Abstract
Mycobacterium tuberculosis has evolved to persist in host macrophages, where it faces a nutrient-poor environment and is exposed to oxidative and nitrosative stress. To defend itself against oxidative/nitrosative stress, M. tuberculosis expresses an NADH-dependent peroxidase and peroxynitrite reductase that is encoded by ahpC, ahpD, lpd, and dlaT. In addition to its central role in the peroxynitrite reductase complex, dlaT (Rv2215) also encodes the E2 component of pyruvate dehydrogenase. Here we demonstrate that inactivation of dlaT in the chromosome of H37Rv resulted in a mutant (H37RvΔdlaT) that displayed phenotypes associated with DlaT's role in metabolism and in defense against nitrosative stress. The H37RvΔdlaT strain showed retarded growth in vitro and was highly susceptible to killing by acidified sodium nitrite. Mouse macrophages readily killed intracellular H37RvΔdlaT organisms, and in mice dlaT was required for full virulence.
Mycobacterium tuberculosis is a major global health threat. Nearly 2 billion people have been infected with M. tuberculosis, and ∼10% of these individuals are predicted to develop active disease at some time in their lives (7). During infection, M. tuberculosis is phagocytosed by alveolar macrophages in the lungs. The bacteria either grow to cause primary tuberculosis or enter a state of latency in which they can persist, sometimes for decades, within the host. The mechanisms that allow M. tuberculosis to survive and persist in the face of a strong host immune response remain largely unidentified.
There is considerable evidence that the production of reactive nitrogen intermediates (RNI) by macrophages is important in the generation of effective immunity against mycobacteria (3, 14, 18). In vitro, RNI are mycobacteriocidal (6, 13), and gamma interferon (IFN-γ)-activated mouse macrophages can kill M. tuberculosis in an inducible nitric oxide synthase (iNOS)-dependent manner (8). Mice deficient in iNOS display markedly enhanced susceptibility to M. tuberculosis (14). In patients with tuberculosis, the expression of iNOS and nitrotyrosine has been demonstrated in the lungs, indicating NO production (4, 22).
The ability of M. tuberculosis to resist the toxicity of RNI has been suggested by several studies. The expression of M. tuberculosis noxR1 in Escherichia coli and Mycobacterium smegmatis conferred resistance to RNI during in vitro culture and during ex vivo growth in macrophages (9); however, an M. tuberculosis noxR1 deletion strain did not show decreased virulence in mice (23). M. tuberculosis noxR3 protected Salmonella enterica serovar Typhimurium from the NO donor S-nitrosoglutathione, acidified nitrite, and hydrogen peroxide (19), but its role during in vivo infection has not been addressed. M. tuberculosis encodes three annotated NO dioxygenase homologues (Hmp, GlbN, and GlbO). Hmp is induced in response to RNI stress (12), but NO dioxygenase activity has not been reported for Hmp. In contrast, the truncated hemoglobins GlbN and GlbO possess NO dioxygenase activity in vitro and have been proposed to protect M. tuberculosis from NO (5, 17). Their role during M. tuberculosis infection in vivo has not been evaluated. The M. tuberculosis peptide methionine sulfoxide reductase (MsrA) catalyzes the reduction of methionine sulfoxide and may be involved in the repair of peroxynitrite-mediated intracellular damage (24).
M. tuberculosis also encodes a four-protein NADH-dependent peroxidase and peroxynitrite reductase (2). In this system, alkyl hydroperoxide reductase subunit C (AhpC) catalyzes the NADH-driven reduction of hydroperoxide and peroxynitrite. Oxidized AhpC is reduced by AhpD, which is regenerated by dihydrolipoamide acyltransferase (DlaT) via reduction of AhpD at its active-site cysteines. Finally, dihydrolipoamide dehydrogenase (Lpd) mediates the reduction of DlaT and completes the catalytic cycle. dlaT (Rv2215) also encodes the E2 component of pyruvate dehydrogenase (PDH) (24a). PDH catalyzes the oxidation of pyruvate by NAD to acetyl-coenzyme A (acetyl-CoA) and CO2. Acetyl-CoA then feeds into the tricarboxylic acid (TCA) cycle. Thus, previous work suggested that DlaT is not only part of M. tuberculosis's antioxidant defense but also critical for its intermediary metabolism. To test this hypothesis, we generated a dlaT knockout strain and tested its ability to grow, resist nitrosative stress, and cause disease in mice. We demonstrate that dlaT is required for optimal growth of M. tuberculosis and for resistance against RNI in vitro. Moreover, the M. tuberculosis ΔdlaT strain is readily killed by murine macrophages and attenuated for virulence in the mouse.
MATERIALS AND METHODS
Mycobacteria and culture conditions.
Wild-type M. tuberculosis H37Rv and derivative strains were grown at 37°C in Middlebrook 7H9 broth containing 0.2% glycerol, 0.5% bovine serum albumin, 0.05% Tween 80, 0.2% dextrose, and 0.085% sodium chloride (7H9-ADNaCl) or on Middlebrook 7H11 agar containing 10% oleic acid-albumin-dextrose-catalase (7H11-OADC). The antibiotics used were 50 μg/ml hygromycin for the H37RvΔdlaT strain and 50 μg/ml hygromycin plus 30 μg/ml kanamycin for the H37RvΔdlaT strain transformed with pMV306-dlaT.
