Eco1a and Eco1b by cephaloridine and cefsulodin; via transformation of the cells into round bodies as a result of the selective inactivation of Eco2 by amdinocillin; via cell filamentation as a result of the selective inactivation of Eco3 by mezlocillin, cefaperazone, cefotaxime, cefuroxime, cephalothin, and aztreonam (data not shown); or via different combinations of these morphological alterations by ampicillin, benzylpenicillin, carbenicillin, and cefoxitin.
The membrane-bound free-standing PBPs Eco4, Eco5, and Eco6 are, in general, less susceptible to the β-lactam antibiotics than the acyltransferase modules of the membrane-bound PBP fusions. However, Eco4 and the PBP fusions Eco1a, Eco1b, Eco2, and Eco3 have comparable susceptibilities to ampicillin, benzylpenicillin, and carbenicillin. Eco5, Eco6, and the PBP fusions Eco1a and Eco1b have comparable susceptibilities to cefoxitin.
From the foregoing, it follows that the essential class A and B PBP fusions present in a bacterial cell each has its own specificity profile for β-lactam antibiotics. Most often, they perform different functions and belong to different subclasses. As shown in the ensuing section, variants of a PBP fusion can arise. The variants belong to the same subclass and presumably perform the same function as the wild-type PBP fusion, but their susceptibilities to the drugs are changed.
Mosaic PBP Fusions
Streptococcus pneumoniae is of known genome sequence (105). It is a paradigm of genetic plasticity and adaptation to the environment via natural transformation (37). DNA sequences that are likely to be functional in a bacterium donor are introduced by homology-dependent recombination in a bacterium acceptor. Factors regulating genetic competence are involved in this process.
S. pneumoniae strains are normally very susceptible to benzylpenicillin (MIC, 0.02 μg of antibiotic ml−1). Determinants conferring reduced susceptibility to β-lactam antibiotics have evolved in commensal Streptococcus spp., presumably by the accumulation of point mutations in genes that code for PBP fusions of classes A and B. The shuffling and capture of DNA sequences from strains that have a low susceptibility to the drugs give rise to S. pneumoniae isolates that carry genes which code for mosaic PBP fusion variants of classes A and/or B having a decreased affinity for one or several β-lactam antibiotics (56, 89, 90, 257). In the laboratory, several independent mutations are necessary for a penicillin-susceptible PBP fusion to be converted into a mosaic PBP fusion.
In nature, Streptococcus oralis and Streptococcus mitis are likely to act as DNA donors (6). As the result of one transformation event with chromosomal DNA from an S. mitis strain having a low susceptibility to penicillin, S. pneumoniae produces an assortment of mosaic PBP fusions that substitute for the wild-type PBP fusions Spn1a, Spn2a, and Spn1b of subclasses A3, A4, and A5 and the PBP fusions Spn2x and Spn2b of subclasses B4 and B5 (91). Mosaic and wild-type PBP fusions with different susceptibilities to β-lactam antibiotics but of the same subclass differ in amino acid residues by up to 15%.
S. pneumoniae strains producing mosaic PBP fusions often manufacture a peptidoglycan which is enriched in interpeptide cross-bridges (64, 65). The inactivation of murMN in S. pneumoniae Pen6 (MIC, 6 μg of benzylpenicillin ml−1), with loss of the enzymes of the pathway to branched peptidoglycan precursors (see the section on lipid II precursors), restores susceptibility to the drug (MIC, 0.03 μg of benzylpenicillin ml−1). N. gonorrhoeae strains that have decreased susceptibility to penicillin have also emerged (212). The mosaic PBP fusions that they produce arise by amino acid substitution, insertion, and exchange of regions of the penicillin-suceptible acyltransferase modules with the homologous regions of resistant PBPs of closely related species.
GROUP II SxxK ACYLTRANSFERASES
The SxxK acyltransferases of group II are indirectly or not implicated in wall peptidoglycan synthesis and metabolism. Many of them help, in one way or another, the SxxK PBP fusions of group I escape penicillin action. Others have very diverse functions. The SxxK acyltransferases of group II occur as free-standing polypeptides and as modules of protein fusions. They illustrate exemplarily the concept of protein superfamily: polypeptide chains adopting the same fold can perform extremely various functions. Often, the SxN motif and/or the KTG motif is modified. The SxxK motif, however, is strictly conserved (Fig. 15).
FIG. 15.
Acyltransferases of the SxxK superfamily of diverse functions not related to wall peptidoglycan metabolism. The proteins are free-standing polypeptides except BlaR of B. licheniformis and DAP of O. anthropi, which are protein fusions. The SxxK acyltransferase module forms the carboxy-terminal domain of BlaR and the amino-terminal domain of DAP. The three-dimensional structures of the proteins marked with an asterisk are known. Motif 1, SxxK, is invariant. Motifs 2 and/or 3 are modified except in PBP R39. Amino acid changes and inserts are underlined. Motif 2 of BlaR is ambiguous.
Streptomyces sp. strain K15 produces a free-standing PBP which acts as a d,d-transpeptidase. SxC substitutes for the SxN motif (171). The mature enzyme is membrane associated but is secreted when overproduced. In aqueous media which contain 55.5 M H2O, proper amino compounds, at millimolar concentrations, compete successfully with water as acceptors of the transfer reaction. With the carbonyl donor-amino acceptor system bisacetyl-l-lysyl-d-alanyl-d-alanine/glycylglycine, the product of the reaction is almost exclusively bisacetyl-l-lysyl-d-alanyl-glycyl-glycine (140, 165). Streptomyces sp. strain R61 and Actinomadura sp. strain R39 secrete free-standing PBPs which act mainly as d,d-carboxypeptidases. In the R61 PBP, YxN substitutes for the SxN motif and HTG substitutes for the KTG motif (57). In the R39 PBP, motifs SxxK, SxN, and KTG are conserved, but a large insert occurs between the SxxK motif and the SxN motif (87).
The primary response of Streptomyces spp. and other soil bacteria to exposure to β-lactam antibiotics might have been to develop a protective mechanism through the secretion of free-standing PBPs with increasing affinities for the drugs (122). Thus, the k+2/K values of the interactions with benzylpenicillin are 135 M−1 s−1 for the K15 enzyme, 18,000 M−1 s−1 for the R61 enzyme, and 300,000 M−1 s−1 for the R39 enzyme.
The conversion of free-standing PBPs into SxxK β-lactamases gave rise to a defense mechanism of great efficiency (125, 126). The SxxK β-lactamases have a very low activity on N-acyl-d-alanyl-d-alanine peptides because the enzyme acylation step is severely rate limiting (195). They react with and rupture the β-lactam amide bond and produce serine ester-linked acyl (penicilloyl, cephalosporoyl,…) enzyme intermediates that are hydrolytically labile. On good β-lactam substrates, the catalytic centers of the serine β-lactamases can turn over 1,000 times or more per second. In β-lactamases of class A, the SxxK, SxN, and KTG motifs are conserved, but an additional motif, ExxLN, occurs in the catalytic center. In β-lactamases of classes C and D, YxN and SxV substitute for the SxN motif, respectively.
Penicillin sensory transducers are components of regulatory pathways leading to the inducible synthesis of penicillin resistance determinants (96, 254). BlaR proteins are implicated in β-lactamase synthesis in Bacillus licheniformis and Staphylococcus aureus. The MecR protein is implicated in synthesis of the penicillin-resistant SxxK protein fusion Sau2a in S. aureus (see next section). The tripartite BlaR and MecR proteins comprise a signal receptor which is at the carboxy end of the polypeptide chains and is exposed on the outer face of the plasma membrane; a four α-helix bundle signal transmitter which is embedded in the membrane; and a cytosolic signal emitter which possesses the HisGluLeuTyrHis consensus of a Zn2+-dependent peptidase.
The Met346-Arg601 penicillin receptor of the B. licheniformis BlaR is related to the Oxa2 β-lactamase by a P value of 6 × 10−36 (identity, 36%). As an independent entity, the BlaR receptor lacks detectable peptidase and β-lactamase activities and behaves as a high-affinity PBP (96), yet signal reception by the full-size BlaR leading to transcription of the β-lactamase-encoding gene does not involve penicilloylation of the serine residue of the SxxK motif of the receptor. In S. aureus, unblocking transcription of the Sau2a-encoding gene is the result of site-specific proteolytic cleavage of the zinc peptidase of the transmitter of MecR, which is activated, and of the repressor, which is inactivated (254).
There are SxxK acyltransferases which are not targeted to peptide bonds extending between two d-alanine residues in an α-position to a carboxylate. Bacillus cereus secretes a peptidase ADP which hydrolyzes the d-phenylalanyl-d-phenylalanyl bond in an endoposition (10). The peptidase has some β-lactamase activity. Its bar code is similar to that of the Streptomyces sp. strain R61 d,d-carboxypeptidase-PBP. The two proteins are related by a P value of 10−48 (identity, 36%). Ochrobactrum anthropi produces intracellularly a d-aminopeptidase, DAP, which acts on d-alanine amide and peptides with a d-alanine residue at the amino end (11, 26). The carbonyl side only of the scissile bond is borne by a d-configured carbon atom. β-Lactam compounds and the tripeptide bisacetyl-l-lysyl-d-alanyl-d-alanine behave as competitive inhibitors.
The catalytic module of the peptidase DAP and the free-standing d,d-carboxypeptidase-PBP of Streptomyces sp. strain R61 are distantly related by a P value of 3 × 10−18 (identity, 26%),yet the two polypeptides adopt the same fold (26). The noncatalytic carboxy-terminal module of the peptidase DAP comprises two eight-stranded β-barrels. A loop protruding from the last β-barrel interacts with the catalytic module. This feature is somewhat reminiscent of the activation of the Streptomyces sp. strain R61 d,d-carboxypeptidase-PBP by elimination of the 26-residue carboxy-terminal segment of the protein precursor, suggesting prosegment occlusion of the catalytic center (62).
Amino acid sequences change more rapidly on the evolutionary time scale than the three-dimensional structures. The Streptomyces sp. strain K15 d,d-transpeptidase-PBP (68), the Streptomyces sp. strain R61 d,d-carboxypeptidase-PBP (124), the TEM β-lactamase of class A (5, 113, 217), the P99 β-lactamase of class C (168), the Oxa-10 β-lactamase of class D (170), the acyltransferase modules of the d-aminopeptidase DAP (26), and the PBP fusion Spn2x of subclass B4 (173) all adopt the same overall fold, indicating a common ancestral origin. The Streptomyces sp. strain K15 d,d-transpeptidase-PBP and the β-lactamases of class A belong to the MEROPS S11 family (190). The Streptomyces sp. strain R61 d,d-carboxypeptidase-PBP, β-lactamases of class C, the B. cereus peptidase ADP, and the catalytic module of the O. anthropi d-aminopeptidase belong to the S12 family. The Actinomadura sp. strain R39 d,d-carboxypeptidase-PBP belongs to the S13 family.
GROUP III SxxK ACYLTRANSFERASES: PENICILLIN-RESISTANT PROTEIN FUSIONS
The enormous variations in the catalytic properties of the SxxK acyltransferases of groups I and II are the result of alterations of the polypeptide chains, sometimes with a great divergence in the amino acid sequences but always with conservation of the polypeptide fold and positioning of secondary structures even well away from the catalytic centers. In light of this versatility, acyltransferases might have gone as far as a change from d,d specificity to l,d specificity. SxxK l,d-acyltransferases catalyzing reactions II and III shown in Fig. 6 are expected to resist most β-lactam antibiotics. The roles that they may play in (3→3) peptidoglycan assembly are discussed in the ensuing sections.
Class B Penr Protein Fusions of Gram-Positive Bacteria
Enterococci and staphylococci produce, in addition to their normal sets of PBP fusions of classes A and B, one protein fusion which has the bar code of class B PBP fusions (Fig. 16) but has a very low affinity for the β-lactam antibiotics (67, 98). The class B penicillin-resistant (Penr) protein fusions which have been tested are inert on both d-alanyl-d-alanine-terminated peptides and thiolester analogues. The k+2/K values of their interactions with β-lactam antibiotics (from ≅1 M−1 s−1 to ≅10 M−1 s−1) are only about 20-fold greater than that observed with bovine serum albumin.
FIG. 16.
Class B Penr protein fusions. Bar code, motifs 1 to 7 and inserts (framed). For protein identifiers, see Table 1.
The linker and acyltransferase modules of the Penr protein fusions Efam5 of Enterococcus faecium (259), Ehi5 and Ehi3r (plasmid borne) of Enterococcus hirae (61, 177), Efas5 of Enterococcus faecalis (206), Sau2a or Sau2′ of Staphylococus aureus (210), and Ssc2 of Staphylococcus sciuri (247, 248) diverged in concert. They fall into a particular subclass, B1 (Fig. 10), to which the protein fusion Bsu3 of Bacillus subtilis (160) also belongs (Fig. 10). Orthologues of Efam5 are also present in Listeria monocytogenes, Listeria innocua, and Clostridium acetobutylicum. Their acyltransferase modules are related to that of Efam5 by P values ranging from 10−87 to 10−66. The linker modules of Penr protein fusions of subclass B1 invariably contain an insert 120 to 130 amino acid residues long upstream from motif 1 (Fig. 16). The inserts of Ehi5 (157) and Sau2a (246) are essential. They probably have their own fold.
Penr protein fusions of subclass B1 are penicillin resistance determinants in exponential-phase cultures of streptococci and staphylococci. They allow the strains that produce them to multiply under conditions under which the acyltransferase modules of class A and B PBP fusions are inactivated by penicillin. The level of resistance, however, is almost always below the level that one would expect on the basis of the very low affinity of the Penr protein fusion. In addition, this alternative mode of bacterial growth in the presence of penicillin is of decreased efficiency. When Efas5-producing E. faecalis cells are collected from penicillin-free medium and inoculated into penicillin-containing medium, 2 to 3 h elapse before the cells start to multiply again. They continue to multiply in the presence of penicillin, but the generation time is twofold greater than that of the control cells in the absence of penicillin (207).
The Penr protein fusions Efam5 of E. faecium and Sau2a of S. aureus have attracted much attention. The Efam5-encoding gene is probably intrinsic to the E. faecium species. All known isolates are Efam5 producers, including those strains which are still susceptible to therapeutically achievable concentrations of penicillin and in which Efam5 is produced at a low basal level. The Efam5-encoding gene belongs to an operon which contains a gene that codes for a protein of the SFR family, indicating that Efam5 is a cell cycle protein. The operon also contains a gene that codes for a Psr protein first identified as a possible repressor of transcription. Recent developments argue against the possibility that Psr plays such a role in E. faecium (196) and E. faecalis (58).
The evolutionary precursor of the Sau2a-encoding gene, mecA, of S. aureus could be a homologue present in S. sciuri, a species found in rodents and primitive mammals (247, 248). Stepwise exposure of a penicillin-susceptible S. sciuri strain in which mecA is silent (MIC, 4 μg of antibiotic ml−1) to increasing concentrations of methicillin gives rise to a methicillin-resistant mutant (MIC, 200 μg of antibiotic ml−1) which, as a result of a point mutation introduced in the −10 consensus of the mecA promoter, produces a protein which reacts with an anti-Sau2a monoclonal antibody. Transduction of S. sciuri mecA into a methicillin-susceptible S. aureus strain (MIC, 4 μg of antibiotic ml−1) results in increased resistance to methicillin, albeit at a lower level (MIC, 12 to 50 μg of antibiotic ml−1) than that of the donor strain. In methicillin-resistant S. aureus strains, mecA is carried by a mobile element called the staphylococcal cassette chromosome SCCmec (110). The mecA sequences appear to be highly conserved. Only two nucleotide positions vary in the genes of methicillin-resistant S. aureus strains and S. sciuri.
The Penr protein fusions Sau2a of S. aureus and Efam5 of E. faecium belong to the same subclass, B1, indicating similarity in function. Their biochemistry audit is far from complete. The mechanisms through which they contribute to the penicillin resistance of cultures in the exponential phase are undetermined. A definite answer will have to take the following observations into account.
E. faecium cells in the exponential phase manufacture a (3→3) peptidoglycan in various proportions of total peptidoglycan (Fig. 1 and 2). As proposed in references 145 and 205, the (3→3) l-lysyl-Nɛ-(d-isoasparaginyl)-l-lysine cross-bridges (Fig. 2) could be made through the sequential actions of a Penr N-acyl-d-alanyl-d-alanine carboxypeptidase catalyzing reaction IIIa and a Penr l,d-transpeptidase catalyzing reaction III (Fig. 6).
