Abstract
A Streptococcus suis surface protein reacting with convalescent-phase sera from pigs clinically infected by S. suis type 2 was identified. The apparent 110-kDa protein, designated Sao, exhibits typical features of membrane-anchored surface proteins of gram-positive bacteria, such as a signal sequence and an LPVTG membrane anchor motif. In spite of high identity with the partially sequenced genomes of S. suis Canadian strain 89/1591 and European strain P1/7, Sao does not share significant homology with other known sequences. However, a conserved avirulence domain that is often found in plant pathogens has been detected. Electron microscopy using an Sao-specific antiserum has confirmed the surface location of the Sao protein on S. suis. The Sao-specific antibody reacts with cell lysates of 28 of 33 S. suis serotypes and 25 of 26 serotype 2 isolates in immunoblots, suggesting its high conservation in S. suis species. The immunization of piglets with recombinant Sao elicits a significant humoral antibody response. However, the antibody response is not reflected in protection of pigs that are intratracheally challenged with a virulent strain in our conventional vaccination model.
Streptococcus suis is an important swine pathogen that causes many pathological conditions, such as arthritis, endocarditis, meningitis, pneumonia, and septicemia (19, 21). It is also an important zoonotic agent for humans in contact with colonized, otherwise healthy pigs or their by-products, causing meningitis and endocarditis (1, 53). Thirty-three serotypes (types 1 to 31, 33, and 1/2) based on capsular antigens are currently known (15-17, 22, 24, 43). Type 2 is considered the most virulent and prevalent type in diseased pigs. The mechanisms involved in the pathogenesis and virulence of S. suis are not completely understood (19), and attempts to control the infection are hampered by the lack of an effective vaccine.
Several approaches have been used to develop vaccines for S. suis. However, little success was achieved because the protection was either serotype or strain dependent, and results in most instances were equivocal (23, 42). For example, some protection with killed whole cells or live avirulent vaccines was reported, but this required repeated immunization, and the protection against heterologous challenges was not evaluated (25, 56). Exposure of young pigs to live virulent strains showed a positive effect in reducing clinical signs characteristics of S. suis infection (52). Since the S. suis capsule plays an important role in virulence, attempts have been made to develop a vaccine based on capsular material. However, this vaccination approach was unsatisfactory because the capsular polysaccharide is poorly immunogenic (9). More recently, interest has shifted toward protein antigens of S. suis as vaccine candidates. Subunit vaccines using suilysin (27) or muramidase-released protein and extracellular protein factor (57) have been shown to protect pigs from homologous and heterologous serotype 2 strains, but their use is hindered by the fact that a substantial number of virulent strains in some geographical regions do not express these proteins (13, 18, 41). Thus, the identification of other antigenic factors, especially surface proteins, would contribute to the development of a subunit vaccine.
In our continued effort to understand the pathogenic mechanism of S. suis and to search for a protein(s) that will be useful in the development of a vaccine, a new surface protein designated Sao (surface antigen one) was identified from a virulent strain of S. suis serotype 2. In this paper, we describe the new surface protein, which is expressed by a number of S. suis serotypes, and evaluate its immunogenicity and protective capacity using a vaccination and challenge trial in pigs.
MATERIALS AND METHODS
Bacterial strains, phage, plasmids, and media.
Reference strain S735 of S. suis serotype 2 was used for genomic library construction. Reference strains of 33 serotypes (types 1 to 31, 33, and 1/2), 26 field strains of serotype 2 from different origins, and five other gram-positive bacteria are listed in Table 1. Phage λZAPII vector and Escherichia coli XL1-Blue MRF′ were obtained from a commercial source (Stratagene, La Jolla, CA). S. suis was grown in Todd-Hewitt broth (Difco, Detroit, MI) or on agar plates (Quelab Laboratories, Montreal, Canada) at 37°C in 5% CO2, while other gram-positive bacteria were grown as recommended by the ATCC. E. coli was grown in either Luria-Bertani (LB) medium alone or LB medium supplemented with 2 g of maltose/liter at 37°C. Where appropriate, E. coli was grown in the presence of 50 μg of ampicillin/ml and 0.8 mM isopropyl-β-d-thiogalactopyranoside (IPTG). The pMal-p vector (New England BioLabs, Pickering, Ontario, Canada) was used to generate the maltose binding protein (MBP)-Sao fusion protein.