Construction and complementation of M. tuberculosis H37RvΔdlaT mutant.
The H37RvΔdlaT strain was constructed by single-step homologous recombination using the specialized transducing phage phae87 (1, 11). Flanking regions of dlaT were amplified by PCR from M. tuberculosis genomic DNA. Primers dlaT-speI-F (5′-TCGGCGACTAGTGCCATGGTGTAGGCCGAAATGGGTT-3′) and dlaT-hindIII-R (5′-GGCCATAAGCTTTGACTCCTCGATCGATCGTCGGTCG-3′ [the complement of the dlaT start codon is underlined]) were used to amplify 500 bp upstream of the start codon. Primers dlaT-kpnI-F (5′-GGCCGAGGTACCACTGTGATGGCCAACGCCGTTGTCG-3′ [the stop codon of dlaT is underlined]) and dlaT-do-R (5′-CATGCCTCTAGACGGAGACAGCACCACTCCGGTCCGG-3′) were used to amplify 508 bp downstream of the stop codon. Both fragments were cloned into plasmid pjsc284 (SpeI and HindIII were used for the upstream fragment, and KpnI and StuI were used for the downstream fragment) to flank the hygromycin cassette and then were sequenced. This plasmid, containing the knockout construct, a lambda cos site, and a single PacI site, was then digested with PacI and packaged into the unique PacI site of the temperature-sensitive phage phae87. The phage was then amplified in M. smegmatis at 30°C and used to infect M. tuberculosis as previously described (11). Southern blotting was used to screen hygromycin-resistant colonies for allelic exchange of the dlaT gene with the hygromycin cassette as a result of homologous recombination. Southern blotting was performed with an Amersham ECL direct nucleic acid labeling and detection system according to the manufacturer's guide.
For complementation, the H37RvΔdlaT strain was transformed with the plasmid pMV306, containing the M. tuberculosis dlaT gene and a 264-bp upstream DNA sequence which may contain the promoter of the gene (pMV306dlaT). The H37RvΔdlaT strain was grown to mid-log phase, collected by centrifugation, washed three times with 10% glycerol, and transformed by electroporation with pMV306-dlaT or the pMV306 vector. Transformants were selected on 7H11-OADC containing 50 μg/ml hygromycin plus 30 μg/ml kanamycin. Complementation of the H37RvΔdlaT strain was confirmed by Western blotting using an anti-DlaT antibody and an anti-lipoic acid antibody (2).
Southern blotting.
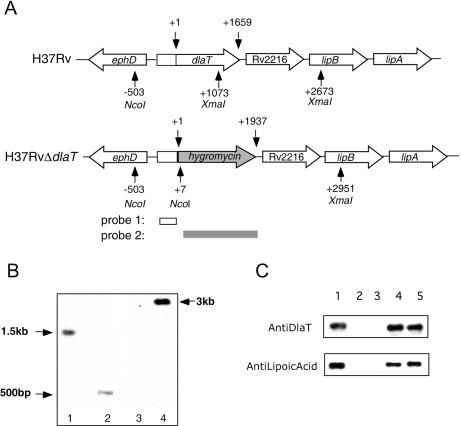
Genomic DNAs were prepared from wild-type H37Rv and one hygromycin-resistant transductant, digested with NcoI and XmaI, separated by agarose gel electrophoresis, and transferred to nylon membranes. The membrane was probed with a 500-bp fragment containing dlaT 5′ flanking sequences, revealing a 1.5-kbp fragment for wild-type M. tuberculosis and a 500-bp fragment for the H37RvΔdlaT mutant. The membrane was reprobed with a 1.93-kbp fragment containing the hyg cassette, revealing a 3-kbp fragment for the H37RvΔdlaT mutant and no hybridization product for wild-type H37Rv.
Preparation of protein lysates and analysis by Western blotting.
Wild-type H37Rv, the H37RvΔdlaT mutant, and the H37RvΔdlaT mutant transformed with pMV306-dlaT were grown to log phase. Bacteria were lysed by bead beating in a buffer containing 25 mM Tris-Cl, 1 mM EDTA, 5 mM MgCl2, 1 mM dithiothreitol, 100 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and 5 μg/ml (each) of leupeptin, aprotinin, and pepstatin. Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a 0.2-μm-pore-size nitrocellulose membrane (Schleicher & Schuell). The membrane was blotted with an anti-DlaT antibody (a kind gift of R. Bryk) and an anti-lipoic acid antibody (a kind gift of L. Szweda) (2).
Analysis of gene expression by quantitative real-time RT-PCR.
The expression of dlaT, Rv2216, lipA, and lipB was examined by quantitative real-time reverse transcription-PCR (RT-PCR). The major housekeeping sigma factor gene sigA was used as an internal control for normalization. Taqman probes for Rv2216, lipA, and lipB were synthesized by Biosearch Technologies (Novato, CA) and labeled with the reporter dye 6-carboxyfluorescein (FAM) at the 5′ end and the black hole quencher (BHQ) at the 3′ end. Molecular beacons (Eurogentec, Seraing, Belgium) for dlaT and sigA were synthesized with a 5′ FAM group and a 3′ [4-(4-dimethylaminophenylazo)benzoic acid] succinimidyl ester group as described online (www.molecular-beacons.org). One hundred nanograms of RNA was transcribed into cDNA at 42°C for 30 min with random hexamers in 20 μl, using 50 U murine leukemia virus reverse transcriptase (Perkin-Elmer, Boston, MA). The cDNA was diluted to 100 μl. For each PCR, 5 μl cDNA was used. PCR was performed in a volume of 15 μl on an ABI PRISM 7900HT sequence detection system (Perkin-Elmer, Boston, MA). The cycling conditions were as follows: 95°C for 5 min, followed by 40 cycles of 95°C for 15 s and 60°C for 30 s.