The presumed Penr d,d-carboxypeptidase could be a Zn2+-dependent peptidase of the EntVanY family (8, 9, 245). VanY is produced by vancomycin-resistant enterococci. VanY homologues occur in Clostridium and Bacillus spp. but not in E. coli or Mycobacterium tuberculosis. Streptomyces albus G secretes a Zn2+-dependent N-acyl-d-alanyl-d-alanine carboxypeptidase functionally equivalent to EntVanY. The Streptomyces enzyme is of known structure (52, 83). His154, Asp161, and His197 are the zinc ligands. Amino acid sequence alignments reveal an equivalent zinc site in EntVanY (137, 151).
The presumed Penr l,d-transpeptidase could be the peptidase responsible for the d-alanine exchange reactions (of the type bisacetyl-l-lysyl-d-alanine + d- [14C]alanine ⇋ bisacetyl-l-lysyl-[14C]-d-alanine + d-alanine) which were identified several decades ago in E. hirae (called Streptococcus faecalis ATCC 9790 in 1974) (40), Gaffkya homari (93-95), Bacillus megaterium (44), and, recently, E. faecium (145). The membrane-associated l,d-peptidase is present at a very low level in wild-type E. hirae cells (40). It assembles properly structured carbonyl donor and amino acceptor peptides into (3→3) Nɛ-l-lysyl-Nɛ-(d-isoasparaginyl)-l-lysyl-linked dimers. It resists benzylpenicillin and is moderately susceptible to cephaloglycine. Almost certainly, it performs covalent catalysis via the formation of an acyl enzyme intermediate. Whether it belongs to the SxxK acyltransferase superfamily or not remains to be established.
The enterococcal Penr d,d-carboxypeptidase and Penr l,d-transpeptidase, working cooperatively with a glycosyltransferase, could be the basic molecular machine required for the formation of polymeric (3→3) peptidoglycan from lipid II precursor molecules. The implication of the subclass B1 Penr protein fusion Efam5 in this multiprotein complex would depend on the genetic backgrounds of the cells. The ampicillin-resistant mutant D344M512 (MIC, 2,000 μg of antibiotic ml−1) and its parental ampicillin-susceptible mutant D344S (MIC, 0.03 μg of antibiotic ml−1) both lack the Efam5-encoding gene (145, 205). In these particular genetic backgrounds, the amounts of (3→3) peptidoglycan made in the absence of Efam5 represent 100% (in mutant D344 M512) and 0.7% (in mutant D344S) of total peptidoglycan. In the genetic background which prevails in wild-type cells, resistance to penicillin is Efam5 concentration dependent. Efam5 might be an essential cell cycle protein that regulates the activities of the (4→3) and (3→3) peptidoglycan assembly molecular machines.
The ability of S. aureus to manufacture a (3→3) peptidoglycan has not been established. It is a possibility worthy of consideration. The amounts of (4→3) peptidoglycan made by Sau2a-producing strains grown in the presence of methicillin are drastically reduced compared to the amount made in the absence of the drug (46), suggesting that a spare Sau2a-dependent peptidoglycan which is not of the (4→3) type substitutes for the missing (4→3) peptidoglycan. Loss of FemX, which adds the first glycine residue to the ɛ-amino group of the l-lysine residue of peptidoglycan precursors, is lethal. In contrast, loss of FemA (197) restores the susceptibility of Sau2a-producing strains to penicillin, suggesting that peptidoglycan precursors having a single glycine substituent at position 3 of the stem peptides lack acceptor activity for the transfer reaction, which is presumably carried out by the acyltransferase module of Sau2a.
Susceptibility to penicillin is also restored by inactivating the gene that codes for the class A PBP fusion Sau2 (176), suggesting that the glycosyltransferase module of the PBP fusion Sau2 and the acyltransferase module of the Penr protein fusion Sau2a function cooperatively in assembly of the putative spare peptidoglycan from lipid II precursor molecules. The implication of a free-standing glycosyltransferase is possible. Uncoupled glycosyltransferases occur in S. aureus and other bacterial species (174, 215).
Class A Penr Protein Fusions of Gram-Negative Bacteria
E. coli produces, in addition to its normal set of PBP fusions of classes A and B, a protein fusion, Eco1c, which has the bar code of a class A PBP fusion (Fig. 17) but escapes acylation by most of the β-lactam antibiotics tested. Eco1c, however, can be detected as a PBP with moxalactam and the iodinated Bolton-Hunter derivative of ampicillin (202, 204). Eco1c has glycosyltransferase activity, and this activity is blocked by moenomycin, indicating that Eco1c can form uncrosslinked peptidoglycan chains from lipid II precursor molecules (202).
FIG. 17.
Class A Penr protein fusions. Bar code, motifs 1 to 9 and extensions (underlined). For protein identifiers, see Table 1. mTgase, free-standing glycosyltransferase of E. coli.
The glycosyltransferase module of Eco1c falls within the continuum of known glycosyltransferase sequences (Fig. 10). It is related to the glycosyltransferase modules of PBP fusions Eco1a and Eco1b by P values of 10−26 (identity, 40%) and 7 × 10−18 (identity, 31%), respectively. Motif 6b at the amino-terminal region of the acyltransferase of Eco1c is not complete. The acyltransferase module falls outside subclasses A1, A2, A3, A4, and A5 (Fig. 10), indicating a distinct function. Eco1c is inert on both d-alanyl-d-alanine-terminated peptide and thiolester analogues. Orthologues of Eco1c, assumed to resist penicillin, occur in many gram-negative bacterial species (Table 3). A common marker is that the usual Arg residue at the amino terminus of motif 3 of the glycosyltransferase module is replaced by another amino acid residue, most often Gly. The acyltransferase modules are related to that of Eco1c by P values ranging from 10−∞ to 10−30 (Table 4). They are related to those of the PBP fusions Eco1a and Eco1b by P values larger than 10−20.
TABLE 3.
Protein orthologues of the SxxK Penr protein fusion Eco1c of E. coli motif 3: GKxxQ in Eco1c and RKxxE in PBP fusions Eco1a and Eco1b
| Bacterial speciesa (reference) | Protein | Config | Motif 3b |
|---|---|---|---|
| Actinobacillus actinomycetemcomitans | Aac1c | 118 | GKxxQ |
| Agrobacterium tumefaciens C58 | Atu1cc | AKxxE | |
| Anabaena 7120 | Ana1c | GKxxE | |
| Bordetella bronchiseptica | Bbr1c | 1155 | AKxxQ |
| Bordetella parapertussis | Bpa1c | 1020 | AKxxQ |
| Bordetella pertussis | Bpe1c | 790 | AKxxQ |
| Burkholderia mallei | Bma1c | 5014 | QKxxQ |
| Burkholderia pseudomallei | Bps1c | 487 | QKxxQ |
| Caulobacter crescentus* (166) | Ccr1c | AKxxQ | |
| Klebsiella pneumoniae | Kpn1c | 1192 | QRxxA |
| Mesorhizobium loti* (117) | Mlo1c | SKxxQ | |
| Methylococcus capsulatus | Mca1c | bmc26 | QKxxQ |
| Nostoc punctiforme | Npu1c | 615 | GKxxE |
| Pasteurella multocida Pm70* (149) | Pmu1c | GKxxE | |
| Pseudomonas syringae PV tomato | Psy1c | E7 | GKxxQ |
| Pseudomonas putida KT 2440 | Ppu1c | 13,538 | GKxxQ |
| Rickettsia montanensis (7) | Rmo1c | GKxxQ | |
| Rickettsia typhi (7) | Rty1c | GKxxQ | |
| Salmonella enterica serovar Typhi CT 18 | Sty1cd | GKxxQ | |
| Salmonella enterica serovar Typhimurium LT2 | Stym1c | 2 | GKxxQ |
| Shewanella putrefaciens 5/9/101 | Spu1c | 93 | GKxxQ |
| Xylella fastidiosa 9a5c* (208) | Xfa1c | GKxxQ | |
| Xylella fastidiosa Dixon | Xfad1c | 286 | GKxxQ |
| Xylella fastidiosa Ann-1 | Xfaa1c | 81 | GKxxQ |
*, genome sequence known.
Motif 3 is GKxxQ in Eco1c and RKxxE in Eco1a and Eco1b.
Accession no. gb|AE007870.
Accession no. emb|AL513382.
TABLE 4.
Similarity indexes (P and I) between the SxxK acyltransferase modules of Eco1c (494 amino acid residues) and Eco1b (397 amino acid residues), used as references, and homologous proteins of gram-negative bacteria
| Bacterial species | Protein | Vs Eco1c
|
Vs Eco1b
|
||||
|---|---|---|---|---|---|---|---|
| P (10−n) | I (%) | Overlap (no. of aa) | P (10−n) | I (%) | Overlap (no. of aa) | ||
| Eco1c orthologues | |||||||
| E. coli K-12 | Eco1c | ∞ | 100 | 1-494 | 7 | 24 | 26-283 |
| S. enterica serovar Typhi CT18 | Sty1c | ∞ | 80 | 1-491 | 8 | 26 | 17-283 |
| P. putida KT2440 | Ppu1c | 153 | 55 | 1-491 | 7 | 24 | 17-283 |
| X. fastidiosa | Xfa1c | 107 | 43 | 1-491 | 9 | 28 | 26-295 |
| M. loti | Mlo1c | 60 | 42 | 4-301 | 11 | 26 | 23-294 |
| P. multocida | Pmu1c | 40 | 26 | 5-485 | 12 | 25 | 26-282 |
| Anabaena sp. strain 7120 | Ana1c | 36 | 33 | 4-305 | 21 | 27 | 4-329 |
| R. typhi | Rty1c | 32 | 31 | 26-280 | 9 | 22 | 14-277 |
| Eco1b orthologues | |||||||
| X. fastidiosa | Xfa1b | 13 | 28 | 1-284 | 49 | 36 | 3-310 |
| P. putida KT2440 | Ppu1b | 13 | 26 | 26-339 | 62 | 38 | 3-343 |
| H. influenzae | Hin1b | 9 | 22 | 21-335 | 80 | 44 | 3-342 |
| P. multocida | Pmu1b | 12 | 23 | 21-332 | 84 | 49 | 3-339 |
| S. enterica serovar Typhi CT18 | Sty1b | 7 | 25 | 26-267 | ∞ | 83 | 2-397 |
| E. coli K-12 | Eco1b | 7 | 24 | 1-267 | ∞ | 100 | 1-397 |
| E. coli K-12 | Eco1a | 9 | 28 | 3-183 | 15 | 27 | 19-210 |
In E. coli, the amount of Eco1c produced 34 h after onset of the stationary phase is low compared to the level produced at mid-exponential phase. It returns to normal after reinitiation of growth (47). The PBP fusions Eco1a, Eco2, and Eco3 behave like Eco1c. Eco1b, however, undergoes less important variations. The production of PBP fusions and free-standing PBPs is frequently growth phase dependent. The gram-negative organism Pseudomonas aeruginosa produces two PBP fusions, Pae3 and Pae3a, that belong to the same subclass, B3 (141). Pae3 and Pae3a are closely related to Eco3 by P values of 10−131 (identity, 45%) and 10−111 (identity, 41%), respectively. Pae3 (encoding gene in the dcw cluster) is produced maximally during the exponential phase of growth and decreases drastically when cells enter the stationary phase. Pae3a (encoding gene 2 Mbp away from the dcw cluster) is produced mainly, if not exclusively, during the stationary phase of growth. Loss of Pae3a is tolerated (141).
In the gram-positive sporeforming organism Bacillus subtilis, the genes encoding the PBP fusions Bsu2b of subclass B4 (249) and BsuVD (encoded by spoVD) of subclass B3 (42) occur in tandem in the dcw cluster (43). The essential Bsu2b is produced during both vegetative growth and sporulation. BsuVD is a mother cell PBP required for spore morphogenesis. These observations suggest that the Penr protein fusion Eco1c of class A is important for E. coli cells that are actively multiplying. Contrary to expectation, Eco1c is dispensable in the exponential phase of growth. Loss of Eco1c (encoding gene in the 57-min region) is harmless, and Eco1c cannot rescue an Eco1ats Eco1b double mutant from death at the restrictive temperature (202).
The above studies refer to a time slice of what is a more global process. They do not include long-term survival and growth under stress conditions. As the generation time of E. coli cells increases from 0.8 h to 6 h, conditions under which the amounts of Eco1a and Eco1b decrease drastically, the proportion of (4→3) peptidoglycan interpeptide linkages remains roughly unchanged (37.4% of total stem peptides versus 41.7%), the proportion of (3→3) peptidoglycan interpeptide linkages increases considerably (15.2% versus 3.1%), and uncrosslinked stem tripeptides represent about 50% of the total peptidoglycan (227). In stationary-phase cells, the peptidoglycan is hypercrosslinked and richer in bound lipoprotein molecules, and these molecules are attached almost exclusively to (3→3) peptide dimers (178).
The peptidoglycan-lipoprotein assemblage is hampered by nocardicin A, cephalosporin C, and cefminox (Fig. 18), the side chains of which terminate in a d-configured NH2-CHR-COOH grouping. These antibiotics cause rapid lysis of E. coli cells even at concentrations below the MICs (226). They are bactericidal for cells at the onset of the stationary phase of growth. They undergo covalent attachment to the peptidoglycan through a transfer reaction in which the d-amino group of the side chain serves as the acceptor. At micromolar concentrations, they prevent the lipoprotein molecules from being linked to the peptidoglycan, destabilizing the bacterial cell envelope.
FIG. 18.
β-Lactam antibiotics with side chains terminating in a d-configured NH2-C*HR-COOH grouping.
The formation of the (3→3) peptidoglycan interpeptide linkages and the covalent attachment of the lipoprotein and β-lactam antibiotic molecules to the peptidoglycan both require the rupture of an l,d-peptide bond in the carbonyl donor. The carbonyl group at the l-center of meso-diaminopimelic acid is then transferred to an amino group borne by a carbon atom which is d-configured or, in the case of the lipoprotein attachment, is located at the end of the lateral chain of a l-lysine residue. The ability of Eco1c to perform acyltransferase activity has not been established, but by combining a glycosyltransferase module and an l,d-acyltransferase module catalyzing reaction II or reaction III (Fig. 6) in a single polypeptide chain, Eco1c could catalyze the three reactions on a competitive basis.
Assuming that the acyltransferase module of Eco1c functions as an N-acyl-l-diaminoacyl-d-dipeptidyl transpeptidase (reaction II in Fig. 6), d-alanyl-d-alanine is released into the periplasm. As discussed in references 136 and 138, the combined actions of a dipeptide transport system, a penicillin-resistant VanX-like Zn2+-dependent d-alanyl-d-alanine dipeptidase, and pyruvate oxidase could enable the import of the released d-alanyl-d-alanine into the cytosol, its hydrolysis into d-alanine, and the oxidation of d-alanine to acetate and CO2, thus supplying energy. Alternatively, d-alanyl-d-alanine could be reutilized by the ligase MurF for lipid II recycling. The possibility also exists that the acyltransferase module of Eco1c functions as an N-acyl-l-diaminoacyl-d-alanine transpeptidase catalyzing reaction III (Fig. 6), after prior action of a penicillin-resistant d,d-carboxypeptidase catalyzing reaction IIIa. As discussed above in the section on class B PBP fusions, E. coli produces several peptidases which perform hydrolytic versions of reaction II and reaction III.
Gene deletion or disruption highlights the importance, if not the essentiality, of the Penr protein class A fusions in gram-negative bacteria. E. coli cells lacking Eco1a, Eco4, Eco5, Eco6, Eco6b, Eco7, EcoAmpC, and EcoAmpH and in which Eco2 and Eco3 are inactivated by amdinocillin and aztreonam, respectively, survive as large spherical bodies (48, 251). In particular genetic backgrounds, the combined activities of the PBP fusion Eco1b and the Penr protein fusion Eco1c may be sufficient to provide E. coli cells with a wall peptidoglycan that is no longer rod-shaped but is of sufficient tensile strength.
The cyanobacterium Anabaena sp. strain PCC7120 performs a higher-plant type of oxygenic photosynthesis. Under aerobic conditions in the presence of nitrate, it grows as filaments comprised of cells which all have the same morphology. In the absence of nitrate, some vegetative cells differentiate into nitrogen-fixing heterocysts. Anabaena sp. strain PCC7120 produces a panoply of PBP fusions (134). It also produces a protein fusion of class A, Ana1c, called PBP2 in reference 134, which is related to Eco1c by a P value of 10−69 (Tables 3 and 4; Fig. 17).