TABLE 1.
Distributions of Sao in S. suis reference strains, isolates of serotype 2, and other bacteria detected by the Sao-specific antibody R44 in Western blots
| Strain, serotype, or isolate no. | Origin | Presence of Sao |
|---|---|---|
| S. suis serotypes (reference strain) | ||
| 1 (5428) | The Netherlands | + |
| 1/2 (2651) | The Netherlands | + |
| 2 (NCTC 10234) | The Netherlands | + |
| 3 (4961) | Denmark | + |
| 4 (6407) | Denmark | + |
| 5 (11538) | Denmark | + |
| 6 (2524) | Denmark | + |
| 7 (8074) | Denmark | + |
| 8 (14636) | Denmark | + |
| 9 (22083) | Denmark | + |
| 10 (4417) | Denmark | + |
| 11 (12814) | Denmark | + |
| 12 (8830) | Denmark | + |
| 13 (10581) | Denmark | − |
| 14 (13730) | The Netherlands | + |
| 15 (NCTC 1046) | The Netherlands | + |
| 16 (2726) | Denmark | − |
| 17 (93A) | Canada | + |
| 18 (NT77) | Canada | + |
| 19 (42A) | Canada | + |
| 20 (86-5192) | USA | − |
| 21 (14A) | Canada | + |
| 22 (88-1861) | Canada | − |
| 23 (89-2479) | Canada | + |
| 24 (88-5299A) | Canada | − |
| 25 (89-3576-3) | Canada | + |
| 26 (89-4109-1) | Canada | + |
| 27 (89-5259) | Canada | + |
| 28 (89-590) | Canada | + |
| 29 (92-1191) | Canada | + |
| 30 (92-1400) | Canada | + |
| 31 (92-4172) | Canada | + |
| 33 (EA1832.92) | Canada | + |
| S. suis serotype 2 isolates | ||
| 89-999 | Canada | + |
| 90-1330 | Canada | + |
| 95-8242 | Canada | + |
| Man 25 | Canada | + |
| Man50 | Canada | + |
| Man63 | Canada | + |
| AAH4 | USA | + |
| AAH5 | USA | + |
| AAH6 | USA | + |
| 1309 | USA | + |
| 88-5955 | USA | + |
| 95-13626 | USA | + |
| 95-16426 | USA | + |
| 95-7220 | USA | + |
| 97-8506 | USA | + |
| SX-332 | USA | + |
| JL 590 | Mexico | + |
| 166 | France | + |
| 96-39247 | France | + |
| 96-49808 | France | + |
| 96-53405 | France | + |
| Italie 57 | Italy | + |
| Italie 68 | Italy | + |
| Italie 69 | Italy | − |
| Italie 228 | Italy | + |
| S735a | The Netherlands | + |
| Streptococcus strains | ||
| S. bovis | ATCC 9809 | − |
| S. equisimilis | ATCC 9542 | − |
| S. intestinalis | ATTC 43492 | − |
| S. pyogenes | ATCC 14289 | − |
| S. uberis | ATCC 6580 | − |
Strain used as a reference in this work.
Antisera.
Convalescent-phase swine sera were collected from pigs clinically infected with S. suis type 2 strain S735. Monospecific anti-Sao serum (R44) was obtained by immunizing New Zealand White rabbits intravenously with 230 μg of purified Sao emulsified with 0.5 ml of Freund's incomplete adjuvant. The rabbits received two booster injections with the same dose of Sao at 2-week intervals and then were bled 10 days after the last booster immunization. Sera were stored at −20°C until used.
Identification, cloning, and sequencing of the sao gene.