The primers, probes, and beacons used were as follows: Rv2216 forward primer, 5′-GCGAATTCCGAAGAACTGCA-3′; reverse primer, 5′-CGCGTGCGGATCGAA-3′; Taqman probe, 5′-FAM-TGGAATCCCGAAAGCGGCGA-BHQ-3′; lipA forward primer, 5′-TCGAACTGCTGATTCCCGAC-3′; reverse primer, 5′-GACTCGAAGACCTCGGCCA-3′; Taqman probe, 5′-FAM-TCAACGGCGAACCAACCCGG-BHQ-3′; lipB forward primer, 5′-CTGCGATTGTGATTTGGCTG-3′; reverse primer, 5′-ACTGCGGCGTCACTGATTC-3′; Taqman probe, 5′-FAM-TCACCGCCATCGTGCCATGC-BHQ-3′; dlaT forward primer, 5′-CCTGACCTTCCTGCCGTTCT-3′; reverse primer, 5′-GCTCGGCGTCGTAGTAGGTG-3′; beacon, 5′-FAM-GCAGCCACATCAACGCTAGCTACAACGAGGGGCTGC-3′; sigA forward primer, 5′-TGCAGTCGGTGCTGGACAC-3′; reverse primer, 5′-CGCGCAGGACCTGTGAGCGG-3′; and beacon, 5′-FAM-CCTCGCGTCGAAGTTGCGCCATCCGAGCGAGG-3′.
Susceptibility of M. tuberculosis strains to in vitro stresses.
To test susceptibility to nitrosative stress, wild-type M. tuberculosis, the H37RvΔdlaT mutant, and the H37RvΔdlaT mutant transformed with pMV306-dlaT were grown to log phase. Cultures were washed with 7H9 medium that had been acidified to pH 5.5 with HCl and centrifuged at 800 rpm for 10 min at room temperature. The supernatants were diluted in 7H9-ADNaCl (pH 6.8) and acidified 7H9-ADNaCl (pH 5.5) that did or did not contain 1.5 mM NaNO2. Cultures were incubated at 37°C. After 4 days, serial dilutions of each culture were plated on 7H11 agar. To test susceptibility to heat and isoniazid (INH), the bacteria were prepared as described above and exposed to 45°C or 60 ng/ml INH in 7H9-ADNaCl (pH 6.8) for 24 h, and then serial dilutions of each culture were plated on 7H11 agar.
Macrophage infection.
Bone marrow cells were flushed from femurs of C57BL/6 mice and iNOS−/− mice (14), differentiated into macrophages in the presence of 20% L-929 fibroblast conditioned medium, and infected with M. tuberculosis from log-phase cultures of wild-type H37Rv, the H37RvΔdlaT strain, and the H37RvΔdlaT strain transformed with pMV306-dlaT at a multiplicity of infection (MOI) of 5. Intracellular survival of M. tuberculosis was assessed as reported previously (8).
Mouse infections and quantification of viable M. tuberculosis in mouse organs.
Mice were infected with logarithmic-phase cultures of M. tuberculosis by the aerosol route, using a Glas-Col inhalation exposure system (Glas-Col Inc., Terre Haute, IN). Animals were exposed for 40 min to an aerosol produced by nebulizing 5 ml of a bacterial suspension in phosphate-buffered saline at a concentration of ∼2 × 107 bacilli/ml. This resulted in an inoculum size of 100 CFU per lung, as determined by plating homogenized lungs onto enriched 7H11 plates at 24 h postinfection.
Mice were sacrificed by inhalation of CO2 under noncrowded conditions. Lungs, spleens, and livers were aseptically removed and homogenized in 4 ml phosphate-buffered saline containing 0.05% Tween 80. On day 1 postinfection, undiluted lung homogenates were plated on 7H11-OADC plates. Plates were incubated at 37°C, and CFU were enumerated 14 to 21 days later. Thereafter, at the indicated time points, mice were sacrificed, their organs were aseptically removed and homogenized, and serial dilutions were plated on 7H11-OADC plates to determine the CFU.
RESULTS
Construction and in vitro characterization of H37RvΔdlaT mutant.
A dlaT (Rv2215) knockout mutant in M. tuberculosis strain H37Rv was generated by single-step homologous recombination, and replacement of dlaT by a hygromycin cassette was confirmed by Southern blotting (Fig. 1A). The loss of DlaT protein expression in the H37RvΔdlaT strain was demonstrated by Western blotting with an anti-DlaT antibody (2) (Fig. 1B). Furthermore, complementation of the H37RvΔdlaT strain with a plasmid containing the dlaT gene (pMV306-dlaT) resulted in DlaT expression, as demonstrated by Western blotting. Lipoylation is necessary for the activity of DlaT (2). Therefore, protein lysates from wild-type H37Rv and the H37RvΔdlaT strain complemented with pMV306-dlaT were analyzed by Western blotting with an anti-lipoic acid antibody. Lipoylated DlaT protein was detected in both the wild-type strain and the H37RvΔdlaT strain transformed with pMV306-dlaT (Fig. 1B). No lipoylated protein was detected in the H37RvΔdlaT strain, confirming that DlaT is the only lipoylated protein in M. tuberculosis detected with this antibody (2). Thus, these data suggest that DlaT in the complemented strain was equipped to be functional.