Disruption of the Ana1c-encoding gene causes no phenotypic differences under aerobic growth conditions in the presence of nitrate. In the absence of nitrate in a plate under nitrogen, the mutant still grows, but the filaments are shorter, they comprise vegetative cells of unequal size, and the heterocyst-like cells are distorted and have a thin cell envelope. In liquid medium without nitrate under aerobic conditions, the mutant dies, indicating that Ana1c plays an essential role in aerobic nitrogen fixation. Ana1c could be involved in the assembly of a particular peptidoglycan that would provide the heterocysts with a cell envelope capable of protecting the nitrogenase against oxygen. HcwA, a peptidoglycan hydrolase probably endowed with N-acetylmuramoyl-l-alanine amidase activity, is also required for heterocyst differentiation (256). The structure of the heterocyst peptidoglycan, however, is not known.
SxxK ACYLTRANSFERASES IN SPECIFIC ORGANISMS
Like E. coli, the mycobacteria manufacture peptidoglycans of the (4→3) and (3→3) types (Fig. 1 and 2). The SxxK acyltransferases of Mycobacterium tuberculosis, Mycobacterium avium, Mycobacterium bovis, and Mycobacterium leprae have been investigated in light of the accumulated knowledge about SxxK acyltransferases of groups I, II, and III. The acyltransferases of M. avium and M. bovis are close orthologues of the M. tuberculosis acyltransferases, and they are not discussed.
The study has been extended to Corynebacterium diphtheriae. Corynebacteria and mycobacteria belong to a suprageneric taxon of mycolic acid-containing actinomycetes. The mycolic acids of corynebacteria are relatively simple mixtures of saturated and unsaturated acids compared to the very complicated mixtures characteristic of mycobacteria (92, 116). C. diphtheriae manufactures a meso-diaminopimelic-containing (4→3) peptidoglycan similar to that of M. tuberculosis. Whether it also manufactures a (3→3) peptidoglycan has not been investigated.
The study has also been extended to Streptomyces coelicolor. Streptomyces spp. manufacture branched glycyl-l,l-diaminopimelic acid peptidoglycans of the (4→3) and (3→3) types (Fig. 1 and 2). They secrete a collection of free-standing PBPs and wall-dissolving enzymes (77). In contrast to mycobacteria, which sometimes branch or form filaments that readily fragment into rods and coccoid bodies, Streptomyces spp. undergo very complex developmental processes (104). They produce branching networks of hyphae both on the surface of a solid substratum and into it to form a mycelium. Septa divide the hyphae into long cells containing several nucleoids. Upon growth completion, aerial filaments divide into unigenomic cells that further metamorphose into spores. S. coelicolor carries a large 8-Mb linear chromosome (192).
The M. tuberculosis (Mtu) and M. leprae (Mle) proteins are denoted according to the numbering of the encoding genes, Rv1 to Rv3923 and Ml1 to Ml2720, respectively. The C. diphtheriae (Cdi) proteins are denoted according to the contig numbering. The S. coelicolor (Sco) proteins are denoted by the cosmid name and the CDS numbering. The assignment of the SxxK acyltransferases to different classes and subclasses rests on the bar codes borne by the proteins, the similarity indexes P and I, the known biochemical properties of several Mycobacterium and Streptomyces acyltransferases, the PBP pattern of M. tuberculosis H37Ra (35), and the environment of the encoding genes. The results are summarized in Table 5.
TABLE 5.
SxxK acyltransferases of M. tuberculosis, M. leprae, C. diphtheriae, and S.coelicolora
| Type | Class | PBP and Penr protein fusions
|
Similarity index vs M. tuberculosis
|
Gene environ- ment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
M. tuberculosis
|
M. leprae | C. diph- theriae | S. coelicolor |
P (10−n)
|
I (%)
|
|||||||||
| Protein | Mr | kDa | M. leprae | C. diphtheriae | S. coelicolor | M. leprae | C. diphtheriae | S. coelicolor | ||||||
| PBP | A | 0050 (Mtu1s) | 71,151 | 94-82 | 2688 (Mle1s) | 378 | H24.23 | ∞ | 173 | 74 | 83 | 51 | 37 | |
| E6.34 | 71 | 35 | ||||||||||||
| K7.12 | 41 | 30 | ||||||||||||
| B3 | 2163 (Mtu3) | 72,506 | 94-82 | 0908 (Mle3) | 101 | 4A10.23 (Sco3) | ∞ | 163 | 82 | 80 | 51 | 33 | dcw | |
| B2 | C88.19 (Sco2) | mre, sfr | ||||||||||||
| B-like I | 0016 | 51,776 | 52 | 0018 | 16 | H69.17 | ∞ | 107 | 80 | 85 | 45 | 36 | sfr | |
| 6G9.32 | 79 | 39 | sfr | |||||||||||
| 2st10A7 | 78 | 35 | ||||||||||||
| Penr | A | 3682 (Mtu1r) | 84,636 | 2308 (Mle1r) | 338 | H17.14 | ∞ | ∞ | 51 | 86 | 48 | 36 | ||
| B-like II | 2864 | 63,015 | 1577 | 230 | ∞ | 118 | 80 | 41 | ||||||
| B-like III | H63.18 | 38* | 27* | |||||||||||
| E87.08 | 27* | 34* | ||||||||||||
| E87.07 | 33* | 33* | ||||||||||||
Similarity indexes P and I apply to full-size protein fusions except for S. coelicolor protein fusions H63.18, E87.08, and E87.07 (*), where similarity with protein fusion Mtu2864 is restricted to the acyltransferase modules.
Class A PBP Fusions of M. tuberculosis, M. leprae, M. smegmatis, and C. diphtheriae
M. tuberculosis, M. leprae, and C. diphtheriae all have the information for a single PBP fusion of class A: Mtu0050 (Mtu1s), Mle2688 (Mle1s), and Cdi378, respectively (Table 5; Fig. 11). Mtu1s (21) and Mle1s (135) have been produced in E. coli with the correct membrane topology. The k+2/K values of the interactions with benzylpenicillin, ampicillin, amoxicillin, cefotaxime, cefuroxime, and ceftriaxone range from ≅100,000 M−1 s−1 to ≅10,000 M−1 s−1. Ticarcillin, piperacilline, methicillin, and moxalactam are not acylating agents. Mtu1s has been obtained in a soluble (ΔG95-Q143) truncated form that adopts an active folded state in terms of penicillin binding. Mle1s is relatively thermolabile, with half-lives of 60 min at 25°C and less than 5 min at 37°C. It catalyzes hydrolysis and aminolysis of thiolester analogues of N-acyl-d-alanyl-d-alanine-terminated peptides.
Mycobacterium smegmatis mc2155 is a fast-growing mycobacterium. Cultures in Luria-Bertani medium under aeration enter the stationary phase after 40 h. As a result of screening for transpositional mutants with permeability defects, an M. smegmatis strain in which a gene was disrupted at a locus coding for the sequence immediately downstream from motif 5, RWxxVL, of a glycosyltransferase module was obtained (22). This glycosyltransferase module is linked to an acyltransferase module which is a close orthologue of the acyltransferase module of Mtu1s (P, 10−152; identity, 79%), indicating that the disrupted gene (accession number AF165523) codes for a PBP fusion, Msm1s, of class A (Fig. 11). For comparison purposes, the acyltransferase module of Msm1s and that of Mtu1r (see the ensuing section) are distantly related by a P value of 9 × 10−13 (identity, 26%).
Compared to the parental strain, the Msm1s-less mutant has a twofold-higher permeability for glycine; it grows more slowly in the exponential phase; the protein content of the culture at the onset of the stationary phase is reduced by 90%; and the mutant has an increased susceptibility to β-lactam antibiotics (MICs, 15 μg of ampicillin ml−1 versus 300 μg of ampicillin ml−1 for the parental strain in the absence of a β-lactamase inactivator).
Chromosomal duplication in M. smegmatis (its genome is nearly 7 Mbp long, i.e., about 50% larger than that of its slow-growing relatives) results in duplicate copies of many genes and is a potent source of genomic dynamics (73). The analysis of the benzylpenicillin-labeled membranes of the Msm1s-less mutant failed to reveal the presence of any residual Msm1s PBP fusion. Considering that the locus at which the Msm1s-encoding gene was disrupted, the amino-terminal region of the disrupted protein is an intact glycosyltransferase module. This module could work cooperatively with the acyltransferase module of a PBP fusion of class B, which itself could be made more readily accessible to the drug because of defects in the cell envelope, explaining why the mutant is viable and is more susceptible to penicillin than the parental strain.
Class A Penr Protein Fusions of M. tuberculosis, M. leprae, M. smegmatis, and C. diphtheriae
M. tuberculosis, M. leprae, and C. diphtheriae all have the information for a single Penr fusion of class A: Mtu3682 (Mtu1r), Mle2308 (Mle1r), and Cdi338, respectively (Table 5 and Fig. 17). The acyltransferase modules of the pair Mtu1r-Mle1r and the pair Mtu1s-Mle1s are distantly related by a P value of ≅10−17 (identity, 25%). Mle1r has been produced in E. coli (16). The k+2/K values of the interactions are 5 to 10 M−1 s−1 with ceftriaxone and benzylpenicillin and 1 M−1 s−1 and even smaller with ampicillin, amoxicillin, cefoxitin, ticarcillin, temocillin, and cephaloridine. Mle1r lacks activity on thiolesters, which are carbonyl donors of Mle1s. It has a high thermostability, with no loss of its low affinity for penicillin after 10 min at 60°C. The sequence Leu174 to Arg314 contains a membrane association site (144). The acyltransferase module can be produced intracellularly in E. coli as inclusion bodies which can be refolded in a form that has the same low affinity for β-lactams as the full-size protein fusion (144). Mtu1r has not been characterized biochemically. In all likelihood, it has the same low affinity for β-lactam antibiotics as its orthologue Mle1r (P, 10−∞; identity, 86%).
M. smegmatis mc2155 survives for very long periods of time, 150 days or more, during either carbon, nitrogen, or phosphorus starvation (209). The viability of cultures in rich Lab-Lemco medium under aeration remains unchanged, about 109 CFU ml−1, for 40 days. It then declines to about 5 × 107 CFU ml−1 at day 120 (121). Like M. tuberculosis and M. leprae, M. smegmatis has the information for a Penr protein fusion, Msm1r, of class A (Fig. 17), which is a close orthologue of Mtu1r (P, 10−∞; identity, 80%). As a result of screening for transpositional mutants with long-term survival defects, an M. smegmatis mutant in which the Msm1r-encoding gene (now identified in contig 2889) is disrupted at a locus coding for motif 1 of the glycosyltransferase module was obtained (121). Compared to the parental strain, cultures of the Msm1r-less mutant had a much impaired viability, 106 CFU ml−1, i.e., 0.1% of the parental value, at day 40. Subsequently, however, viability returned and reached the level of the parental strain at day 120. Other mutants also impaired in long-term survival but in which the predicted functions of the disrupted genes are in biotin and polyketide biosynthesis behave the same way (121).
M. smegmatis manufactures peptidoglycans of the (4→3) and (3→3) types (see the Introduction). Loss of the PBP fusion Msm1s of class A is deleterious to M. smegmatis cells in the exponential phase, indicating that Msm1s is an important component of the molecular machine implicated in (4→3) peptidoglycan synthesis from lipid II precursor molecules. Loss of the Penr protein fusion Msm1r of class A is very damaging for cells in long-term stationary phase. A (3→3) peptidoglycan especially adapted to survival under starvation conditions might be synthesized from lipid II by Msm1r.
Subclass B3 and B-Like I PBP Fusions of M. tuberculosis, M. leprae, and C. diphtheriae
M. tuberculosis, M. leprae, and C. diphtheriae all have the information for a protein fusion of class B: Mtu2163 (Mtu3), Mle0908 (Mle3), and Cdi101, respectively (Table 5; Fig. 12). The linker and acyltransferase modules of Mtu3 diverged in concert and fall into the same subclass, B3 (Fig. 10). The acyltransferase module of Mtu3 is more closely related to the acyltransferase modules of Eco3 of E. coli and Pae3 of P. aeruginosa of subclass B3 (P, 10−32 and 3 × 10−48, respectively; identity, 30% and 34%, respectively) than to the acyltransferase module of Eco2 of subclass B2 (P, 9 × 10−13; identity, 25%). In M. tuberculosis, Rv2163 codes for Mtu3; Rv2150c and Rv2154c code for protein homologues of the E. coli cell septation proteins FtsZ and FtsW, respectively; Rv2151c codes for a protein of the FtsQ-like family; and Rv2152c, Rv2153c, Rv2155c, Rv2156c, Rv2157c, and Rv2158c code for protein homologues of the E. coli MurC, MurG, MurD, MurX (i.e., MraY), MurF, and MurE, respectively. Altogether, they form the M. tuberculosis dcw cluster (Fig. 5). On the basis of these predictive studies (and in the absence of direct biochemical data), Mtu2163 (Mtu3), Mle0908 (Mle3), and Cdi101 are provisionally identified as PBP fusions of subclass B3.
M. tuberculosis, M. leprae, and C. diphtheriae all have the information for another protein fusion of class B: Mtu0016, Mle0018, and Cdi16, respectively (Table 5; Fig. 12). The acyltransferase modules of Mtu0016 and Mtu3 of subclass B3 are distantly related by a P value of 10−13 (identity, 25%), indicating that they belong to different subclasses of class B. In contrast to the linker module of Mtu3, which is 147 amino acid residues long, the linker module of Mtu0016 is 110 amino acid residues long, it lacks motif 1 but contains motifs 2 and 3. Mtu0016 and the 484-amino-acid PBP fusion SgrpbpA of Streptomyces griseus (114) are related by a P value of 10−72 (identity, 36%) over the entire sequences. Both the Mtu0016- and SgrpbpA-encoding genes are located in the neighborhood of a gene that codes for a protein of the SFR family. Disruption of the SgrpbpA-encoding gene has no obvious effects on the growth and sporulation of S. griseus. Streptomyces spp. have a plethora of PBPs. SgrpbpA and other PBP fusions may substitute for each other. On the basis of these predictive studies, Mtu0016, Mle0018, and Cdi16 are provisionally identified as PBP fusions of a particular subclass, B-like I.
Class B-Like II Penr Protein Fusions of M. tuberculosis, M. leprae, and C. diphtheriae
M. tuberculosis, M. leprae, and C. diphtheriae all have the information for a protein fusion: Mtu2864, Mle1577, and Cdi230, respectively (Table 5, Fig. 16). They each have a not-well-conserved motif 1, lack motifs 2 and 3, and contain a 127-amino-acid insert located between the membrane anchor and the presumed motif 1, a structural feature which is reminiscent of the Penr protein fusions of subclass B1. M. tuberculosis does not produce any detectable PBPs migrating at a middle distance between the 82-kDa and 52-kDa PBPs on sodium dodecyl sulfate gels (35).
It is likely that Mtu2864 (theoretical Mr 63,015) does not bind penicillin. Consistently, its acyltransferase module is distantly related to the acyltransferase module of the PBP fusion Mtu3 by a P value of 10−15 (identity, 32%). It is related to the acyltransferase module of the Penr protein fusion SclpcbR of Streptomyces clavuligerus (encoding gene downstream from the isopenicillin synthase-encoding gene of the cephamycin cluster) (172) by a P value of 5 × 10−38 (identity, 35%). SclpcbR is essential in stationary-phase cultures, conditions under which cephamycin is produced. On the basis of these predictive studies, Mtu2864, Mle1577, and Cdi230 are provisionally identified as Penr protein class B-like II fusions.
Free-Standing PBPs, β-Lactamases, and Related Polypeptides of M. tuberculosis and M. leprae
The polypeptides fall into four groups (Fig. 14). M. tuberculosis has the information for three free-standing polypeptides, Mtu3627, Mtu3330, and Mtu2911, and M. leprae has the information for two free-standing polypeptides, Mle0211 and Mle0691, which bear the bar codes SxxK, SxN, and KTG (Fig. 14). They are probably auxiliary cell cycle proteins implicated in (4→3) peptidoglycan synthesis. Mtu3330 and Mle0691 are orthologues belonging to the MEROPS S11 family. Mtu3627 and Mle0211 are also orthologues, belonging to the MEROPS S13 family. Like Eco4, they contain a large insert between the SxxK motif and the SxN motif. An M. smegmatis PBP has been isolated (15). Its apparent molecular mass, 49.5 kDa, is similar to those of Eco4, Mtu3627, and Mle0211. It has a great affinity for benzylpenicillin, ampicillin, and cefoxitine. It catalyzes hydrolysis and aminolysis of bisacetyl-l-lysyl-d-alanyl-d-alanine (15, 159).