Chromosomal DNA from S. suis strain S735 was isolated as previously described (48). Purified chromosomal DNA was partially digested with the restriction enzyme EcoRI, and the resulting fragments were electrophoresed in a 1% agarose gel. Fragments in the 6- to 10-kb size range were extracted from the gel and ligated to the EcoRI arms of the λZAPII vector, and the vector was encapsidated using Gigapack II packaging extract (Stratagene). The recombinant phages were used to infect E. coli XL1-Blue MRF′, which was then plated onto LB agar. The resulting plaques were lifted onto nitrocellulose membranes (Bio-Rad, Mississauga, Ontario, Canada). The membranes were blocked with Tris-buffered saline containing 2% skim milk and sequentially incubated with convalescent-phase swine sera from S. suis serotype 2 infections, peroxidase-conjugated rabbit anti-swine immunoglobulin G (IgG) (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), and O-phenylenediamine. The positive plaques were purified to homogeneity. The sequence of the insert was determined using T3 and T7 promoters as primers in the DNA Sequencing Facility, University of Maine (Orono, ME). The nucleotide and amino acid sequences deduced from open reading frames (ORFs) were analyzed using programs available on the Internet.
The DNA fragment containing the gene encoding mature Sao was amplified from purified chromosomal DNA of strain S735 by PCR primers P1 (5′-ATGGATCCATTGAAGGCCGCTCGGCACAAGAAGTAAAA-3′) and P2 (5′-CCAAGTCGACTTATAATTTACGTTTACGTGTA-3′), which contained BamHI and SalI restriction sites, respectively. PCR was performed for 5 min at 94°C, followed by 30 cycles of 1 min at 94°C, 30 s at 56°C, and 1 min at 72°C. The resulting PCR fragment was cloned into the BamHI and SalI sites of the pMAL-p expression vector. The recombinant plasmid containing the sao gene was named pORF3.
Expression and purification of recombinant Sao protein.
The purified plasmid pORF3 was used to transform E. coli XL1-Blue by electroporation with a Genepulse II apparatus (Bio-Rad) following the manufacturer's recommendations. This recombinant strain was grown in LB medium plus glucose (2 g/liter) and ampicillin (50 μg/ml). For overexpression, the culture was inoculated from an overnight culture with the starting optical density at 600 nm adjusted to 0.1. The culture was incubated with agitation until the optical density at 600 nm was approximately 0.8, and then IPTG (0.8 mM) was added in order to induce production of the MBP-Sao fusion protein. After 2 h of induction, the fusion protein was found in the bacterial periplasm as well as in the cytoplasm. Bacterial lysates were used for purification of the Sao protein.
The fusion protein was purified by affinity chromatography using an amylose resin (New England Biolabs) following the manufacturer's instructions. The E. coli cell pellet was suspended in affinity column binding buffer (20 mM Tris-HCl, 50 mM NaCl, pH 7.4), and cells were lysed using a French pressure cell press (SLM Instruments, Inc., Urbana, IL). After filtration through a 0.45-μm membrane, the supernatant of the E. coli lysate was applied to the amylose resin. The MBP-Sao fusion protein was eluted with 1% maltose in binding buffer, and protein-containing fractions were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The purified fusion protein was cleaved with protease factor Xa (New England Biolabs) at a concentration of 20 μg/mg protein and then applied to a Mono-Q column (Amersham Pharmacia Biotech, Baie d'Urfee, Canada). The recombinant Sao protein devoid of its MBP carrier was eluted from the column by using a linear NaCl gradient (0 to 0.4 M NaCl in 20 mM Tris-HCl, pH 7.4). The Sao-containing fractions were combined and dialyzed against phosphate-buffered saline (PBS). The purity of recombinant Sao was evaluated by SDS-PAGE, and the concentration of the protein was determined with a Bio-Rad protein assay kit used according to the manufacturer's instructions. The NH2-terminal amino acid sequence of recombinant Sao was determined using automated Edman degradation as previously described (47).
SDS-PAGE and Western immunoblotting.
SDS-PAGE was performed as described by Laemmli (28). Cell lysates or purified protein was separated in a 10% acrylamide gel, and the gel was then stained with Coomassie brilliant blue R250 (Sigma, St. Louis, Mo.). Prestained low-molecular-mass markers (Bio-Rad) were used to determine the apparent molecular masses of proteins. Alternatively, Western blotting of proteins transferred to nitrocellulose membranes was performed essentially as described by Burnette (6).
Immunoelectron microscopy.