FIG. 1.
Construction and in vitro characterization of H37RvΔdlaT mutant. (A) Genomic context of dlaT and schematic organization of dlaT knockout. The hybridization sites of the two probes used for Southern blot analysis are shown, and the positions of NcoI and XmaI restriction sites are indicated. Restriction of chromosomal DNA with NcoI and XmaI resulted in a 1.5-kbp fragment for wild-type H37Rv and a 500-bp fragment for the H37RvΔdlaT mutant when probed with a 500-bp fragment containing the 5′ flanking sequences of the dlaT gene (probe 1). If the DNAs were probed with a 1.93-kbp fragment containing the hyg cassette (probe 2), a 3-kbp fragment was expected for the H37RvΔdlaT mutant. (B) Southern blot of genomic DNAs digested with NcoI and XmaI. The blot was probed with probe 1, revealing the expected 1.5-kbp fragment for wild-type M. tuberculosis (lane 1) and the 500-bp fragment for a hygromycin-resistant transductant in which dlaT was disrupted with the hyg marker (lane 2). The same blot was also probed with probe 2, revealing a 3-kbp fragment of H37RvΔdlaT DNA (lane 4) but no band for wild-type H37Rv DNA (lane 3). (C) Western blot of M. tuberculosis lysates with anti-DlaT antibody (1:10,000) and anti-lipoic acid antibody (1:10,000). Lane 1, wild-type H37Rv; lane 2, H37RvΔdlaT mutant; lane 3, H37RvΔdlaT mutant transformed with pMV306; lane 4, H37RvΔdlaT mutant transformed with pMV306-dlaT; lane 5, recombinant DlaT protein (50 ng).
Retarded growth of H37RvΔdlaT mutant in vitro and susceptibility to nitrosative stress.
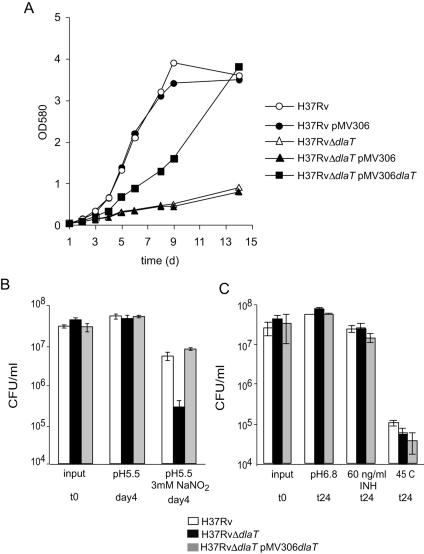
Work by Sassetti et al. suggested that dlaT is essential for optimal growth of M. tuberculosis in vitro (20). Consistent with this, the H37RvΔdlaT mutant showed severely retarded growth in vitro (Fig. 2A). This growth phenotype was partially reversed in the complemented strain. We hypothesized that the deletion of dlaT may have caused polar effects on Rv2216, lipB, and lipA, which are located downstream of dlaT on the M. tuberculosis chromosome (Fig. 1A). We tested the expression of these genes in wild-type H37Rv, the H37RvΔdlaT mutant, and the H37RvΔdlaT mutant transformed with pMV306-dlaT by quantitative real-time RT-PCR (Table 1). The expression of lipB and lipA was not affected in the H37RvΔdlaT mutant; however, the expression of Rv2216 was reduced eight- and fivefold in the H37RvΔdlaT mutant and the H37RvΔdlaT strain complemented with pMV306-dlaT, respectively. As expected, dlaT was not expressed in the H37RvΔdlaT strain, whereas expression levels were similar in the wild type and the complemented mutant. These data suggest that reduced expression of Rv2216 caused the growth defect of the H37RvΔdlaT strain complemented with pMV306-dlaT.
FIG. 2.
Retarded growth of H37RvΔdlaT mutant in vitro and susceptibility to nitrosative stress. (A) Growth of wild-type H37Rv (open circles), H37Rv transformed with pMV306 (filled circles), the H37RvΔdlaT mutant (open triangles), the H37RvΔdlaT mutant transformed with pMV306 (filled triangles), and the H37RvΔdlaT mutant transformed with pMV306-dlaT (filled squares) in liquid medium. Results are representative of three experiments. (B) Survival of H37Rv (white bars), the H37RvΔdlaT mutant (black bars), and the H37RvΔdlaT mutant transformed with pMV306-dlaT (gray bars) in liquid medium at pH 5.5 and liquid medium at pH 5.5 containing 3 mM NaNO2. Data are averages of two experiments, both performed in triplicates, and error bars represent standard errors. (C) Resistance of H37Rv (white bars), the H37RvΔdlaT mutant (black bars), and the H37RvΔdlaT mutant transformed with pMV306-dlaT (gray bars) to heat (45°C) and INH (60 ng/ml). Data are averages of triplicates, and error bars represent standard deviations (SDs).