M. tuberculosis but not M. leprae has the information for three free-standing polypeptides, Mtu0907, Mtu1730, and Mtu1922, which bear the modified bar codes SxxK, YxN, and HxG (Fig. 14), indicating that they are not related to wall peptidoglycan biochemistry. They belong to the MEROPS S12 family. The P values for the pair Mtu1730-Mtu0907 and the pair Mtu1730-Mtu1922 are 4 × 10−23 (identity, 30%) and 3 × 10−18 (identity, 29%), respectively. Mtu1730 is related to the free-standing PBP4 of Nocardia lactamdurans (encoding gene within the cluster involved in cephamycin biosynthesis) by a P value of 10−45 (identity, 34%). Mtu1922 is related (for more than 80% of its entire sequence) to the Streptomyces sp. strain R61 d,d-carboxypeptidase-PBP (P, 2 × 10−19; identity, 29%), the endopeptidase ADP of B. cereus (P, 8 × 10−20; identity, 29%); and the catalytic module of the d-aminopeptidase DAP of O. anthropi (P, 5 × 10−18; identity, 28%) (Fig. 15).
M. tuberculosis produces a β-lactamase of class A, Mtu2068 (Fig. 14), which is related to a β-lactamase of Nocardia lactamdurans by a P value of 9 × 10−62 (identity, 50%). M. leprae apparently lacks the information for a β-lactamase of class A similar to Mtu2068. However, M. leprae isolated from the tissues of experimentally infected armadillos treated with benzylpenicillin 6 months or more before sacrifice has β-lactamase activity (181).
M. tuberculosis has the information for a free-standing polypeptide, Mtu1367, which has motif 1, SxxK, and motif 2, YxH, but lacks motif 3 (Fig. 14). Mtu1367 is related to a triacylglycerol lipase of Pseudomonas sp. strain 109 by a P value of 2 × 10−34 (identity, 36%). In M. leprae, pseudogene Ml0528 carries the same information.
PBP Fusions and Penr Protein Fusions of S. coelicolor
S. coelicolor has the information for three class A PBP fusions, ScoH24.23, ScoE6.34, and ScoK7.12 (Fig. 11). They are orthologues of Mtu1s (P ≤ 10−40) (Table 5).
S. coelicolor has the information for one PBP fusion of subclass B3, Sco4A10.23 (Fig. 12). Sco4A10.23 is an orthologue of Mtu3 (P, 10−82) (Table 5). In S. coelicolor, 4A10.23c codes for Sco4A10.23 (Sco3), and 4A10.22c to 4A10.15c code for protein homologues of E. coli MurE, MurF, MraY, MurD, FtsW, MurG, FtsQ, and FtsZ (192). Altogether, they form a cluster equivalent to the cell septation dcw cluster of E. coli (Fig. 5). An S. coelicolor FtsZ null mutant is viable and produces aerial hyphae. It is unable to produce spores, indicating that FtsZ is required for the hyphae to undergo septation into uninucleoid cells that differentiate into spores (152). The manipulation of a developmentally controlled FtsZ promoter also generates a nonsporulating S. coelicolor strain (66).
S. coelicolor has the information for three PBP fusions of subclass B-like I, ScoH69.17, Sco6G9.32, and Sco2st10A7 (Fig. 12). They are orthologues of Mtu0016 (P ≅ 10−80) (Table 5).
S. coelicolor has the information for one PBP fusion of subclass B2, ScoC88.19 (Sco2) (Fig. 12). Sco2 has no equivalent in M. tuberculosis, M. leprae, or C. diphtheriae. The linker and acyltransferase modules of Sco2 diverged in concert in a way that indicates relatedness with Eco2 of subclass B2 (Fig. 10). In addition, the acyltransferase module of Sco2 is more closely related to the acyltransferase module of Eco2 (P, 6 × 10−31; identity, 30%) than to the acyltransferase module of Eco3 of subclass B3 (P, 7 × 10−11; identity, 21%). In S. coelicolor (32), C88.19c codes for Sco2; C88.22c, C88.21c, and C88.20c code for protein homologues of the E. coli actin-like MreB, MreC, and MreD, respectively; and C88.18c codes for a protein of the SFR family. Altogether, they form a cluster equivalent to the rod shape-determining mre cluster of E. coli (241).
S. coelicolor has the information for a protein fusion, ScoH17.14 (Fig. 17), which is an orthologue of the Penr protein fusion Mtu1r of class A (P, 10−51) (Table 5).
Finally, S. coelicolor has the information for three protein fusions, ScoH63.18, ScoE87.08, and ScoE87.07 (Fig. 16), the acyltransferase modules of which are related to the acyltransferase module of the Penr protein fusion Mtu2864 of class B-like II (by P values ranging from 10−27 to 10−38) (Table 5). The linker modules, however, lack statistically significant similarity with the linker modules of the Penr protein fusions of class B-like II. On this basis, ScoH63.18, ScoE87.08, and ScoE87.07 are identified provisionally as Penr protein fusions of a particular class B-like III.
Detected versus Predicted PBPs of M. tuberculosis H37Ra
M. tuberculosis H37Ra produces four PBPs of 94, 82, 52, and 37 kDa (35). The 94- and 82-kDa PBPs migrate close to each other on sodium dodecyl sulfate gels because of poor resolution of large proteins. Their molecular masses are almost certainly overestimated. Thus, disruption of the M. smegmatis gene encoding the 715-amino-acid PBP fusion Msm1s (theoretical Mr 74,459) causes the disappearance from the gel of a protein band of 95 kDa (22). Identifying the experimentally detected PBPs with the predicted PBP fusions of class A, class B, class B-like I, and free-standing PBPs remains somewhat ambiguous. It is likely that the M. tuberculosis 94- to 82-kDa protein bands comprise the PBP fusions Mtu1s of class A (theoretical Mr 71,119) and Mtu3 of subclass B3 (theoretical Mr 72,506). The 52-kDa PBP sometimes appears as a doublet (35). The 52-kDa protein band probably comprises the PBP fusion Mtu0016 of subclass B-like I (theoretical Mr 51,777) and the free-standing PBP Mtu3627 (theoretical Mr 46,835), the equivalent of Eco4. In turn, the 37-kDa PBP is probably Mtu3330 (theoretical Mr 41,683), the equivalent of Eco5 or Eco6 in E. coli.
The specificity profiles of the 94-, 82-, 52-, and 37-kDa PBPs for β-lactam antibiotics have been expressed in k+2/K values (Table 2) from the published IC50 values (35). Imipenem is a potent inactivator of the 94- to 82-kDA PBP fusions (k+2/K values from 2,000 to 6,000 M−1 s−1). The 52-kDa PBP fusion is less susceptible to the β-lactams.
WHERE NEXT?
Bacterial cell walls were isolated for the first time in the early 1950s (200). Many facets of the wall biochemistry and mode of action of penicillin have been disclosed. Bacteria that are actively multiplying, conditions under which they manufacture a wall peptidoglycan of the (4→3) type, are killed by β-lactam antibiotics. SxxK d,d-acyltransferases implicated in the assembly of the polymer are immobilized by the drugs in the form of stable, inactive PBPs. Class A PBP fusions comprised of a glycosyltransferase module and a d,d-acyltransferase module of class A, class B PBP fusions comprised of a linker (peptide recognition) module and a d,d-acyltransferase module of class B, free-standing d,d-acyltransferases-PBPs, and a set of non-penicillin-binding cell cycle proteins assemble into a morphological apparatus that ensures the formation of a nascent (4→3) peptidoglycan from lipid II precursor molecules and the remodeling of the wall polymer throughout cell expansion and division. PBP fusions of classes A and B are globally the lethal targets of the β-lactam antibiotics.
In spite of these advances, we are still in the reductionist approach of dissecting the constituents of the morphogenic apparatus. The known molecular parts will have to be reassembled into functional entities in order to resolve the issues of the process. The modes of internal communication, transmission of the directives eliciting the right responses, and coordination of many events from DNA replication to cell division represent work for years to come. Even the question of how the different assortments of PBPs determine cell growth and division in exponential-phase cultures of different bacterial species is left open.
One PBP fusion each in classes A and B is probably the minimal set required for a bacterium, such as the coccus-shaped N. meningitidis, to multiply. The rod-shaped E. coli has two class A PBP fusions (they can substitute for each other) and two class B PBP fusions (one of which, Eco3, can substitute for the other, Eco2, in particular genetic backgrounds). M. tuberculosis, M. leprae, and C. diphtheriae have one PBP fusion each in classes A, B, and B-like I. Class B-like I PBP fusions, which have an incomplete linker module, may be typical of the actinomycetes.
S. coelicolor undergoes complex developmental processes. It has the information for three PBPs of class A, one PBP fusion each in subclasses B2 and B3, and three class B-like I PBP fusions. The genus Chlamydia is unusual in many respects (81). These gram-negative bacteria are susceptible to penicillin. They undergo binary fission as reticulate bodies within the chlamydial inclusion. They have the information for the synthesis of a lipid II precursor similar to that of E. coli (216). However, they are peptidoglycanless. Consistently, they lack the information for class A PBP fusions. They produce two class B PBP fusions which perhaps manufacture a wall glycanless-peptide polymer in a penicillin-sensitive manner (81).
Phylogenetically distant bacterial species, such as E. faecium, E. coli, other gram-negative bacteria, Mycobacterium spp., and Streptomyces spp., have the dual ability to manufacture a (4→3) peptidoglycan in a penicillin-susceptible manner and a (3→3) peptidoglycan in a penicillin-resistant manner. The conjuncture is that, basically, the polymeric (3→3) peptidoglycans are formed from lipid II precursor molecules by the combined activities of a glycosyltransferase and a penicillin-resistant acyltransferase that has undergone a change from the d,d specificity (reaction I in Fig. 6) to the l,d specificity (reaction II or III in Fig. 6). The results of the studies reported here lead to the conclusion that the (3→3) peptidoglycan-synthesizing machines are different in different bacterial species. They raise a host of problems that remain to be worked on.
E. faecium has two acyltransferase activities, a Penr N-acyl-d-alanyl-d-alanine d,d-carboxypeptidase and a Penr N-acyl-l-lysyl-d-alanine-cleaving transpeptidase, that would be required to make (3→3) l-lysyl-Nɛ-(d-isoasparaginyl)-l-lysine cross-bridges (Fig. 2) according to reactions IIIa and III shown in Fig. 6. The activities have been detected, but the enzymes have not been isolated and characterized biochemically; the Penr d,d-carboxypeptidase could be a Zn2+-dependent hydrolase, and the glycosyltransferase remains hypothetical.
E. faecium also produces a Penr SxxK protein fusion, Efam5, of subclass B1. The encoding gene is in the neighborhood of a gene that codes for an SFR protein, suggesting that in exponential-phase cultures, Efam5 plays the role of a cell cycle protein in (3→3) peptidoglycan metabolism similar to the roles that the class B PBP fusions play in (4→3) peptidoglycan metabolism. Consistent with this view, E. faecium cells in the exponential phase of growth exhibit various levels of resistance to penicillin in an Efam5 concentration-dependent manner, and they manufacture a (3→3) peptidoglycan in various proportions of total peptidoglycan. In particular genetic backgrounds, however, Efam5 is dispensable. An Efam5-less mutant which manufactures exclusively a (3→3) peptidoglycan during growth has been isolated. It has a MIC for ampicillin of greater than 2 mg of antibiotic ml−1.
What is true for E. faecium is probably true for other enterococci and the staphylococci. Like Efam5 in E. faecium, the Penr SxxK protein fusion Sau2a of subclass B1 is a penicillin resistance determinant in exponential-phase cultures of S. aureus. However, the ability of S. aureus to manufacture a (3→3) peptidoglycan has not been established.
The (3→3) peptidoglycan-synthesizing machines of E. coli, M. tuberculosis, M. leprae, and M. smegmatis appear to be different from that of E. faecium in terms of composition and growth phase dependency. E. coli and the mycobacteria all produce a Penr SxxK protein fusion of class A, Eco1c, Mtu1r, Mle1r, and Msm1r, respectively. By combining a glycosyltransferase module and a Penr acyltransferase module having the l,d specificity, the Penr protein class A fusions would have the activities required to carry out (3→3) peptidoglycan assembly from lipid II precursor molecules in a penicillin-resistant manner, either directly, according to reaction II shown in Fig. 6, or after the action of a Penr d,d-carboxypeptidase, according to reaction III.
The Penr protein class A fusions are not penicillin resistance determinants in exponential-phase cultures of E. coli and Mycobacterium spp., conditions under which they multiply in a penicillin-susceptible manner. Eco1c does not rescue an E. coli strain that has the double mutation Eco1ats Eco1b from death at the nonpermissive temperature. Wild-type E. coli cells manufacture a (3→3) peptidoglycan in increasing proportions of total peptidoglycan and become increasingly more resistant to penicillin when they divide at decreasing growth rates and when they enter the stationary phase. An M. smegmatis mutant that lacks Msm1r shows much impaired viability in long-term survival, conditions under which the wild-type strain manufactures large amounts of (3→3) peptidoglycan (see the Introduction). These observations lead to the plausible conclusion that (3→3) peptidoglycan synthesis and intrinsic resistance to penicillin at the level of the target proteins are dependent on class A Penr protein fusions in E. coli and mycobacteria exposed to adverse growth conditions.
Stationary phase and long-term survival are not synonymous with no metabolism and no cell division as it occurs in sporulation. The stringent response is a vast transcriptional program that encompasses many genes. It is initiated by increased concentrations of ppGpp and pppGpp, made by RelA and SpoT in E. coli and by Rel in M. tuberculosis (33, 182). E. coli lacking RelA and SpoT cannot grow in minimal medium and hardly survives in stationary phase. One induced regulon concerns genes whose expression depends on the rpoS-encoded sigma factor σs. Mutations in rpoS and other unlinked loci confer on cells in aged cultures a competitive advantage that is restricted to the stationary phase over cells of a young population, so that the mutants take over the bacterial population (253).
Many genes form a defensive system aimed at avoiding the damaging effects of ongoing respiratory activity (167). Superoxide dismutases are important under aerobic carbon starvation. An alkylhydroperoxide reductase is required under aerobic phosphate starvation (158). About 2% of the genome is under the control of the nitrogen regulatory protein C, which activates operons whose products minimize the effects of nitrogen-limiting conditions (258). The peptidyl prolyl isomerase SurA, involved in protein folding in the periplasm, is essential for stationary-phase survival (133, 225).
Likewise, Rel is essential to M. tuberculosis. Persistent cells incorporate radioactive uridine into RNA and produce 16s RNA and mRNA transcripts (107). The glyoxylate shunt enzyme isocitrate lyase is essential (153). The mRNA level of the sigma factor SigJ-encoding gene is upregulated 20-fold in 100-day cultures compared to exponential-phase cultures (106). Stationary-phase cultures of M. smegmatis are a dynamic population of cells. Growth and cell division occur and mutations accumulate which lead to the emergence of variants more adapted to survival (209). In light of these data, a shift of peptidoglycan synthesis from the (4→3) type to the (3→3) type could be the first known response to starvation and other stress conditions that would affect wall peptidoglycan metabolism.
According to the proposed scenarios, lipid II precursor molecules perform dual functions. The SxxK d,d-acyltransferase modules of the PBP fusions examine the stem pentapeptides, search for d-alanyl-d-alanine sequences, and use them as carbonyl donors for the synthesis of (4→3) interpeptide linkages or cross-bridges in a penicillin-susceptible manner (reaction I in Fig. 6). The SxxK l,d-acyltransferase modules of the Penr protein fusions would search for l-diaminoacyl-d-alanyl-d-alanine or l-diaminoacyl-d-alanine sequences and use them as carbonyl donors for the synthesis of (3→3) interpeptide linkages or cross-bridges (reactions II and III in Fig. 6) in a penicillin-resistant manner. These transfer reactions differ from the transfer reactions through which many surface proteins are attached covalently to the peptidoglycan in gram-positive bacteria. In this case, cysteine transferases, called sortases (109, 150), identify the signal sequence LPxTG at the carboxy end of the surface protein as the carbonyl donor, cleave the threonyl-glycine bond, and transfer the carbonyl of the threonine residue to the ω-amino group at position 3 of peptidoglycan precursor molecules.
Little is known about the Penr SxxK protein fusions beyond their cursory description. Further studies may well reshape our views on peptidoglycan biochemistry, offer a clue to the mycobacterial paradox, and provide much-needed information for a full understanding of the intrinsic resistance to penicillin of important bacterial pathogens.