S. suis S735 was grown in 5 ml of Todd-Hewitt broth overnight, centrifuged, and resuspended in 500 μl of PBS (pH 8.0). Twenty microliters of the bacterial suspension was placed on nickel-Formvar grids (INRS, Institut Armand Frappier, Laval, Canada) and allowed to partially air dry. After being blocked for 30 min with 10% normal donkey serum in dilution buffer (PBS-1% bovine serum albumin-1% Tween 20, pH 8.0), the grids were soaked in 50 μl of Sao-specific rabbit serum or control rabbit anti-MBP serum (New England Biolabs) diluted 1/25 in dilution buffer for 2 h at room temperature. The grids were washed three times with PBS-1% Tween 20, transferred to 50 μl of 12-nm colloidal gold-Affinipure donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) diluted 1/30 in dilution buffer, and incubated for 1 h at room temperature. After three washes with PBS-1% Tween 20 and one wash with distilled water, bacteria were stained with 1% phosphotungstic acid and examined with an electron microscope (Philips 201) at an accelerating voltage of 60 kV.
Immunization and protection study.
Pigs were used to perform the immunization and protection assay at the Vaccine and Infectious Disease Organization (University of Saskatchewan, Saskatoon, Canada) in accordance with principles outlined in the Guide to the Care and Use of Experimental Animals of the Canadian Council on Animal Care, using a protocol that was approved by the University Committee on Animal Care (32). Three-week-old piglets with an average weight of 8.23 kg from a herd that is free of S. suis serotype 2 were randomly assigned to two groups of eight. The pigs were injected intramuscularly twice at a 3-week interval with 1 ml of either 100 μg purified Sao mixed with 30% Emulsigen-Plus (MVP Laboratories, Ralston, NE) adjuvant or 30% Emulsigen-Plus in physiological saline as a control. Eleven days after the second injection, the immunized and control animals were challenged by aerosol with 1 ml (4.6 × 106 CFU) of a log-phase culture of the virulent S. suis strain 166, which has been confirmed to be highly virulent (3). Blood samples were collected prior to each injection and challenge for determinations of antibody responses. Pigs were monitored daily for clinical signs, body temperature, and mortality for 10 days after challenge. All pigs were examined postmortem for gross pathology, and blood was cultured to detect the presence of S. suis bacteremia.
ELISA.
Sao-specific total serum IgG and IgG isotypes (IgG1 and IgG2) of immunized piglets were determined by an enzyme-linked immunosorbent assay (ELISA). Polysorb plates (Nunc, Rochester, NY) were coated overnight at 4°C with 100 μl per well of purified recombinant Sao at a concentration of 0.3 μg/ml in carbonate buffer. After three washes with PBS containing 0.05% Tween 20 (PBST), the plates were blocked with 5% skim milk in PBST for 1 h at 37°C. For determinations of total IgG, swine sera from the control and vaccine groups were diluted 1/5,000 in PBST and added to appropriate wells in duplicate at 100 μl per well. After incubation for 1 h at 37°C and three washes, bound antibodies were detected by incubation for 1 h at 37°C with peroxidase-conjugated goat anti-swine IgG heavy-plus-light-chain antiserum (Jackson ImmunoResearch Laboratories). For IgG1 and IgG2 detection, optimally diluted swine sera from the Sao-immunized group were added at 100 μl per well. Mouse anti-porcine IgG1 or IgG2 (Serotec, Kidlington, Oxford, United Kingdom) was used as the primary antibody, and peroxidase-conjugated goat anti-mouse IgG heavy-plus-light-chain antiserum (Serotec) was used as the secondary antibody. The plates were developed with tetramethyl benzidine substrate (Zymed, San Francisco, CA). Absorbance was measured at 450 nm in an ELISA reader (Power Wave 340; Bio-Tek Instruments, Inc., Winooski, VT). Results were expressed as means ± standard deviations. Statistical significance was determined by Student's t test.
Nucleotide sequence accession number.
The sequence of the gene encoding the Sao protein of S. suis has been assigned GenBank accession number AY864331.
RESULTS
Identification of the sao gene.