TABLE 1.
Expression of dlaT, Rv2216, lipA, and lipB in wild-type and mutant M. tuberculosis strains
| M. tuberculosis strain | Gene expression relative to sigAa
|
|||
|---|---|---|---|---|
| dlaT | Rv2216 | lipA | lipB | |
| H37Rv | 0.21 ± 0.04 | 0.41 ± 0.05 | 1.95 ± 0.26 | 0.31 ± 0.05 |
| H37Rv ΔdlaT mutant | Not detected | 0.05 ± 0.01b | 2.61 ± 0.29 | 0.48 ± 0.06 |
| H37Rv ΔdlaT mutant complemented with pMV306dlaT | 0.31 ± 0.07 | 0.08 ± 0.01b | 2.65 ± 0.37 | 0.38 ± 0.06 |
RNAs were assayed by quantitative real-time RT-PCR with early-log-phase cultures of M. tuberculosis strains. Gene expression is reported as the copy number divided by the copy number of sigA mRNA. Data are means ± standard errors from six replicates derived from two independent experiments.
Significantly different (P < 0.0001) from value for H37Rv, as calculated by two-tailed, unpaired Student's t test. The expression of lipA and lipB in the H37RvΔdlaT mutant and the H37RvΔdlaT mutant complemented with pMV306dlaT was not significantly different from that in H37Rv.
To address the hypothesis that DlaT is required for resistance to nitrosative stress, wild-type and mutant bacteria were exposed to pH 5.5, with or without 3 mM NaNO2, for 4 days and then plated on agar to assess killing. In control cultures with no nitrite, the mutant survived as well as the wild-type strain after exposure to pH 5.5. In contrast, after exposure to nitrite, there was 20-fold more killing of the mutant than of the wild-type strain (Fig. 2B). The complemented strain showed as much resistance to nitrite as the wild-type strain. To test if DlaT protects specifically against nitrosative stress or if it also protects against other forms of stress, H37Rv, the H37RvΔdlaT mutant, and the complemented mutant were exposed to heat and to treatment with the antituberculous drug INH (Fig. 2C). As expected, the H37RvΔdlaT mutant was not more susceptible than wild-type H37Rv to exposure to heat or treatment with INH. These data demonstrate that DlaT protects M. tuberculosis against RNI but has no role in resistance to heat or INH.
Survival of H37RvΔdlaT mutant in resting and activated macrophages in vitro.
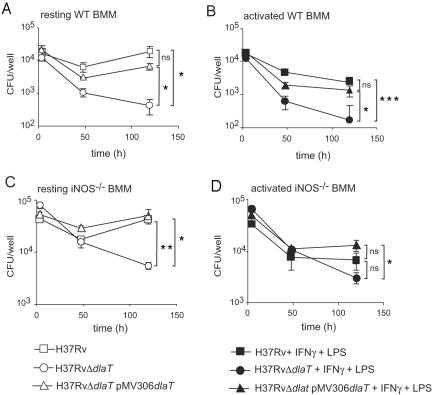
Activated macrophages can kill intracellular M. tuberculosis via the production of RNI and via other iNOS-independent defense mechanisms, such as LRG-47-controlled phagosome maturation (3, 14, 15). To test whether dlaT is required for M. tuberculosis to infect and replicate in macrophages, bone marrow-derived macrophages were prepared from wild-type C57BL/6 and iNOS−/− mice and infected with wild-type H37Rv, the H37RvΔdlaT strain, or the H37RvΔdlaT strain complemented with pMV306-dlaT at an MOI of 5. Unlike wild-type M. tuberculosis, which persisted in resting macrophages, the H37RvΔdlaT mutant was killed (Fig. 3A). Even iNOS−/− macrophages killed the H37RvΔdlaT mutant, suggesting that the susceptibility of the H37RvΔdlaT mutant to resting macrophages cannot be ascribed to NO (Fig. 3C). Thus, the disruption of dlaT sensitized M. tuberculosis to more macrophage-associated stresses than those that are dependent on macrophage activation or on iNOS. This was also observed with IFN-γ-activated macrophages (both wild-type and iNOS−/−), which killed the H37RvΔdlaT mutant more efficiently than wild-type H37Rv. Complementation of the H37RvΔdlaT mutant with dlaT rescued the mutant from killing by macrophages (Fig. 3A and B, C). These data demonstrate that dlaT is required for M. tuberculosis to survive in macrophages in vitro.
FIG. 3.
Survival of H37RvΔdlaT mutant in resting and activated macrophages. Bone marrow-derived macrophages (BMM) from wild-type C57BL/6 mice (A and B) or iNOS−/− mice (C and D) were infected with wild-type H37Rv (squares), the H37RvΔdlaT mutant (circles), or the H37RvΔdlaT mutant transformed with pMV306-dlaT (triangles) at an MOI of 5 either directly (A and C) or after activating the cells with IFN-γ (100 U/ml) and lipopolysaccharide (10 ng/ml) for 24 h (B and D). Cells were washed 4 h after infection to remove non-cell-associated bacteria and cultured in complete Dulbecco's modified Eagle's medium. Infected cells were lysed at the indicated time points and plated on 7H11-OADC for CFU counts. Error bars indicate SDs. Results are representative of three experiments. Asterisks indicate that bacterial counts were significantly different between H37Rv and the H37RvΔdlaT mutant or between H37Rv and the H37RvΔdlaT mutant transformed with pMV306-dlaT, as calculated by two-tailed, unpaired Student's t test (*, P < 0.05; **, P < 0.005; ***, P < 0.001; NS, not significant).