Acknowledgments
This work was supported in part by the Belgian program on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Services fédéraux des affaires scientifiques, techniques et culturelles (PAI no. P4/03). C.G. is Chercheur qualifié of the Fonds National de la Recherche Scientifique (FNRS, Brussels).
Figure 9, lower part, is a gift from Georges Dive (this laboratory), and Figure 13 is a gift from Robert Brasseur (Faculté Universitaire des Sciences Agronomiques, Gembloux), and we thank them. We thank Joyoti Basu from the Bose Institute, Calcutta, Martine Nguyen-Distèche, Jacques Coyette, Paulette Charlier, Eveline Fonzé, Michaël Delmarcelle, Albert Demonceau, and André Piette for discussion, and the reviewers for their interest and comments.
REFERENCES
- 1.Adam, M., C. Fraipont, N. Rhazi, M. Nguyen-Distèche, B. Lakaye, J. M. Frère, B. Devreese, J. Van Beeumen, Y. van Heijenoort, J. van Heijenoort, and J. M. Ghuysen. 1997. The bimodular G57-V577 polypeptide chain of the class B penicillin-binding protein 3 of Escherichia coli catalyzes peptide bond formation from thiolesters and does not catalyze glycan chain polymerization from lipid II intermediate. J. Bacteriol. 179:6005-6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addinall, S. G., and J. Lutkenhaus. 1996. FtsZ-spirals and -arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Mol. Microbiol. 22:231-237. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., and W. Gish. 1996. Local alignment statistics. Methods Enzymol. 266:460-480. [DOI] [PubMed] [Google Scholar]
- 4.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambler, R. P., A. F. W. Coulson, J. M. Frère, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A β-lactamases. Biochem. J. 276:269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amoroso, A., D. Demares, M. Mollerach, G. Gutkind, and J. Coyette. 2001. All detectable high-molecular-mass penicillin-binding proteins are modified in a high-level β-lactam-resistant clinical isolate of Streptococcus mitis. Antimicrob. Agents Chemother. 45:2075-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson, J. O., and S. G. Andersson. 2001. Pseudogenes, junk DNA, and the dynamics of Rickettsia genomes. Mol. Biol. Evol. 18:829-839. [DOI] [PubMed] [Google Scholar]
- 8.Arthur, M., F. Depardieu, L. Cabanié, P. Reynolds, and P. Courvalin. 1998. Requirement of the VanY and VanX dd-peptidases for glycopeptide resistance in enterococci. Mol. Microbiol. 30:819-830. [DOI] [PubMed] [Google Scholar]
- 9.Arthur, M., F. Depardieu, H. A. Snaith, P. E. Reynolds, and P. Courvalin. 1994. Contribution of VanY dd-carboxypeptidase to glycopeptide resistance in Enterococcus faecalis by hydrolysis of peptidoglycan precursors. Antimicrob. Agents Chemother. 38:1899-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asano, Y., H. Ito, T. Dairi, and Y. Kato. 1996. An alkaline d-stereospecific endopeptidase with β-lactamase activity from Bacillus cereus. J. Biol. Chem. 22:30256-30262. [DOI] [PubMed] [Google Scholar]
- 11.Asano, Y., Y. Kato, A. Yamada, and K. Kondo. 1992. Structural similarity of d-aminopeptidase to carboxypeptidases dd and β-lactamases. Biochemistry 31:2316-2328. [DOI] [PubMed] [Google Scholar]
- 12.Azuma, I., D. W. Thomas, A. Adam, J. M. Ghuysen, R. Bonaly, J. F. Petit, and E. Lederer. 1970. Occurrence of N-glycolylmuramic acid in bacterial cell walls. A preliminary survey. Biochim. Biophys. Acta 208:444-451. [DOI] [PubMed] [Google Scholar]
- 13.Baquero, M. R., M. Bouzon, J. C. Quintela, J. A. Ayala, and F. Moreno. 1996. dacD, an Escherichia coli gene encoding a novel penicillin-binding protein (PBP6b) with dd-carboxypeptidase activity. J. Bacteriol. 178:7106-7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbour, A. G. 1981. Properties of penicillin-binding proteins in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 19:316-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu, J., R. Chattopadhyay, M. Kundu, and P. Chakrabarti. 1992. Purification and partial characterization of a penicillin-binding protein from Mycobacterium smegmatis. J. Bacteriol. 174:4829-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu, J., S. Mahapatra, M. Kundu, S. Mukhopadhyay, M. Nguyen-Distèche, P. Dubois, B. Joris, J. Van Beeumen, S. T. Cole, P. Chakrabarti, and J. M. Ghuysen. 1996. Identification and overexpression in Escherichia coli of a Mycobacterium leprae gene, pon1, encoding a high-molecular-mass class A penicillin-binding protein, PBP1. J. Bacteriol. 178:1707-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck, B. D., and J. T. Park. 1977. Basis for the observed fluctuation of carboxypeptidase II activity during the cell cycle in BUG 6, a temperature-sensitive division mutant of Escherichia coli. J. Bacteriol. 130:1292-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begg, K. J., S. J. Dewar, and W. O. Donachie. 1995. A new Escherichia coli cell division gene ftsK. J. Bacteriol. 177:6211-6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begg, K. J., A. Takasuga, D. H. Edwards, S. J. Dewar, B. G. Spratt, H. Adachi, T. Ohta, H. Matsuzawa, and W. D. Donachie. 1990. The balance between different peptidoglycan precursors determines whether Escherichia coli cells will elongate or divide. J. Bacteriol. 172:6697-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berthet, F. X., J. Rauzier, E. M. Lim, W. Philipp, B. Gicquel, and D. Portnoï. 1995. Characterization of the Mycobacterium tuberculosis erp gene encoding a potential cell surface protein with repetitive structures. Microbiology 141:2123-2130. [DOI] [PubMed] [Google Scholar]
- 21.Bhakta, S., and J. Basu. 2002. Overexpression, purification and biochemical characterization of a class A high-molecular-mass penicillin-binding protein (PBP), PBP1* and its soluble derivative from Mycobacterium tuberculosis. Biochem. J. 361:635-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billman-Jacobe, H., R. E. Haites, and R. L. Coppel. 1999. Characterization of a Mycobacterium smegmatis mutant lacking penicillin binding protein 1. Antimicrob. Agents Chemother. 43:3011-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blasco, B., A. G. Pisabarro, and M. A. de Pedro. 1988. Peptidoglycan biosynthesis in stationary-phase cells of Escherichia coli. J. Bacteriol. 170:5224-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blattner, F. R., G. Plunkett 3rd, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 25.Bloom, B. R. 1992. Back to a frightening future. Nature 358:538-539. [DOI] [PubMed] [Google Scholar]
- 26.Bompard-Gilles, C., H. Remaut, V. Villeret, T. Prange, L. Fanuel, M. Delmarcelle, B. Joris, J. M. Frère, and J. Van Beeumen. 2000. Crystal structure of a D-aminopeptidase from Ochrobactrum anthropi, a new member of the penicillin-recognizing enzyme family. Structure Fold. Des. 15:971-980. [DOI] [PubMed] [Google Scholar]
- 27.Botta, G. A., and J. T. Park. 1981. Evidence of involvement of penicillin-binding protein 3 in murein synthesis during septation, but not during cell elongation. J. Bacteriol. 145:333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyle, D. S., M. M. Khattar, S. G. Addinall, J. Lutkenhaus, and W. D. Donachie. 1997. ftsW is an essential cell-division gene in Escherichia coli. Mol. Microbiol. 24:1263-1273. [DOI] [PubMed] [Google Scholar]
- 29.Braun, V. 1975. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim. Biophys. Acta 415:335-377. [DOI] [PubMed] [Google Scholar]
- 30.Brennan, P. J., and H. Nikaido. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64:29-63. [DOI] [PubMed] [Google Scholar]
- 31.Buddelmeijer, N., N. Judson, D. Boyd, J. J. Mekalanos, and J. Beckwith. 2002. YgbQ, a cell division protein in Escherichia coli and Vibrio cholerae, localizes in codependent fashion with FtsL to the division site. Proc. Natl. Acad. Sci. USA 99:6316-6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger, A., K. Sichler, G. Kelemen, M. Buttner, and W. Wohlleben. 2000. Identification and characterization of the mre gene region of Streptomyces coelicolor A3(2). Mol. Gen. Genet. 263:1053-1060. [DOI] [PubMed] [Google Scholar]
- 33.Cashel, M., D. R. Gentry, V. J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1488-1496. In F. C. Neidhardt, R. Curtis, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 34.Chalut, C., X. Charpentier, M. H. Remy, and J. M. Masson. 2001. Differential responses of Escherichia coli cells expressing cytoplasmic domain mutants of penicillin-binding protein 1b after impairment of penicillin-binding proteins 1a and 3. J. Bacteriol. 183:200-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chambers, H. F., D. Moreau, D. Yajko, C. Miick, C. Wagner, C. Hackbarth, S. Kocagöz, E. Rosenberg, W. K. Hadley, and H. Nikaido. 1995. Can penicillins and other β-lactam antibiotics be used to treat tuberculosis? Antimicrob. Agents Chemother. 39:2620-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen, J. C., M. Minev, and J. Beckwith. 2002. Analysis of ftsQ mutant alleles in Escherichia coli: complementation, septal localization, and recruitment of downstream cell division proteins. J. Bacteriol. 184:695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claverys, J. P., M. Prudhomme, I. Mortier-Barrière, and B. Martin. 2000. Adaptation to the environment: Streptococcus pneumoniae, a paradigm for recombination-mediated genetic plasticity? Mol. Microbiol. 35:251-259. [DOI] [PubMed] [Google Scholar]
- 38.Cole, S. T., et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 39.Cole, S. T., et al. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 40.Coyette, J., H. R. Perkins, I. Polacheck, G. D. Shockman, and J. M. Ghuysen. 1974. Membrane-bound dd-carboxypeptidase and ld-transpeptidase of Streptococcus faecalis ATCC 9790. Eur. J. Biochem. 44:459-468. [DOI] [PubMed] [Google Scholar]
- 41.Curtis, N. A., D. Orr, G. W. Ross, and M. G. Boulton. 1979. Affinities of penicillins and cephalosporins for the penicillin-binding proteins of Escherichia coli K-12 and their antibacterial activity. Antimicrob. Agents Chemother. 16:533-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daniel, R. A., S. Drake, C. E. Buchanan, R. Scholle, and J. Errington. 1994. The Bacillus subtilis spoVD gene encodes a mother-cell-specific penicillin-binding protein required for spore morphogenesis. J. Mol. Biol. 235:209-220. [DOI] [PubMed] [Google Scholar]
- 43.Daniel, R. A., E. J. Harry, and J. Errington. 2000. Role of penicillin-binding protein PBP2B in assembly and functioning of the division machinery of Bacillus subtilis. Mol. Microbiol. 35:299-311. [DOI] [PubMed] [Google Scholar]
- 44.DasGupta, H., and D. P. Fan. 1979. Purification and characterization of a carboxypeptidase-transpeptidase of Bacillus megaterium acting on the tetrapeptide moiety of the peptidoglycan. J. Biol. Chem. 254:5672-5683. [PubMed] [Google Scholar]
- 45.Davies, C., S. W. White, and R. A. Nicholas. 2001. Crystal structure of a deacylation-defective mutant of penicillin-binding protein 5 at 2.3-Å resolution. J. Biol. Chem. 276:616-623. [DOI] [PubMed] [Google Scholar]
- 46.de Jonge, B. L. M., and A. Tomasz. 1993. Abnormal peptidoglycan produced in a methicillin-resistant strain of Staphylococcus aureus grown in the presence of methicillin: functional role for penicillin-binding protein 2A in cell wall synthesis. Antimicrob. Agents Chemother. 37:342-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de la Rosa, E. J., M. A. de Pedro, and D. Vázquez. 1982. Modification of penicillin-binding proteins of Escherichia coli associated with changes in the state of growth of the cells. FEMS Microbiol. Lett. 14:91-94. [Google Scholar]
- 48.Denome, S. A., P. K. Elf, T. A. Henderson, D. E. Nelson, and K. D. Young. 1999. Escherichia coli mutants lacking all possible combinations of eight penicillin binding proteins: viability, characteristics, and implications for peptidoglycan synthesis. J. Bacteriol. 181:3981-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Pedro, M. A., W. D. Donachie, J. V. Höltje, and H. Schwarz. 2001. Constitutive septal murein synthesis in Escherichia coli with impaired activity of the morphogenetic proteins RodA and penicillin-binding protein 2. J. Bacteriol. 183:4115-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deretic, V., and R. A. Fratti. 1999. Mycobacterium tuberculosis phagosome. Mol. Microbiol. 31:1603-1609. [DOI] [PubMed] [Google Scholar]
- 51.de Rosa, R., and B. Labedan. 1998. The evolutionary relationships between the two bacteria Escherichia coli and Haemophilus influenzae and their putative last common ancestor. Mol. Biol. Evol. 15:17-27. [DOI] [PubMed] [Google Scholar]
- 52.Dideberg, O., P. Charlier, G. Dive, B. Joris, J. M. Frère, and J. M. Ghuysen. 1982. Structure at 2.5 Å resolution of a Zn++-containing d-alanyl-d-alanine-cleaving carboxypeptidase. Nature 299:469-470. [DOI] [PubMed] [Google Scholar]
- 53.Dive, G., D. Dehareng, and J. M. Ghuysen. 1994. A detailed study of a molecule into a molecule: the N-acetyl-l-tryptophanamide in active site model of the α-chymotrypsin. J. Am. Chem. Soc. 116:2548-2556. [Google Scholar]
- 54.Dive, G., D. Dehareng, and D. Peeters. 1996. Proposition for the acylation mechanism of serine proteases: a one-step process? Int. J. Quantum Chem. 58:85-107. [Google Scholar]
- 55.Donachie, W. D. 2001. Co-ordinate regulation of the Escherichia coli cell cycle; or, the cloud of unknowing. Mol. Microbiol. 40:779-785. [DOI] [PubMed] [Google Scholar]
- 56.Dowson, C. G., T. J. Goffey, and B. G. Spratt. 1994. Origin and molecular epidemiology of penicillin binding protein-mediated resistance to β-lactam antibiotics. Trends Microbiol. 2:361-366. [DOI] [PubMed] [Google Scholar]
- 57.Duez, C., C. Piron-Fraipont, B. Joris, J. Dusart, M. S. Urdea, J. A. Martial, J. M. Frère, and J. M. Ghuysen. 1987. Primary structure of the Streptomyces R61 exocellular dd-peptidase. 1. Cloning into Streptomyces lividans and nucleotide sequence of the gene. Eur. J. Biochem. 162:509-518. [DOI] [PubMed] [Google Scholar]
- 58.Duez, C., W. Zorzi, F. Sapunaric, A. Amoroso, I. Thamm, and J. Coyette. 2001. The penicillin resistance of Enterococcus faecalis JH2-2r results from an overproduction of the low affinity penicillin-binding protein PBP4 and does not involve a psr-like gene. Microbiology 147:2561-2569. [DOI] [PubMed] [Google Scholar]
- 59.Edwards, D. H., and W. D. Donachie. 1993. Construction of a triple deletion of penicillin-binding proteins 4, 5, and 6 in Escherichia coli, p. 369-374. In M. A. de Pedro, J. V. Höltje, and W. Löffelhardt (ed.), Bacterial growth and lysis. Plenum Press, New York, N.Y.