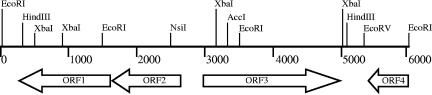
An S. suis chromosomal library was constructed from S. suis strain S735 in λZAPII and screened using convalescent-phase swine sera from S. suis serotype 2-infected animals. One clone, λSS735, which expressed a protein with an apparent molecular mass of 110 kDa that was strongly reactive against the convalescent-phase swine sera, was selected for further characterization. The schematic organization of the inserted DNA of λSS735 is presented in Fig. 1. DNA sequence analysis of the 6,057-bp insert revealed four ORFs. This gene cluster was found in the partially sequenced genomes of S. suis Canadian strain 89/1591 (NZ_AAFA00000000) and European strain P1/7 (NC_004549), with the same organization. The deduced amino acid sequences of both ORF1 and ORF2 showed identities ranging from 60 to 80% with a glycosyl transferase, and ORF4 showed identities ranging from 50 to 75% with a catabolite control protein A from many bacterial species, most of them belonging to the genus Streptococcus. ORF3 encodes a 670-amino-acid protein, designed Sao, with a predicted pI of 6.0 and a calculated molecular mass of 74.8 kDa. A comparison of the amino acid sequence of Sao with those in available databases revealed no significant homology with other proteins. Subcloning analysis of the sao sequence in the pMal-p vector revealed that Sao reacted strongly with the convalescent-phase swine sera, suggesting that Sao is the immunogenic protein.
FIG. 1.
Schematic representation and partial restriction map of the DNA insert of recombinant phage λSS735. Numbers indicate distances (in base pairs) from the 5′ end.
Sao is a novel C-terminally-anchored surface protein of S. suis.
The 2,010-bp sao gene starts with an ATG codon which is preceded by a putative Shine-Dalgarno sequence (GAAAGGA) 10 bp upstream of the start codon and terminates with a TAA codon (Fig. 2). An analysis of the predicted Sao amino acid sequence revealed a hydrophobic core of 15 amino acids at the N terminus and a putative signal-peptidase cleavage site between Ala29 and Gln30. Ten repeats of a 27-amino-acid sequence with a strong consensus pattern separated by 3-amino-acid spacers were detected within the carboxyl half of the protein. Immediately C-terminal from the repeat region is a cell wall-associated region, which spans 49 amino acid residues and is characterized by a high percentage of threonine residues (20.4%). This threonine-rich region is immediately followed by an LPVTG consensus motif typical of membrane-anchored surface proteins of many gram-positive bacteria. Beginning four amino acids C-terminal from the membrane anchor motif, a second hydrophobic segment of 16 amino acids was identified, which is followed by four positively charged amino acid residues at the C-terminal end of the protein (Fig. 2).
FIG. 2.
Nucleotide sequence and deduced amino acid sequence for the gene encoding the Sao protein of S. suis. The Shine-Dalgarno sequence is shown in italics. The initiation codon, ATG, and the stop codon, TAA, are shown in bold. The two hydrophobic segments at both the N- and C-terminal ends of Sao are underlined. The vertical arrow indicates the cleavage site of the potential signal peptidase. R1 to R10 indicate the beginnings of the repeating units. The potential cell wall-associated region is underlined with a dashed line. The LPVTG membrane anchor motif is boxed, and the charged C-terminal tail is indicated.
An analysis of the amino acid composition revealed a region lacking aromatic residues between Glu272 and Thr630 which spans all of the repeat sequences. Furthermore, a conserved domain search using BLAST identified an avirulence domain in the Lys319-to-Val601 region, which exhibits similarity with the AvrXa7 avirulence factor from the plant pathogen Xanthomonas oryzae pv. oryzae (59), with 20% identity (Fig. 3). If conservative amino acid substitutions are taken into consideration, the similarity is 48%.
FIG. 3.
Amino acid sequence alignment of the region from Lys319 to Val601 of Sao with the region from Lys475 to Val793 of AvrXa7 of Xanthomonas oryzae pv. oryzae. Double dots indicate identical residues, and single dots represent conserved substitutions.
Production of recombinant Sao.