Attenuation of H37RvΔdlaT mutant in C57BL/6 mice.
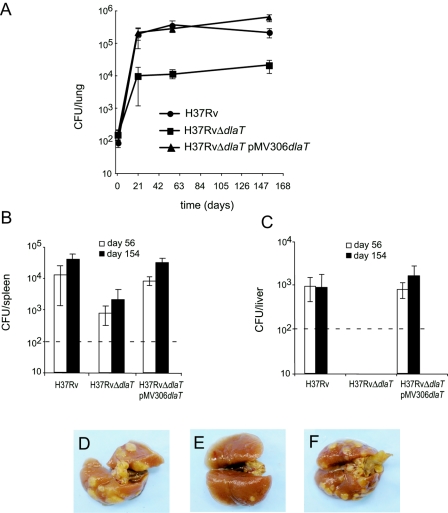
The impact of dlaT deficiency on the virulence of M. tuberculosis was assessed in a mouse model of tuberculosis. Wild-type C57BL/6 mice were infected by the aerosol route with 100 CFU per mouse, and bacterial loads in the organs were determined at selected time points. The H37RvΔdlaT mutant showed a 20-fold reduced viability in the lungs compared with wild-type M. tuberculosis at 3 weeks postinfection and persisted at this attenuated level for the following 19 weeks (Fig. 4A). Bacterial loads in the spleen and liver were examined at 8 weeks and 22 weeks postinfection. The H37RvΔdlaT mutant was attenuated 20-fold in spleens (Fig. 4B). No CFU were recovered from livers of mice infected with the H37RvΔdlaT mutant, but due to the detection limit of 100 CFU, the exact extent of attenuation in the liver was not determined (Fig. 4C). The reduced bacterial loads in mice infected with the H37RvΔdlaT mutant resulted in attenuated gross lung pathology. At 22 weeks postinfection, tuberculous nodules were distinct in lungs of H37Rv-infected mice but were hardly visible in lungs of mice infected with the H37RvΔdlaT mutant (Fig. 4D). The virulence of the H37RvΔdlaT mutant was fully restored by complementation with a plasmid containing the dlaT gene (Fig. 4A to E).
FIG. 4.
Attenuation of H37RvΔdlaT mutant in C57BL/6 mice. (A to C) Wild-type C57BL/6 mice were infected by the aerosol route with 100 CFU of wild-type H37Rv (squares), the H37RvΔdlaT mutant (circles), or the H37RvΔdlaT mutant transformed with pMV306-dlaT (triangles). At the indicated time points, four mice from each group were sacrificed, and bacteria were enumerated in the lungs (A), spleens (B), and livers (C). The detection limit was 100 CFU/organ (indicated by dashed lines). Error bars indicate SDs, and results are representative of two experiments. (D to G) Lung pathology 22 weeks after infection with wild-type H37Rv (D), the H37RvΔdlaT mutant (E), and the H37RvΔdlaT mutant transformed with pMV306-dlaT (F).
In summary, dlaT is important for optimal growth in vitro and confers resistance to nitrosative stress as well as to unidentified, iNOS-independent stresses in macrophages. Our results also demonstrate that dlaT is critical for M. tuberculosis to survive in primary mouse macrophages and to gain full virulence in vivo.
DISCUSSION
RNI produced by activated macrophages are potent antimicrobial agents that are able to damage DNA and lipids and to disrupt the activity of proteins containing Fe-S clusters, transition metals, hemes, thiols, or tyrosyl groups. However, M. tuberculosis can survive and replicate in macrophages. This is not only due to its ability to arrest the formation of phagolysosomes (10) but may also depend on its ability to detoxify host-derived RNI. In addition, M. tuberculosis needs to adapt metabolically to the intracellular environment, which was inferred to be nutrient poor (21).
Rv2215/dlaT was originally annotated “sucB” and thought to encode the dihydrolipoamide succinyltransferase (E2) component of α-ketoglutarate dehydrogenase. However, Tian et al. reported recently that M. tuberculosis lacks α-ketoglutarate dehydrogenase activity and that dlaT encodes dihydrolipoamide acyltransferase, which together with the pyruvate dehydrogenase E1 component (AceE) and dihydrolipoamide dehydrogenase (Lpd) constitutes PDH in M. tuberculosis (25). They demonstrated that purified AceE, DlaT, and Lpd could reconstitute PDH in vitro. Furthermore, PDH activity was lost in the H37RvΔdlaT strain. This deprives the bacteria of acetyl-CoA, which is central to the intermediary metabolism of M. tuberculosis. Accordingly, the H37RvΔdlaT mutant showed severely retarded growth when cultured in 7H9 medium with glucose and glycerol as carbon sources. This growth defect was partially complemented when the mutant was transformed with a plasmid expressing DlaT, demonstrating that the inactivation of dlaT inhibits in vitro growth. Quantitative real-time PCR analysis of genes located downstream of dlaT revealed reduced expression of Rv2216, a gene of unknown function, in mutant and complemented M. tuberculosis. The deletion of dlaT did not affect the expression of lipA and lipB, which are located downstream of Rv2216. Western blot analysis showed that DlaT in the complemented strain was lipoylated, which also suggests that the functions of the predicted lipoate biosynthesis proteins LipA and LipB were not affected. The expression of dlaT itself was similar in the wild-type and complemented strains, excluding the possibility that altered expression of DlaT may have caused dominant negative effects that affected the growth of the complemented mutant in vitro. These analyses therefore suggest that a polar effect on Rv2216 might be responsible for the in vitro growth defect of the complemented mutant. Importantly, a reduced expression of Rv2216 did not contribute to any of the other observed phenotypes of the dlaT mutant, because all other defects of the dlaT mutant were fully complemented in the H37RvΔdlaT strain transformed with pMV306-dlaT.