- 60.Ehlert, K., M. Tschierske, C. Mopi, W. Schröder, and B. Berger-Bächi. 2000. Site-specific serine incorporation by Lif and Epr into positions 3 and 5 of the staphylococcal peptidoglycan interpeptide bridge. J. Bacteriol. 182:2635-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Kharroubi, A., P. Jacques, G. Piras, J. Van Beeumen, J. Coyette, and J. M. Ghuysen. 1991. The Enterococcus hirae R40 penicillin-binding protein 5 and the methicillin-resistant Staphylococcus aureus penicillin-binding protein 2′ are similar. Biochem. J. 280:463-469. [PMC free article] [PubMed] [Google Scholar]
- 62.Fanuel, L., B. Granier, J. M. Wilkin, C. Bellefroid-Bourguignon, B. Joris, J. Knowles, E. Komives, J. Van Beeumen, J. M. Ghuysen, and J. M. Frère. 1994. The precursor of the Streptomyces R61 dd-peptidase containing a C-terminal extension is inactive. FEBS Lett. 351:49-52. [DOI] [PubMed] [Google Scholar]
- 63.Fattorini, L., G. Orefici, S. H. Jin, G. Scardaci, G. Amicosante, N. Franceschini, and I. Chopra. 1992. Resistance to β-lactams in Mycobacterium fortuitum. Antimicrob. Agents Chemother. 36:1068-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Filipe, S. R., E. Severina, and A. Tomasz. 2000. Distribution of the mosaic structured murM genes among natural populations of Streptococcus pneumoniae. J. Bacteriol. 182:6798-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filipe, S. R., and A. Tomasz. 2000. Inhibition of the expression of penicillin resistance in Streptococcus pneumoniae by inactivation of cell wall muropeptide branching genes. Proc. Natl. Acad. Sci. USA 97:4891-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flärdh, K., E. Leibovitz, M. J. Buttner, and K. F. Chater. 2000. Generation of a nonsporulating strain of Streptomyces coelicolor A3(2) by the manipulation of a developmentally controlled ftsZ promoter. Mol. Microbiol. 38:737-749. [DOI] [PubMed] [Google Scholar]
- 67.Fontana, R., A. Grossato, L. Rossi, Y. R. Cheng, and G. Satta. 1985. Transition from resistance to hypersusceptibility to β-lactam antibiotics associated with loss of a low-affinity penicillin-binding protein in a Streptococcus faecium mutant highly resistant to penicillin. Antimicrob. Agents Chemother. 28:678-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fonzé, E., M. Vermeire, M. Nguyen-Distèche, R. Brasseur, and P. Charlier. 1999. The crystal structure of a penicilloyl-serine transferase of intermediate penicillin-sensitivity. The dd-transpeptidase of Streptomyces K15. J. Biol. Chem. 274:21853-21860. [DOI] [PubMed] [Google Scholar]
- 69.Frère, J. M., C. Duez, J. M. Ghuysen, and J. Vandekerkhove. 1976. Occurrence of a serine residue in the penicillin-binding site of the exocellular dd-carboxypeptidase-transpeptidase from Streptomyces R61. FEBS Lett. 70:257-260. [DOI] [PubMed] [Google Scholar]
- 70.Frère, J. M., J. M. Ghuysen, and H. R. Perkins. 1975. Interaction between the exocellular dd-carboxypeptidase-transpeptidase from Streptomyces R61, substrate and β-lactam antibiotics. A choice of models. Eur. J. Biochem. 57:353-359. [DOI] [PubMed] [Google Scholar]
- 71.Frère, J. M., J. M. Ghuysen, J. Degelaen, A. Loffet, and H. R. Perkins. 1975. Fragmentation of benzylpenicillin after interaction with the exocellular dd-carboxypeptidases-transpeptidases of Streptomyces R61 and R39. Nature 258:168-170. [DOI] [PubMed] [Google Scholar]
- 72.Frère, J. M., J. M. Ghuysen, H. Vanderhaeghe, P. Adriaens, and J. Degelaen. 1976. Fate of thiazolidine ring during fragmentation of penicillin by the exocellular dd-carboxypeptidase-transpeptidase of Streptomyces R61. Nature 260:451-454. [DOI] [PubMed] [Google Scholar]
- 73.Galamba, A., K. Soetaert, X. M. Wang, J. De Bruyn, P. Jacobs, and J. Content. 2001. Disruption of adhC reveals a large duplication in the Mycobacterium smegmatis mc2155 genome. Microbiology 147:3281-3294. [DOI] [PubMed] [Google Scholar]
- 74.Galleni, M., G. Amicosante, and J. M. Frère. 1988. A survey of the kinetic parameters of class C β-lactamases. Cephalosporins and other β-lactam compounds. Biochem. J. 255:123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gatfield, J., and J. Pieters. 2000. Essential role for cholesterol in entry of mycobacteria into macrophages. Science 288:1647-1650. [DOI] [PubMed] [Google Scholar]
- 76.Gérard, P., T. Vernet, and A. Zapun. 2002. Membrane topology of the Streptococcus pneumoniae FtsW division protein. J. Bacteriol. 184:1925-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghuysen, J. M. 1968. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol. Rev. 32:425-464. [PMC free article] [PubMed] [Google Scholar]
- 78.Ghuysen, J. M. 1991. Serine β-lactamases and penicillin-binding proteins. Annu. Rev. Microbiol. 45:37-67. [DOI] [PubMed] [Google Scholar]
- 79.Ghuysen, J. M. 1994. Molecular structures of penicillin-binding proteins and β-lactamases. Trends Microbiol. 2:372-380. [DOI] [PubMed] [Google Scholar]
- 80.Ghuysen, J. M., and G. Dive. 1994. Biochemistry of the penicilloyl serine transferases. New Comprehensive Biochem. Bacterial Cell Wall 27:103-130. [Google Scholar]
- 81.Ghuysen, J. M., and C. Goffin. 1999. Lack of cell wall peptidoglycan versus penicillin sensitivity: new insights into the chlamydial anomaly. Antimicrob. Agents Chemother. 43:2339-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghuysen, J. M., J. M. Frère, M. Leyh-Bouille, M. Nguyen-Distèche, and J. Coyette. 1986. Active-site-serine d-alanyl-d-alanine-cleaving-peptidase-catalyzed acyl-transfer reactions. Procedures for studying the penicillin-binding proteins of bacterial plasma membranes. Biochem. J. 235:159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghuysen, J. M., J. Lamotte-Brasseur, B. Joris, and G. D. Shockman. 1994. Binding site-shaped repeated sequences of Streptomyces albus G dd-peptidase, Bacillus subtilis Cwla amidase. Corynebacterium acetobutylicum lysozyme and Bacillus spp. ORF-L 3 gene product. FEBS Lett. 342:23-28. [DOI] [PubMed] [Google Scholar]
- 84.Goffin, C., and J. M. Ghuysen. 1998. Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs. Microbiol. Mol. Biol. Rev. 62:1079-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gondré, B., B. Flouret, and J. van Heijenoort. 1973. Release of d-alanyl-d-alanine from the precursor of the cell wall peptidoglycan by a peptidase of Escherichia coli K12. Biochimie 55:685-691. [DOI] [PubMed] [Google Scholar]
- 86.Gordon, E., N. Mouz, E. Duée, and O. Dideberg. 2000. The crystal structure of the penicillin-binding protein 2x from Streptococcus pneumoniae and its acyl-enzyme form: implication in drug resistance. J. Mol. Biol. 299:477-485. [DOI] [PubMed] [Google Scholar]
- 87.Granier, B., C. Duez, S. Lepage, S. Englebert, J. Dusart, O. Dideberg, J. Van Beeumen, J. M. Frère, and J. M. Ghuysen. 1992. Primary and predicted secondary structures of the Actinomadura R39 extracellular dd-peptidase, a penicillin-binding protein (PBP) related to the Escherichia coli PBP4. Biochem. J. 282:781-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gustafson, J., A. Strässle, H. Hächler, T. H. Kayser, and B. Berger-Bächi. 1994. The femC locus of Staphylococcus aureus required for methicillin resistance includes the glutamine synthetase operon. J. Bacteriol. 176:1460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hakenbeck, R., and J. Coyette. 1998. Resistant penicillin-binding proteins. Cell. Mol. Life Sci. 54:332-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hakenbeck, R., T. Grebe, D. Zähner, and J. B. Stock. 1999. β-Lactam resistance in Streptococcus pneumoniae: penicillin-binding proteins and non-penicillin-binding proteins. Mol. Microbiol. 33:673-678. [DOI] [PubMed] [Google Scholar]
- 91.Hakenbeck, R., A. König, I. Kern, M. van der Linden, W. Keck, D. Billot-Klein, R. Legrand, B. Schoot, and L. Gutmann. 1998. Acquisition of five high-Mr penicillin-binding protein variants during transfer of high-level β-lactam resistance from Streptococcus mitis to Streptococcus pneumoniae. J. Bacteriol. 180:1831-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamid, M. E., D. E. Minnikin, and M. Goodfellow. 1993. A simple chemical test to distinguish mycobacteria from other mycolic acid-containing actinomycetes. J. Gen. Microbiol. 139:2203-2213. [DOI] [PubMed] [Google Scholar]
- 93.Hammes, W. P. 1978. The ld-carboxypeptidase activity in Gaffkya homari. The target of the action of d-amino acids or glycine on the formation of wall-bound peptidoglycan. Eur. J. Biochem. 91:501-507. [DOI] [PubMed] [Google Scholar]
- 94.Hammes, W. P., and H. Seidel. 1978. The activities in vitro of dd-carboxypeptidase and ld-carboxypeptidase of Gaffkya homari during biosynthesis of peptidoglycan. Eur. J. Biochem. 84:141-147. [DOI] [PubMed] [Google Scholar]
- 95.Hammes, W. P., and H. Seidel. 1978. The ld-carboxypeptidase activity in Gaffkya homari. The target of the action of certain β-lactam antibiotics on the formation of wall-bound peptidoglycan. Eur. J. Biochem. 91:509-515. [DOI] [PubMed] [Google Scholar]
- 96.Hardt, K., B. Joris, S. Lepage, R. Brasseur, J. O. Lampen, J. M. Frère, A. L. Fink, and J. M. Ghuysen. 1997. The penicillin sensory-transducer BlaR involved in the inducibility of β-lactamase synthesis in Bacillus licheniformis is embedded in the plasma membrane via a four α-helix bundle. Mol. Microbiol. 23:935-944. [DOI] [PubMed] [Google Scholar]
- 97.Harry, E. J. 2001. Bacterial cell division: regulating Z-ring formation. Mol. Microbiol. 40:795-803. [DOI] [PubMed] [Google Scholar]
- 98.Hartman, B. J., and A. Tomasz. 1984. Low-affinity penicillin-binding protein associated with β-lactam resistance in Staphylococcus aureus. J. Bacteriol. 158:513-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Henderson, T. A., K. D. Young, S. A. Denome, and P. K. Elf. 1997. AmpC and AmpH, proteins related to the class C β-lactamases, bind penicillin and contribute to the normal morphology of Escherichia coli. J. Bacteriol. 179:6112-6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Henriques, A. O., P. Glaser, P. J. Piggot, and C. P. Moran Jr. 1998. Control of cell shape and elongation by the rodA gene in Bacillus subtilis. Mol. Microbiol. 28:235-247. [DOI] [PubMed] [Google Scholar]
- 101.Herbert, D., C. N. Paramasivan, P. Venkatesan, G. Kubendiran, R. Prabhakar, and D. A. Mitchison. 1996. Bactericidal action of ofloxacin, sulbactam-ampicillin, rifampin, and isoniazid on logarithmic- and stationary-phase cultures of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 40:2296-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Higashi, Y., J. L. Strominger, and C. C. Sweeley. 1967. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc. Natl. Acad. Sci. USA 65:441-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holland, P. W. 1999. The effect of gene duplication on homology. Novartis Found. Symp. 222:226-236. [PubMed] [Google Scholar]
- 104.Hopwood, D. A. 1999. Forty years of genetics with Streptomyces: from in vivo through in vitro to in silico. Microbiology 145:2183-2202. [DOI] [PubMed] [Google Scholar]
- 105.Hoskins, J., et al. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu, Y., and A. R. M. Coates. 2001. Increased levels of sigJ mRNA in late stationary phase cultures of Mycobacterium tuberculosis detected by DNA array hybridisation. FEMS Microbiol. Lett. 202:59-65. [DOI] [PubMed] [Google Scholar]
- 107.Hu, Y., J. A. Mangan, J. Dhillon, K. M. Sole, D. A. Mitchison, P. D. Butcher, and A. R. M. Coates. 2000. Detection of mRNA transcripts and active transcription in persistent Mycobacterium tuberculosis induced by exposure to rifampin or pyrazinamide. J. Bacteriol. 182:6358-6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ikeda, M., T. Sato, M. Wachi, H. K. Jung, F. Ishino, Y. Kobayashi, and M. Matsuhashi. 1989. Structural similarity among Escherichia coli FtsW and RodA proteins and Bacillus subtilis SpoVE protein, which function in cell division, cell elongation, and spore formation, respectively. J. Bacteriol. 171:6375-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ilangovan, U., H. Ton-That, J. Iwahara, O. Schneewind, and R. T. Clubb. 2001. Structure of sortase, the transpeptidase that anchors proteins to the cell wall of Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 98:6056-6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ito, T., Y. Katayama, K. Asada, N. Mori, K. Tsutsumimoto, C. Tiensasitorn, and K. Hiramatsu. 2001. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1323-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Janczura, E., M. Leyh-Bouille, C. Cocito, and J. M. Ghuysen. 1981. Primary structure of the wall peptidoglycan of leprosy-derived corynebacteria. J. Bacteriol. 145:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jarlier, V., and H. Nikaido. 1994. Mycobacterial cell wall: structure and role in natural resistance to antibiotics. FEMS Microbiol. Lett. 123:11-18. [DOI] [PubMed] [Google Scholar]
- 113.Jelsch, C., L. Mourey, J. M. Masson, and J. P. Samama. 1993. Crystal structure of E. coli TEM1 β-lactamase at 1.8 Å resolution. Proteins 16:364-383. [DOI] [PubMed] [Google Scholar]
- 114.Jiang, H., and K. E. Kendrick. 2000. Cloning and characterization of the gene encoding penicillin-binding protein A of Streptomyces griseus. FEMS Microbiol. Lett. 193:63-68. [DOI] [PubMed] [Google Scholar]
- 115.Joseleau-Petit, D., D. Thévenet, and R. D'Ari. 1994. ppGpp Concentration, growth without PBP2 activity, and growth-rate control in Escherichia coli. Mol. Microbiol. 13:911-917. [DOI] [PubMed] [Google Scholar]
- 116.Kaneda, K., S. Imaizumi, S. Mizuno, T. Baba, M. Tsukamura, and I. Yano. 1988. Structure and molecular species composition of three homologous series of α-mycolic acids from Mycobacteria spp. J. Gen. Microbiol. 134:2213-2229. [DOI] [PubMed] [Google Scholar]
- 117.Kaneko, T., et al. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7:331-338. [DOI] [PubMed] [Google Scholar]
- 118.Karlin, S., and S. F. Altschul. 1993. Applications and statistics for multiple high-scoring segments in molecular sequences. Proc. Natl. Acad. Sci. USA 90:5873-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kato, J.-i., H. Suzuki, and Y. Hirota. 1985. Dispensability of either penicillin-binding protein-1a or -1b involved in the essential process for cell elongation in Escherichia coli. Mol. Gen. Genet. 200:272-277. [DOI] [PubMed] [Google Scholar]
- 120.Keck, W., A. M. van Leeuwen, M. Huber, and E. Wm. Goodell. 1990. Cloning and characterization of mepA, the structural gene of the penicillin-insensitive murein endopeptidase from Escherichia coli. Mol. Microbiol. 4:209-219. [DOI] [PubMed] [Google Scholar]
- 121.Keer, J., M. J. Smeulders, K. M. Gray, and H. D. Williams. 2000. Mutants of Mycobacterium smegmatis impaired in stationary-phase survival. Microbiology 146:2209-2217. [DOI] [PubMed] [Google Scholar]
- 122.Kelly, J. A., O. Dideberg, P. Charlier, J. P. Wéry, M. Libert, P. C. Moews, J. R. Knox, C. Duez, C. Fraipont, B. Joris, J. Dusart, J. M. Frère, and J. M. Ghuysen. 1986. On the origin of bacterial resistance to penicillin. Comparison of a β-lactamase and a penicillin target. Science 231:1429-1437. [DOI] [PubMed] [Google Scholar]
- 123.Kelly, J. A., and A. P. Kuzin. 1995. Refined crystallographic structure of a dd-peptidase penicillin target enzyme at 1.6 Å resolution. J. Mol. Biol. 254:223-236. [DOI] [PubMed] [Google Scholar]
- 124.Kelly, J. A., A. P. Kuzin, P. Charlier, and E. Fonzé. 1998. X-ray studies of enzymes that interact with penicillin. Cell. Mol. Life Sci. 54:353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Knott-Hunziker, V., K. Redhead, S. Petursson, and S. G. Waley. 1980. β-Lactamase action: isolation of an active-site serine peptide from the Pseudomonas enzyme and a penicillin. FEBS Lett. 121:8-10. [Google Scholar]
- 126.Knott-Hunziker, V., S. G. Waley, B. S. Orlek, and P. G. Sammes. 1979. Penicillinase active sites: labelling of serine-44 in β-lactamase I by 6β-bromopenicillanic acid. FEBS Lett. 99:59-61. [DOI] [PubMed] [Google Scholar]