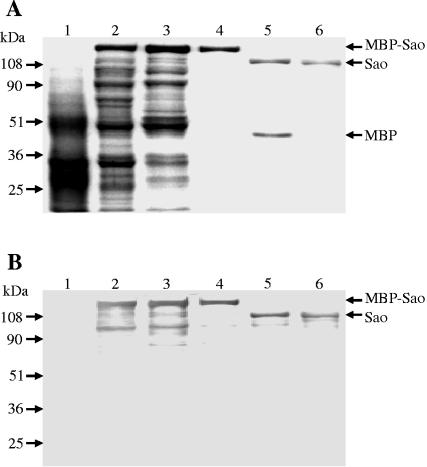
The sequence coding for the mature Sao protein was amplified by PCR and ligated into the IPTG-inducible pMAL-p vector. The resulting recombinant plasmid was expressed in E. coli XL1-Blue. As shown in Fig. 4A, induction of the E. coli recombinants harboring the malE-sao fusion gene led to the expression of an approximately 150-kDa MBP-Sao fusion protein which was absent from noninduced E. coli cells. One characteristic of Sao is a region of 10 repeating amino acid sequences in which aromatic substitutions are absent. The fusion protein was mostly found in the cytoplasm of E. coli cells; however, a truncated MBP-Sao fusion protein in which the repeating region was deleted was completely transported into the periplasmic space (data not shown), suggesting that this region somehow interfered with MBP localization.
FIG. 4.
Expression of MBP-Sao fusion protein in E. coli XL1-Blue and purification of recombinant mature Sao. A Coomassie-stained gel (A) and Western blot analysis (B) of the corresponding samples probed with convalescent-phase swine sera show the E. coli whole-cell lysate before (lane 1) and after (lane 2) induction with IPTG, the supernatant of the E. coli lysate (lane 3), the affinity-purified MBP-Sao fusion protein (lane 4), Sao and MBP cleaved by factor Xa (lane 5), and recombinant Sao devoid of MBP purified by anion-exchange chromatography (lane 6). The molecular masses are indicated on the left.
The fusion protein was purified by affinity chromatography and showed a single protein band of approximately 150 kDa upon SDS-PAGE (Fig. 4A). The purified fusion protein was proteolytically cleaved with factor Xa, yielding an apparently 110-kDa Sao protein and the expected 45-kDa MBP (Fig. 4A). The Sao protein devoid of MBP was obtained by subsequent purification by anion-exchange chromatography, yielding a single protein band, as visualized by SDS-PAGE (Fig. 4A). In a Western blot, both the MBP-Sao fusion protein and the purified recombinant Sao protein demonstrated specific reactivity to the convalescent-phase swine sera used for screening of the genomic library (Fig. 4B). The identity of purified Sao was confirmed by N-terminal protein sequencing. The protein concentration was adjusted to 1 mg/ml.
Cell surface expression of Sao in S. suis.
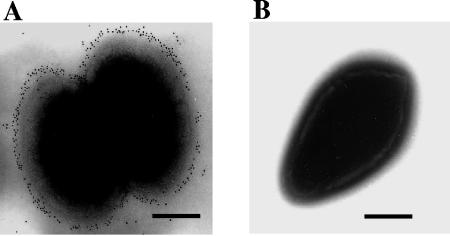
Immunoelectron microscopy using a monospecific polyclonal anti-Sao antibody, R44, confirmed the surface location of Sao on S. suis (strain S735) cells. Immunogold particles were found on almost all observed bacterial cells treated with Sao-specific antibody. An example of the typical pattern of distribution of immunogold particles on the S. suis cell surface is shown in Fig. 5A. Rabbit anti-MBP serum was used as a control and did not show any labeling (Fig. 5B). This indicates that the Sao protein is expressed homogeneously on the cellular surface.
FIG. 5.
Immunoelectron microscopy of S. suis. The surface location of Sao on S. suis is demonstrated using a monospecific Sao antiserum and a gold-conjugated secondary antibody (A). No labeling was found in the control bacterial cell (B). Bars, 200 nm.
Distribution of Sao among S. suis strains.