In addition to its central role in intermediary metabolism, DlaT supports M. tuberculosis's antioxidant defense by constituting an NADH-dependent mycobacterial peroxynitrite reductase, together with AhpC, AhpD, and Lpd (2). As a result, the H37RvΔdlaT mutant was very susceptible to nitrosative stress. When acidified sodium nitrite was present in the culture medium, the H37RvΔdlaT mutant was killed 20-fold more than wild-type M. tuberculosis. Furthermore, the H37RvΔdlaT mutant was more susceptible to killing by mouse macrophages. In contrast to wild-type M. tuberculosis, which persisted in resting macrophages and was only killed in IFN-γ-activated macrophages, the H37RvΔdlaT mutant was readily killed, even in resting macrophages. This suggests that the poor survival of the H37RvΔdlaT mutant in macrophages cannot just be ascribed to the toxic effects of macrophage-derived RNI. Accordingly, the absence of iNOS did not significantly impair the macrophage bactericidal activity against the H37RvΔdlaT mutant. Interestingly, IFN-γ-activated iNOS−/− macrophages also killed wild-type H37Rv. This is in contrast to previous results from this laboratory when we used a clinical isolate of M. tuberculosis, strain 1254 (8), and never observed iNOS-independent killing. However, with H37Rv we have seen IFN-γ-dependent, iNOS-independent killing of intracellular H37Rv several times, but not always, suggesting that iNOS-independent killing of M. tuberculosis may depend on the strain and the experimental conditions. So far, we have not been able to identify the experimental conditions that lead to activation of this killing mechanism.
We also demonstrated that dlaT is important for the full virulence of M. tuberculosis in a mouse model of tuberculosis. Growth of the H37RvΔdlaT mutant in mouse lungs, spleens, and livers was attenuated >10-fold compared to that of wild-type H37Rv. The H37RvΔdlaT strain transformed with pMV306-dlaT was as virulent as the wild-type strain, demonstrating that the absence of DlaT was solely responsible for the loss of virulence. The H37RvΔdlaT mutant was able to replicate in mouse lungs, although at a rate significantly lower than that of wild-type M. tuberculosis. Macrophages readily killed intracellular H37RvΔdlaT organisms in vitro; however, the mutant persisted in vivo. It is conceivable that during growth in vivo, M. tuberculosis has access to different energy and carbon sources than those available during growth in vitro. During the chronic phase of infection, M. tuberculosis switches its metabolism to use fatty acids as major carbon and energy sources (16, 26). In vivo, the H37RvΔdlaT mutant might use the glyoxylate shunt to allow anaplerotic maintenance of the TCA cycle and uptake of carbon by gluconeogenesis. Under these conditions, glycolysis is decreased, and thus PDH activity is not essential. This would also explain the ability of the H37RvΔdlaT mutant to persist in mouse lungs without a further loss of viability. In addition, other metabolic pathways or defense mechanisms may be induced in vivo which make M. tuberculosis less dependent on DlaT for metabolism and antioxidant defense.
Even though the M. tuberculosis H37RvΔdlaT strain was not eliminated in vivo, it was not able to cause significant pathology in infected mice. Targeting DlaT in combination with drugs that inhibit other pathways, for example, inhibition of the glyoxylate shunt, might be a successful approach to improve tuberculosis therapy.
Acknowledgments
This work was supported by a Cancer Research Institute predoctoral fellowship training grant (to S. Shi) and NIH grant HL68525 (to S. Ehrt). The Department of Microbiology and Immunology is supported by the William Randolph Hearst Foundation.
We thank Carl Nathan for many helpful suggestions and discussions, Carl Nathan and Dirk Schnappinger for critically reading the manuscript, William Jacobs for providing the mycobacteriophage phae87, Ruslana Bryk for providing recombinant DlaT and the anti-DlaT antibody, and L. Szweda for the gift of anti-lipoic acid antibody.