- 127.Kohlrausch, U., and J. V. Höltje. 1991. Analysis of murein and murein precursors during antibiotic-induced lysis of Escherichia coli. J. Bacteriol. 173:3425-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kopp, U., M. Roos, J. Wecke, and H. Labischinski. 1996. Staphylococcal peptidoglycan interpeptide bridge biosynthesis: a novel antistaphylococcal target? Microb. Drug Resist. 2:29-41. [DOI] [PubMed] [Google Scholar]
- 129.Korat, B., H. Mottl, and W. Keck. 1991. Penicillin-binding protein 4 of Escherichia coli: molecular cloning of the dacB gene, controlled overexpression, and alterations in murein composition. Mol. Microbiol. 5:675-684. [DOI] [PubMed] [Google Scholar]
- 130.Kraulis, P. J. 1991. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24:946-950. [Google Scholar]
- 131.Lamotte-Brasseur, J., G. Dive, and J. M. Ghuysen. 1984. On the structural analogy between d-alanyl-d-alanine terminated peptides and β-lactam antibiotics. Eur. J. Med. Chem. 19:319-330. [Google Scholar]
- 132.Lamotte, J., G. Dive, and J. M. Ghuysen. 1991. Conformational analysis of β and γ-lactam antibiotics. Eur. J. Med. Chem. 26:43-50. [Google Scholar]
- 133.Lazar, S. W., M. Almirón, A. Tormo, and R. Kolter. 1998. Role of the Escherichia coli SurA protein in stationary-phase survival. J. Bacteriol. 180:5704-5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lázaro, S., F. Fernández-Piñas, E. Fernández-Valiente, A. Blanco-Rivero, and F. Leganés. 2001. pbpB, a gene coding for a putative penicillin-binding protein, is required for aerobic nitrogen fixation in the Cyanobacterium anabaena spp. strain PCC7120. J. Bacteriol. 183:628-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lepage, S., P. Dubois, T. K. Ghosh, B. Joris, S. Mahapatra, M. Kundu, J. Basu, P. Chakrabarti, S. T. Cole, M. Nguyen-Distèche, and J. M. Ghuysen. 1997. Dual multimodular class A penicillin-binding proteins in Mycobacterium leprae. J. Bacteriol. 179:4627-4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lessard, I. A. D., S. D. Pratt, D. G. McCafferty, D. E. Bussiere, C. Hutchins, B. L. Wanner, L. Katz, and C. T. Walsh. 1998. Homologs of the vancomycin resistance d-Ala-d-Ala dipeptidase VanX in Streptomyces toyocaensis, Escherichia coli and Synechocystis: attributes of catalytic efficiency, stereoselectivity and regulation with implications for function. Chem. Biol. 5:489-504. [DOI] [PubMed] [Google Scholar]
- 137.Lessard, I. A. D., and C. T. Walsh. 1999. Mutational analysis of active-site residues of the enterococcal d-Ala-d-Ala dipeptidase VanX and comparison with Escherichia coli d-Ala-d-Ala ligase and d-Ala-d-Ala carboxypeptidase VanY. Chem. Biol. 6:177-187. [DOI] [PubMed] [Google Scholar]
- 138.Lessard, I. A. D., and C. T. Walsh. 1999. VanX, a bacterial d-alanyl-d-alanine dipeptidase: resistance, immunity, or survival function? Proc. Natl. Acad. Sci. USA 96:11028-11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Leyh-Bouille, M., R. Bonaly, J. M. Ghuysen, R. Tinelli, and D. Tipper. 1970. ll-Diaminopimelic acid containing peptidoglycans in walls of Streptomyces spp. and of Clostridium perfringens (type A). Biochemistry 9:2944-2952. [DOI] [PubMed] [Google Scholar]
- 140.Leyh-Bouille, M., M. Nguyen-Distèche, S. Pirlot, A. Veithen, C. Bourguignon, and J. M. Ghuysen. 1986. Streptomyces K15 dd-peptidase-catalyzed reactions with suicide β-lactam carbonyl donors. Biochem. J. 235:177-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liao, X., and R. E. W. Hancock. 1997. Identification of a penicillin-binding protein 3 homolog, PBP3x, in Pseudomonas aeruginosa: gene cloning and growth phase-dependent expression. J. Bacteriol. 179:1490-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Löwe, J., and L. A. Amos. 1998. Crystal structure of the bacterial cell-division protein FtsZ. Nature 391:203-206. [DOI] [PubMed] [Google Scholar]
- 143.Lutkenhaus, J., and S. G. Addinall. 1997. Bacterial cell division and the Z ring. Annu. Rev. Biochem. 66:93-116. [DOI] [PubMed] [Google Scholar]
- 144.Mahapatra, S., S. Bhakta, J. Ahamed, and J. Basu. 2000. Characterization of derivatives of the high-molecular-mass penicillin-binding protein (PBP) 1 of Mycobacterium leprae. Biochem. J. 350:75-80. [PMC free article] [PubMed] [Google Scholar]
- 145.Mainardi, J. L., R. Legrand, M. Arthur, B. Schoot, J. van Heijenoort, and L. Gutmann. 2000. Novel mechanism of β-lactam resistance due to by-pass of dd-transpeptidation in Enterococcus faecium. J. Biol. Chem. 275:16490-16496. [DOI] [PubMed] [Google Scholar]
- 146.Margolin, W. 2000. Themes and variations in prokaryotic cell division. FEMS Microbiol. Rev. 24:531-548. [DOI] [PubMed] [Google Scholar]
- 147.Markiewicz, Z., J. K. Broome-Smith, U. Schwarz, and B. G. Spratt. 1982. Spherical Escherichia coli due to elevate levels of d-alanine carboxypeptidase. Nature 297:702-704. [DOI] [PubMed] [Google Scholar]
- 148.Marrec-Fairley, M., A. Piette, X. Gallet, R. Brasseur, H. Hara, C. Fraipont, J. M. Ghuysen, and M. Nguyen-Distèche. 2000. Differential functions of amphiphilic peptide segments of the cell septation penicillin-binding protein 3 of Escherichia coli. Mol. Microbiol. 37:1019-1031. [DOI] [PubMed] [Google Scholar]
- 149.May, B. J., Q. Zhang, L. L. Li, M. L. Paustian, T. S. Whittam, and V. Kapur. 2001. Complete genomic sequence of Pasteurella multocida, Pm70. Proc. Natl. Acad. Sci. USA 98:3460-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mazmanian, S. K., H. Ton-That, and O. Schneewind. 2001. Sortase-catalyzed anchoring of surface proteins to the cell call of Staphylococcus aureus. Mol. Microbiol. 40:1049-1057. [DOI] [PubMed] [Google Scholar]
- 151.McCafferty, D. G., I. A. D. Lessard, and C. T. Walsh. 1997. Mutational analysis of potential zinc-binding residues in the active site of the enterococcal d-Ala-d-Ala dipeptidase VanX. Biochemistry 36:10498-10505. [DOI] [PubMed] [Google Scholar]
- 152.McCormick, J. R., E. P. Su, A. Driks, and R. Losick. 1994. Growth and viability of Streptomyces coelicolor mutant for the cell division gene ftsZ. Mol. Microbiol. 14:243-254. [DOI] [PubMed] [Google Scholar]
- 153.McKinney, J. D., K. Höner zu Bentrup, E. J. Muñoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, Jr., and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735-738. [DOI] [PubMed] [Google Scholar]
- 154.Mercer, K. L. N., and D. S. Weiss. 2002. The Escherichia coli cell division protein FtsW is required to recruit its cognate transpeptidase, FtsI (PBP3), to the division site. J. Bacteriol. 184:904-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Merritt, E. A., and D. J. Bacon. 1997. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277:505-524. [DOI] [PubMed] [Google Scholar]
- 156.Metz, R., S. Henning, and W. P. Hammes. 1986. LD-carboxypeptidase activity in Escherichia coli. II. Isolation, purification and characterization of the enzyme from E. coli K12. Arch. Microbiol. 144:181-186. [DOI] [PubMed] [Google Scholar]
- 157.Mollerach, M., P. Partoune, J. Coyette, and J. M. Ghuysen. 1996. Importance of the E46-D160 polypeptide segment of the non-penicillin-binding module for the stability of the low-affinity, multimodular class B penicillin-binding protein 5 of Enterococcus hirae. J. Bacteriol. 178:1774-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moreau, P. L., F. Gérard, N. W. Lutz, and P. Cozzone. 2001. Non-growing Escherichia coli cells starved for glucose or phosphate use different mechanisms to survive oxidative stress. Mol. Microbiol. 39:1048-1060. [DOI] [PubMed] [Google Scholar]
- 159.Mukherjee, T., D. Basu, S. Mahapatra, C. Goffin, J. Van Beeumen, and J. Basu. 1996. Biochemical characterization of the 49 kDa penicillin-binding protein of Mycobacterium smegmatis. Biochem. J. 320:197-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Murray, T., D. L. Popham, and P. Setlow. 1996. Identification and characterization of pbpC, the gene encoding Bacillus subtilis penicillin-binding protein 3. J. Bacteriol. 178:6001-6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nagasawa, H., Y. Sakagami, A. Suzuki, H. Suzuki, H. Hara, and Y. Hirota. 1989. Determination of the cleavage site involved in C-terminal processing of penicillin-binding protein 3 of Escherichia coli. J. Bacteriol. 171:5890-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nakagawa, J., S. Tamaki, S. Tomioka, and M. Matsuhashi. 1984. Functional biosynthesis of cell wall peptidoglycan by polymorphic bifunctional polypeptides. Penicillin-binding protein 1Bs of Escherichia coli with activities of transglycosylase and transpeptidase. J. Biol. Chem. 259:13937-13946. [PubMed] [Google Scholar]
- 163.Nanninga, N. 2001. Cytokinesis in prokaryotes and eukaryotes: common principles and different solutions. Microbiol. Mol. Biol. Rev. 65:319-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nelson, D. E., and K. D. Young. 2001. Contributions of PBP5 and dd-carboxypeptidase penicillin binding proteins to maintenance of cell shape in Escherichia coli. J. Bacteriol. 183:3055-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nguyen-Distèche, M., M. Leyh-Bouille, S. Pirlot, J. M. Frère, and J. M. Ghuysen. 1986. Streptomyces K15 dd-peptidase-catalyzed reactions with ester and amide carbonyl donors. Biochem. J. 235:167-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nierman, W. C., et al. 2001. Complete genome sequence of Caulobacter crescentus. Proc. Natl. Acad. Sci. USA 98:4136-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nyström, T. 1999. Starvation, cessation of growth and bacterial aging. Curr. Opin. Microbiol. 2:214-219. [DOI] [PubMed] [Google Scholar]
- 168.Oefner, C., A. D'Arcy, J. J. Daly, K. Gubernator, R. L. Charnas, I. Heinze, C. Hubschwerlen, and F. K. Winkler. 1990. Refined crystal structure of β-lactamase of Citrobacter freundii indicates a mechanism for β-lactam hydrolysis. Nature (London) 343:284-288. [DOI] [PubMed] [Google Scholar]
- 169.Orengo, C. A., D. T. Jones, and J. M. Thornton. 1994. Protein superfamilies and domain superfolds. Nature (London) 372:631-634. [DOI] [PubMed] [Google Scholar]
- 170.Paetzel, M., F. Danel, L. de Castro, S. C. Mosimann, M. G. P. Page, and N. C. J. Strynadka. 2000. Crystal structure of the class D β-lactamase Oxa-10. Nat. Struct. Biol. 7:918-925. [DOI] [PubMed] [Google Scholar]
- 171.Palomeque-Messia, P., S. Englebert, M. Leyh-Bouille, M. Nguyen-Distèche, C. Duez, S. Houba, O. Dideberg, J. Van Beeumen, and J. M. Ghuysen. 1991. Amino acid sequence of the penicillin-binding protein/dd-peptidase of Streptomyces K15. Predicted secondary structures of the low-Mr penicillin-binding proteins of class A. Biochem. J. 279:223-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Paradkar, A. S., K. A. Aidoo, A. Wong, and S. E. Jensen. 1996. Molecular analysis of a β-lactam resistance gene encoded within the cephamycin gene cluster of Streptomyces clavuligerus. J. Bacteriol. 178:6266-6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Parès, S., N. Mouz, Y. Pétillot, R. Hakenbeck, and O. Dideberg. 1996. X-ray structure of Streptococcus pneumoniae PBP2x, a primary penicillin target enzyme. Nat. Struct. Biol. 3:284-289. [DOI] [PubMed] [Google Scholar]
- 174.Park, W., and M. Matsuhashi. 1984. Staphylococcus aureus and Micrococcus luteus peptidoglycan transglycosylases that are not penicillin-binding proteins. J. Bacteriol. 157:538-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pedersen, L. B., E. R. Angert, and P. Setlow. 1999. Septal localization of penicillin-binding protein 1 in Bacillus subtilis. J. Bacteriol. 181:3201-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pinho, M. G., S. R. Filipe, H. De Lancastre, and A. Tomasz. 2001. Complementation of the essential peptidoglycan transpeptidase function of penicillin-binding protein 2 (PBP2) by the drug resistance protein PBP2A in Staphylococcus aureus. J. Bacteriol. 183:6525-6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Piras, G., D. Raze, A. El Kharroubi, D. Hastir, S. Englebert, J. Coyette, and J. M. Ghuysen. 1993. Cloning and sequencing of the low-affinity penicillin-binding protein 3r-encoding gene of Enterococcus hirae S185: modular design and structural organization of the protein. J. Bacteriol. 175:2844-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pisabarro, A. G., M. A. de Pedro, and D. Vazquez. 1985. Structural modifications in the peptidoglycan of Escherichia coli associated with changes in the state of growth of the culture. J. Bacteriol. 161:238-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pisabarro, A. G., R. Prats, D. Vazquez, and A. Rodriguez-Tébar. 1986. Activity of penicillin-binding protein 3 from Escherichia coli. J. Bacteriol. 168:199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Prabhakaran, K., E. B. Harris, and B. Randhawa. 1999. Bactericidal action of ampicillin/sulbactam against intracellular mycobacteria. Int. J. Antimicrob. Agents 13:133-135. [DOI] [PubMed] [Google Scholar]
- 181.Prabhakaran, K., E. B. Harris, R. M. Sanchez, and R. C. Hastings. 1987. β-Lactamase synthesis in Mycobacterium leprae. Microbios 49:183-188. [PubMed] [Google Scholar]
- 182.Primm, T. P., S. J. Andersen, V. Mizrahi, D. Avarbock, H. Rubin, and C. E. Barry III. 2000. The stringent response of Mycobacterium tuberculosis is required for long-term survival. J. Bacteriol. 182:4889-4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Quintela, J. C., M. Caparrós, and M. A. de Pedro. 1995. Variability of peptidoglycan structural parameters in Gram-negative bacteria. FEMS Microbiol. Lett. 125:95-100. [DOI] [PubMed] [Google Scholar]
- 184.Quintela, J. C., M. A. de Pedro, P. Zollner, G. Allmaier, and F. Garcia-del Portillo. 1997. Peptidoglycan structure of Salmonella typhimurium growing within cultured mammalian cells. Mol. Microbiol. 23:693-704. [DOI] [PubMed] [Google Scholar]
- 185.Quinting, B., J. M. Reyrat, D. Monnaie, G. Amicosante, V. Pelicic, B. Gicquel, J. M. Frère, and M. Galleni. 1997. Contribution of β-lactamase production to the resistance of mycobacteria to β-lactam antibiotics. FEBS Lett. 406:275-278. [DOI] [PubMed] [Google Scholar]
- 186.Ramakrishnan, L., N. A. Federspiel, and S. Falkow. 2000. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science 288:1436-1439. [DOI] [PubMed] [Google Scholar]
- 187.Rambukkana, A. 2001. Molecular basis for the peripheral nerve predilection of Mycobacterium leprae. Curr. Opin. Microbiol. 4:21-27. [DOI] [PubMed] [Google Scholar]
- 188.Rambukkana, I. 2000. How does Mycobacterium leprae target the peripheral nervous system? Trends Microbiol. 8:23-28. [DOI] [PubMed] [Google Scholar]
- 189.Randhawa, B., E. B. Harrris, and K. Prabhakaran. 1999. Bactericidal action of oral ampicillin/sulbactam against Mycobacterium leprae. J. Antimicrob. Chemother. 44:279-281. [DOI] [PubMed] [Google Scholar]
- 190.Rawlings, N. D., and A. J. Barrett. 2000. MEROPS: the peptidase database. Nucleic Acids Res. 28:323-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Reddy, P. S., A. Raghavan, and D. Chatterjee. 1995. Evidence of a ppGpp-binding site in Escherichia coli RNA polymerase: proximity relationship with the rifampicin-binding domain. Mol. Microbiol. 15:255-265. [DOI] [PubMed] [Google Scholar]
- 192.Redenbach, M., H. M. Kieser, D. Denapaite, A. Eichner, J. Cullum, H. Kinashi, and D. A. Hopwood. 1996. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol. Microbiol. 21:77-96. [DOI] [PubMed] [Google Scholar]
- 193.Reeck, G. R., C. de Haën, D. C. Teller, R. F. Doolittle, W. M. Fitch, R. E. Dickerson, P. Chambon, A. D. McLachlan, E. Margoliash, T. H. Jukes, and E. Zuckerland. 1987. ≪Homology≫ in proteins and nucleic acids: a terminology muddle and a way out of it. Cell 50:667.. [DOI] [PubMed] [Google Scholar]
- 194.Rhazi, N. 2000. Etude du mécanisme catalytique des dd-peptidases bactériennes. Ph.D. thesis. University of Liège, Liège, Belgium.