To evaluate the expression of Sao among reference strains of different serotypes of S. suis and serotype 2 field strains, whole-cell preparations of bacteria were applied to Western blots and detected by the Sao-specific antibody R44. As shown in Table 1, R44 reacted with 28 of 33 S. suis serotypes and 25 of 26 tested serotype 2 isolates from different geographic origins in North America and Europe. Five strains of other Streptococcus species were used to demonstrate that Sao was not expressed by other streptococci.
Immunogenicity of Sao and protection of pigs from challenge with S. suis.
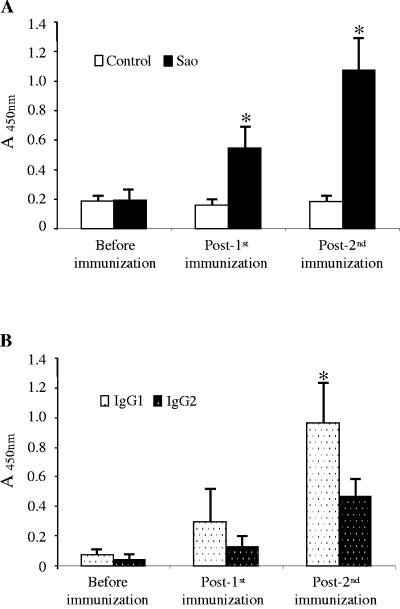
Groups of eight piglets were immunized twice intramuscularly with 100 μg of either purified recombinant Sao emulsified with an adjuvant or the adjuvant only. The immunization of pigs with Sao triggered an antigen-specific response (Fig. 6A). An analysis of corresponding sera obtained from control animals and experimental animals before immunization clearly indicated that there was no Sao-specific antibody, since only background ELISA values were recorded. Only 2 weeks after the first injection, Sao elicited a significant IgG response that was obviously enhanced by the second immunization. Assessments of IgG isotypes demonstrated that while both IgG1 and IgG2 isotypes were induced in sera of Sao-immunized animals, the IgG1 response dominated over the IgG2 response (Fig. 6B), suggesting that this immunization protocol with Sao mainly induced a Th2-like immune response. An aerosol challenge of the pigs with S. suis strain 166 resulted in steady increases in clinical scores starting from day 2 after the challenge, and there was no significant effect of the vaccination on clinical signs (data not shown). Three pigs from each group died or were euthanized due to high clinical scores prior to the end of the experiment. S. suis bacteremia was found in all dead pigs and was not detected in the surviving pigs. As summarized in Table 2, fewer pigs in the vaccinated group showed evidence of arthritis postmortem than those in the control group.
FIG. 6.
Antibody responses after vaccination of piglets with Sao. (A) Total Sao-specific IgG in sera was measured by ELISA, showing that a single injection of Sao elicits a significant IgG response that is obviously enhanced by the booster injection. (B) An ELISA for serum IgG isotypes in Sao-immunized pigs shows that IgG1 levels are consistently higher than IgG2 levels. The results are expressed as means of absorbance values with standard errors. *, P ≤ 0.05.
TABLE 2.
Protection of pigs following challenge with S. suis strain 166
| Vaccination group (n = 8) | No. of arthritic pigs | No. of bacteremic pigs | No. of surviving pigs |
|---|---|---|---|
| Emulsigen-Plus (control) | 6 | 3 | 5 |
| Emulsigen-Plus + Sao | 4 | 3 | 5 |
DISCUSSION
S. suis infection is a major cause of sudden death of pigs and is also increasingly becoming a human health concern due to its zoonotic capabilities. Attempts to control the infection are hampered by the lack of effective vaccines which could control the diversity of strains and serotypes that cause disease. The identification and characterization of potential cell surface targets are some of the strategies and prerequisites for the development of an effective vaccine. In this work, we identified a new C-terminally anchored surface protein, designated Sao, from S. suis type 2. This protein contains 670 amino acids with a calculated molecular mass of 74.8 kDa, which is less than that estimated by SDS-PAGE (110 kDa). The reason for the discrepancy between the apparent and theoretical sizes of Sao is unknown. However, a truncated Sao protein from which the Lys319-Val601 region was deleted migrated by SDS-PAGE as a 46-kDa band, which is consistent with the predicted molecular mass of 44 kDa (data not shown). Therefore, the aberrant migration of Sao in SDS gels is determined by the Lys319-Val601 sequence. Spanning most repeats of Sao, this region is characterized by the absence of aromatic amino acids and by high contents of charged amino acid residues (31%) and acidic residues (19%). A number of proteins have been reported to migrate anomalously by SDS-PAGE due to their unusual amino acid composition (14, 38, 50). A high percentage of acidic residues within the E. coli FtsY protein was reported to result in a >70% deviation in apparent size (92 kDa) from its theoretical molecular mass (54 kDa) (14). Such a deviation was also noted for highly charged proteins, such as the S. suis extracellular protein EF (110 versus 85 kDa) (50) and the Xenopus oocyte RNA-binding proteins p54 (54 versus 36 kDa) and p56 (56 versus 37 kDa) (38). Alternatively, the aberrant migration could be due to posttranslational modification of the proteins. Although the sao gene shares strong homology with ORFs in the partially sequenced genomes of S. suis Canadian strain 89/1591 and European strain P1/7, no significant homology at the protein level was detected with any other known sequences, indicating that Sao is a novel surface protein.