Editor: J. L. Flynn
REFERENCES
- 1.Bardarov, S., J. Kriakov, C. Carriere, S. Yu, C. Vaamonde, R. A. McAdam, B. R. Bloom, G. F. Hatfull, and W. R. Jacobs, Jr. 1997. Conditionally replicating mycobacteriophages: a system for transposon delivery to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 94:10961-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryk, R., C. D. Lima, H. Erdjument-Bromage, P. Tempst, and C. Nathan. 2002. Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science 295:1073-1077. [DOI] [PubMed] [Google Scholar]
- 3.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi, H. S., P. R. Rai, H. W. Chu, C. Cool, and E. D. Chan. 2002. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 166:178-186. [DOI] [PubMed] [Google Scholar]
- 5.Couture, M., S. R. Yeh, B. A. Wittenberg, J. B. Wittenberg, Y. Ouellet, D. L. Rousseau, and M. Guertin. 1999. A cooperative oxygen-binding hemoglobin from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 96:11223-11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darwin, K. H., S. Ehrt, J. C. Gutierrez-Ramos, N. Weich, and C. F. Nathan. 2003. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science 302:1963-1966. [DOI] [PubMed] [Google Scholar]
- 7.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 8.Ehrt, S., D. Schnappinger, S. Bekiranov, J. Drenkow, S. Shi, T. R. Gingeras, T. Gaasterland, G. Schoolnik, and C. Nathan. 2001. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J. Exp. Med. 194:1123-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrt, S., M. U. Shiloh, J. Ruan, M. Choi, S. Gunzburg, C. Nathan, Q. Xie, and L. W. Riley. 1997. A novel antioxidant gene from Mycobacterium tuberculosis. J. Exp. Med. 186:1885-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn, J. L., and J. Chan. 2003. Immune evasion by Mycobacterium tuberculosis: living with the enemy. Curr. Opin. Immunol. 15:450-455. [DOI] [PubMed] [Google Scholar]
- 11.Glickman, M. S., J. S. Cox, and W. R. Jacobs, Jr. 2000. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 5:717-727. [DOI] [PubMed] [Google Scholar]
- 12.Hu, Y., P. D. Butcher, J. A. Mangan, M. A. Rajandream, and A. R. Coates. 1999. Regulation of hmp gene transcription in Mycobacterium tuberculosis: effects of oxygen limitation and nitrosative and oxidative stress. J. Bacteriol. 181:3486-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long, R., B. Light, and J. A. Talbot. 1999. Mycobacteriocidal action of exogenous nitric oxide. Antimicrob. Agents Chemother. 43:403-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacMicking, J. D., R. J. North, R. LaCourse, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacMicking, J. D., G. A. Taylor, and J. D. McKinney. 2003. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science 302:654-659. [DOI] [PubMed] [Google Scholar]
- 16.McKinney, J. D., K. Honer zu Bentrup, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, Jr., and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735-738. [DOI] [PubMed] [Google Scholar]
- 17.Milani, M., A. Pesce, H. Ouellet, M. Guertin, and M. Bolognesi. 2003. Truncated hemoglobins and nitric oxide action. IUBMB Life 55:623-627. [DOI] [PubMed] [Google Scholar]
- 18.Nathan, C. F., and S. Ehrt. 2004. Nitric oxide in tuberculosis, p. 215-235. In W. N. Rom and S. M. Garay (ed.), Tuberculosis, 2nd ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 19.Ruan, J., G. St. John, S. Ehrt, L. Riley, and C. Nathan. 1999. noxR3, a novel gene from Mycobacterium tuberculosis, protects Salmonella typhimurium from nitrosative and oxidative stress. Infect. Immun. 67:3276-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sassetti, C. M., D. H. Boyd, and E. J. Rubin. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48:77-84. [DOI] [PubMed] [Google Scholar]
- 21.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schon, T., G. Elmberger, Y. Negesse, R. H. Pando, T. Sundqvist, and S. Britton. 2004. Local production of nitric oxide in patients with tuberculosis. Int. J. Tuberc. Lung Dis. 8:1134-1137. [PubMed] [Google Scholar]
- 23.Stewart, G. R., S. Ehrt, L. W. Riley, J. W. Dale, and J. McFadden. 2000. Deletion of the putative antioxidant noxR1 does not alter the virulence of Mycobacterium tuberculosis H37Rv. Tuber. Lung Dis. 80:237-242. [DOI] [PubMed] [Google Scholar]
- 24.St. John, G., N. Brot, J. Ruan, H. Erdjument-Bromage, P. Tempst, H. Weissbach, and C. Nathan. 2001. Peptide methionine sulfoxide reductase from Escherichia coli and Mycobacterium tuberculosis protects bacteria against oxidative damage from reactive nitrogen intermediates. Proc. Natl. Acad. Sci. USA 98:9901-9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Tian, J., R. Bryk, M. Itoh, M. Suematsu, and C. Nathan. 2005. Variant tricarboxylic acid cycle in Mycobacterium tuberculosis: identification of alpha-ketoglutarate decarboxylase. Proc. Natl. Acad. Sci. USA 102:10670-10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian, J., R. Bryk, S. Shi, H. Erdjument-Bromage, P. Tempst, and C. Nathan. 2005. Mycobacterium tuberculosis appears to lack alpha-ketoglutarate dehydrogenase and encodes pyruvate dehydrogenase in widely separated genes. Mol. Microbiol. 57:859-868. [DOI] [PubMed] [Google Scholar]
- 26.Timm, J., F. A. Post, L. G. Bekker, G. B. Walther, H. C. Wainwright, R. Manganelli, W. T. Chan, L. Tsenova, B. Gold, I. Smith, G. Kaplan, and J. D. McKinney. 2003. Differential expression of iron-, carbon-, and oxygen-responsive mycobacterial genes in the lungs of chronically infected mice and tuberculosis patients. Proc. Natl. Acad. Sci. USA 100:14321-14326. [DOI] [PMC free article] [PubMed] [Google Scholar]