- 195.Rhazi, N., M. Galleni, M. I. Page, and J. M. Frère. 1999. Peptidase activity of β-lactamases. Biochem. J. 341:409-413. [PMC free article] [PubMed] [Google Scholar]
- 196.Rice, L. B., L. L. Carias, R. Hutton-Thomas, F. Sifaoui, L. Gutmann, and S. D. Rudin. 2001. Penicillin-binding protein 5 and expression of ampicillin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 45:1480-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rohrer, S., K. Ehlert, M. Tschierske, H. Labischinski, and B. Berger-Bächi. 1999. The essential Staphylococcus aureus gene fmhB is involved in the first step of peptidoglycan pentaglycine interpeptide formation. Proc. Natl. Acad. Sci. USA 96:9351-9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Romeis, T., and J. V. Höltje. 1994. Penicillin-binding protein 7/8 of Escherichia coli is a dd-endopeptidase. Eur. J. Biochem. 224:597-604. [DOI] [PubMed] [Google Scholar]
- 199.Ropp, P. A., and R. A. Nicholas. 1997. Cloning and characterization of the ponA gene encoding penicillin-binding protein 1 from Neisseria gonorrhoeae and Neisseria meningitidis. J. Bacteriol. 179:2783-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Salton, M. R. J. 1994. The bacterial cell envelope—a historical perspective. New Comprehensive Biochem. Bacterial Cell Wall 27:1-22. [Google Scholar]
- 201.Schaible, U. E., K. Hagens, K. Fischer, H. L. Collins, and S. H. E. Kaufmann. 2000. Intersection of group I CD1 molecules and mycobacteria in different intracellular compartments of dendritic cells. J. Immunol. 164:4843-4852. [DOI] [PubMed] [Google Scholar]
- 202.Schiffer, G., and J. V. Höltje. 2039. 1999. Cloning and characterization of PBP1C, a third member of the multimodular class A penicillin-binding proteins of Escherichia coli. J. Biol. Chem. 274:32031-32033. [DOI] [PubMed] [Google Scholar]
- 203.Schleifer, K. H., and O. Kandler. 1972. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 36:407-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Schwarz, U., K. Seeger, F. Wengenmayer, and H. Strecker. 1981. Penicillin-binding proteins of Escherichia coli identified with a 125I-derivative of ampicillin. FEMS Microbiol. Lett. 10:107-109. [Google Scholar]
- 205.Sifaoui, F., M. Arthur, L. Rice, and L. Gutmann. 2001. Role of penicillin-binding protein 5 in expression of ampicillin resistance and peptidoglycan structure in Enterococcus faecium. Antimicrob. Agents Chemother. 45:2594-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Signoretto, C., M. Boaretti, and P. Canepari. 1994. Cloning, sequencing and expression in Escherichia coli of the low-affinity penicillin-binding protein of Enterococcus faecalis. FEMS Microbiol. Lett. 123:99-106. [DOI] [PubMed] [Google Scholar]
- 207.Signoretto, C., M. Boaretti, and P. Canepari. 1998. Peptidoglycan synthesis by Enterococcus faecalis penicillin binding protein 5. Arch. Microbiol. 170:185-190. [DOI] [PubMed] [Google Scholar]
- 208.Simpson, A. J., et al. 2000. The genome sequence of the plant pathogen Xylella fastidiosa. The Xylella fastidiosa consortium of the organization for nucleotide sequencing and analysis. Nature 406:151-157. [DOI] [PubMed] [Google Scholar]
- 209.Smeulders, M. J., J. Keer, R. A. Speight, and H. D. Williams. 1999. Adaptation of Mycobacterium smegmatis to stationary phase. J. Bacteriol. 181:270-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Song, M. D., M. Wachi, M. Doi, F. Ishino, and M. Matsuhashi. 1987. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 221:167-171. [DOI] [PubMed] [Google Scholar]
- 211.Spratt, B. G. 1975. Distinct penicillin-binding proteins involved in the division, elongation, and shape of Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 72:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Spratt, B. G. 1988. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature 332:173-176. [DOI] [PubMed] [Google Scholar]
- 213.Spratt, B. G., V. Jobanputra, and W. Zimmermann. 1977. Binding of thienamycin and clavulanic acid to the penicillin-binding proteins of Escherichia coli K-12. Antimicrob. Agents Chemother. 12:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Spratt, B. G., and A. B. Pardee. 1975. Penicillin-binding proteins and cell shape in Escherichia coli. Nature 254:516-517. [DOI] [PubMed] [Google Scholar]
- 215.Spratt, B. G., J. Zhou, M. Taylor, and M. J. Merrick. 1996. Monofunctional biosynthetic peptidoglycan transglycosylases. Mol. Microbiol. 19:639-640. [DOI] [PubMed] [Google Scholar]
- 216.Storey, C., and I. Chopra. 2001. Affinities of β-lactams for penicillin binding proteins of Chlamydia trachomatis and their antichlamydial activities. Antimicrob. Agents Chemother. 45:303-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Strynadka, N. C. J., H. Adachi, S. E. Jensen, K. Johns, A. Sielecki, C. Betzel, K. Sutoh, and M. N. James. 1992. Molecular structure of the acyl-enzyme intermediate in β-lactam hydrolysis at 1.7 Å resolution. Nature 359:700-705. [DOI] [PubMed] [Google Scholar]
- 218.Sturgill-Koszycki, S., P. H. Schlesinger, P. Chakraborty, P. L. Haddix, H. L. Collins, A. K. Fok, R. D. Allen, S. L. Gluck, J. Heuser, and D. G. Russel. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263:678-681. [DOI] [PubMed] [Google Scholar]
- 219.Suzuki, H., J. Kato, Y. Sakagami, M. Mori, A. Suzuki, and Y. Hirota. 1987. Conversion of the α component of penicillin-binding protein 1b to the β component in Escherichia coli. J. Bacteriol. 169:891-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Suzuki, H., Y. Nishimura, and Y. Hirota. 1978. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc. Natl. Acad. Sci. USA 75:664-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Templin, M. F., A. Ursinus, and J. V. Höltje. 1999. A defect in cell wall recycling triggers autolysis during the stationary growth phase of Escherichia coli. EMBO J. 18:4108-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Terrak, M., T. K. Gosh, J. van Heijenoort, J. Van Beeumen, M. Lampilas, J. Aszodi, J. A. Ayala, J. M. Ghuysen, and M. Nguyen-Distèche. 1999. The catalytic, glycosyltransferase and acyltransferase modules of the cell wall peptidoglycan-polymerizing penicillin-binding protein 1b of Escherichia coli. Mol. Microbiol. 34:350-364. [DOI] [PubMed] [Google Scholar]
- 223.Tettelin, H., et al. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 224.Tipper, D. J., and J. L. Strominger. 1965. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-d-alanyl-d-alanine. Proc. Natl. Acad. Sci. USA 54:1133-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tormo, A., M. Almirón, and R. Kolter. 1990. surA, an Escherichia coli gene essential for survival in stationary phase. J. Bacteriol. 172:4339-4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tsuruoka, T., A. Tamura, A. Miyata, T. Takei, S. Inouye, and M. Matsuhashi. 1985. Second lytic target of β-lactam compounds that have a terminal d-amino acid residue. Eur. J. Biochem. 151:209-216. [DOI] [PubMed] [Google Scholar]
- 227.Tuomanen, E., and R. Cozens. 1987. Changes in peptidoglycan composition and penicillin-binding proteins in slowly growing Escherichia coli. J. Bacteriol. 169:5308-5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ursinus, A., H. Steinhaus, and J. V. Höltje. 1992. Purification of a nocardicin A-sensitive ld-carboxypeptidase from Escherichia coli by affinity chromatography. J. Bacteriol. 174:441-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.van den Ent, F., L. A. Amos, and J. Löwe. 2001. Prokaryotic origin of the actin cytoskeleton. Nature 413:39-41. [DOI] [PubMed] [Google Scholar]
- 230.Van der Linden, M. P. G., L. Hoan, M. A. Hoyer, and W. Keck. 1992. Possible role of Escherichia coli penicillin-binding protein 6 in stabilization of stationary-phase peptidoglycan. J. Bacteriol. 174:7572-7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.van Heijenoort, J. 1996. Murein synthesis, p. 1025-1034. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 232.van Heijenoort, J. 2001. Formation of the glycan chains in the synthesis of bacterial peptidoglycan. Glycobiology 11:25R-36R. [DOI] [PubMed] [Google Scholar]
- 233.van Heijenoort, Y., M. Gómez, M. Derrien, J. Ayala, and J. van Heijenoort. 1992. Membrane intermediates in the peptidoglycan metabolism of Escherichia coli: possible roles of PBP1b and PBP3. J. Bacteriol. 174:3549-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.van Heijenoort, J., and L. Gutmann. 2000. Correlation between the structure of the bacterial peptidoglycan monomer unit, the specificity of transpeptidation, and susceptibility to β-lactams. Proc. Natl. Acad. Sci. USA 97:5028-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.van Heijenoort, Y., and J. van Heijenoort. 1980. Biosynthesis of the peptidoglycan of Escherichia coli K-12: properties of the in vitro polymerization by transglycosylation. FEBS Lett. 110:241-244. [DOI] [PubMed] [Google Scholar]
- 236.via, L. E., D. Deretic, R. J. Ulmer, N. S. Hibler, L. A. Huber, and V. Deretic. 1997. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272:13326-13331. [DOI] [PubMed] [Google Scholar]
- 237.Vicente, M., and J. Errington. 1996. Structure, function and controls in microbial division. Mol. Microbiol. 20:1-7. [DOI] [PubMed] [Google Scholar]
- 238.Vinella, D., R. D'Ari, and P. Bouloc. 1992. Penicillin binding protein 2 is dispensible in Escherichia coli when ppGpp synthesis is induced. EMBO J. 11:1493-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Vocadlo, D. J., G. J. Davies, R. Laine, and S. G. Withers. 2001. Catalysis by hen egg-white lysozyme proceeds via a covalent intermediate. Nature 412:835-838. [DOI] [PubMed] [Google Scholar]
- 240.Voladri, R. K., D. L. Lakey, S. H. Hennigan, B. E. Menzies, K. M. Edwards, and D. S. Kernodle. 1998. Recombinant expression and characterization of the major β-lactamase of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 42:1375-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wachi, M., M. Doi, Y. Okada, and M. Matsuhashi. 1989. New mre genes mreC and mreD, responsible for formation of the rod shape of Escherichia coli cells. J. Bacteriol. 171:6511-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Weber, B., K. Ehlert, A. Diehl, P. Reichmann, H. Labischinski, and R. Hakenbeck. 2000. The fib locus in Streptococcus pneumoniae is required for peptidoglycan crosslinking and PBP-mediated β-lactam resistance. FEMS Microbiol. Lett. 188:81-85. [DOI] [PubMed] [Google Scholar]
- 243.Weiss, D. S., J. C. Chen, J. M. Ghigo, D. Boyd, and J. Beckwith. 1999. Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J. Bacteriol. 181:508-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wietzerbin, J., B. C. Das, J. F. Petit, E. Lederer, M. Leyh-Bouille, and J. M. Ghuysen. 1974. Occurrence of d-alanyl-(d)-meso-diaminopimelic acid and meso-diaminopimelyl-meso-diaminopimelic acid interpeptide linkages in the peptidoglycan of Mycobacteria. Biochemistry 13:3471-3476. [DOI] [PubMed] [Google Scholar]
- 245.Wright, G. D., C. Molinas, M. Arthur, P. Courvalin, and C. T. Walsh. 1992. Characterization of VanY, a dd-carboxypeptidase from vancomycin-resistant Enterococcus faecium BM4147. Antimicrob. Agents Chemother. 36:1514-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wu, C. Y. E., W. E. Alborn, Jr., J. E. Flokowitsch, J. Hoskins, S. Ünal, L. C. Blaszczak, D. A. Preston, and P. L. Skatrud. 1994. Site-directed mutagenesis of the mecA gene from a methicillin-resistant strain of Staphylococcus aureus. J. Bacteriol. 176:443-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wu, S. W., H. de Lencastre, and A. Tomasz. 2001. Recruitment of the mecA gene homologue of Staphylococcus sciuri into a resistance determinant and expression of the resistant phenotype in Staphylococcus aureus. J. Bacteriol. 183:2417-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wu, S., C. Piscitelli, H. de Lencastre, and A. Tomasz. 1996. Tracking the evolutionary origin of the methicillin resistance gene: cloning and sequencing of a homologue of mecA from a methicillin susceptible strain of Staphylococcus sciuri. Drug Resist. 2:435-441. [DOI] [PubMed] [Google Scholar]
- 249.Yanouri, A., R. A. Daniel, J. Errington, and C. E. Buchanan. 1993. Cloning and sequencing of the cell division gene pbpB, which encodes penicillin-binding protein 2B in Bacillus subtilis. J. Bacteriol. 175:7604-7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Young, D. B., and K. Duncan. 1995. Prospects for new interventions in the treatment and prevention of mycobacterial disease. Annu. Rev. Microbiol. 49:641-673. [DOI] [PubMed] [Google Scholar]
- 251.Young, K. D. 2001. Approaching the physiological functions of penicillin-binding proteins in Escherichia coli. Biochimie 83:99-102. [DOI] [PubMed] [Google Scholar]
- 252.Yousif, S. Y., J. K. Broome-Smith, and B. G. Spratt. 1985. Lysis of Escherichia coli by β-lactam antibiotics: deletion analysis of the role of penicillin-binding proteins 1A and 1B. J. Gen. Microbiol. 131:2839-2845. [DOI] [PubMed] [Google Scholar]
- 253.Zambrano, M. M., D. A. Siegele, M. Almirón, A. Tormo, and R. Kolter. 1993. Microbial competition: Escherichia coli mutants that take over stationary-phase cultures. Science 259:1757-1759. [DOI] [PubMed] [Google Scholar]
- 254.Zhang, H. Z., C. J. Hackbarth, K. M. Chansky, and H. F. Chambers. 2001. A proteolytic transmembrane signaling pathway and resistance to β-lactams in staphylococci. Science 291:1962-1965. [DOI] [PubMed] [Google Scholar]
- 255.Zhang, Q. Y., and B. G. Spratt. 1989. Nucleotide sequence of the penicillin-binding protein 2 gene of Neisseria meningitidis. Nucleic Acids Res. 17:5383.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhu, J., K. Jäger, T. Black, K. Zarka, O. Koksharova, and C. P. Wolk. 2001. HcwA, an autolysin, is required for heterocyst maturation in Anabaena spp. strain PCC7120. J. Bacteriol. 183:6841-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zighelboim, S., and A. Tomasz. 1980. Penicillin-binding proteins of multiply antibiotic-resistant South African strains of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 17:434-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zimmer, D. P., E. Soupene, H. L. Lee, V. F. Wendisch, A. B. Khodursky, B. J. Peter, R. A. Bender, and S. Kustu. 2000. Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proc. Natl. Acad. Sci. USA 97:14674-14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zorzi, W., X. Y. Zhou, O. Dardenne, J. Lamotte, D. Raze, J. Pierre, L. Gutmann, and J. Coyette. 1996. Structure of the low-affinity penicillin-binding protein 5 PBP5fm in wild-type and highly penicillin-resistant strains of Enterococcus faecium. J. Bacteriol. 178:4948-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]