An analysis of the predicted amino acid sequence revealed that Sao possesses all the typical features of a membrane-anchored surface protein of gram-positive bacteria, including an N-terminal signal sequence, repeating sequences, an LPVTG consensus motif, and a positively charged C-terminal tail. Immediately C-terminal from the repeat region is a threonine-rich sequence. This region is found in many other surface proteins of gram-positive bacteria (5, 54), although they do not share a high degree of sequence identity. Since this threonine-rich region is immediately followed by the LPVTG consensus motif typical of membrane-anchored surface proteins of S. suis and many other gram-positive bacteria and since the region is proximal to the second hydrophobic domain, which is located in the cell membrane, it would be expected to lie within the peptidoglycan layer of the cell wall. The LPXTG motif has been found to be highly conserved among all C-terminally anchored proteins examined so far (11, 36, 40, 44, 45, 51). However, while positions 1, 2, 4, and 5 are nearly completely conserved, position 3 is variable, with several amino acid substitutions, predominantly A, Q, E, T, N, D, K, and L (10). Sao adds to the variety of amino acids in this location and has valine in the X position.
The domain spanning the repeat sequence in Sao shares some homology with the AvrXa7 avirulence factor from the plant pathogen Xanthomonas oryzae pv. oryzae. The avr genes have been extensively studied in bacterial plant pathogens (29). The products of avr genes are targets of the type III secretion system and have been shown to act as ligands to bind specifically to R proteins of plant host cells, resulting in activation of the plant defense response, which often involves a hypersensitive response (29). Whether such an interaction exists between animal pathogens and host cells is unknown. AvrA from Salmonella enterica serovar Typhimurium and the YopJ protein from Yersinia pseudotuberculosis, both animal pathogens, also have sequence similarity with the avirulence protein AvrRxv from the plant pathogen Xanthomonas campestris pv. vesicatoria and with Y410 from Rhizobium sp. (12, 20). The YopJ protein has been shown to be presented to the host via a type III secretion system and to induce apoptosis in macrophages (7). Whether this region of Sao plays a functional role in the interaction between S. suis and host cells remains to be established.
The immunization of pigs elicited a rapid Sao-specific humoral antibody response that was significantly boosted by a subsequent injection. However, the antibody to Sao did not confer protection against a heterologous challenge using S. suis strain 166. A discrepancy between the antibody response and protection has been reported for some other surface antigens of gram-positive bacteria, such as a streptococcal fibronectin binding protein (Sfb1) (35), pneumococcal surface protein A (PspA) (37), group B polysaccharide (34), and the M-like protein of Streptococcus equi (49). The reason why antibodies against Sao were not protective against the challenge with S. suis 166 is unclear. Emulsigen-Plus was used as an adjuvant in this study, because it creates an antigen depot at the site of inoculation from which the antigen is slowly released, provides prolonged stimulation to the immune system, and is used in effective, commercially available vaccines for swine (31, 55). However, recent evidence showed that vaccines formulated with Emulsigen triggered a predominantly IgG1 response with a very weak Th1-type immune response (26, 39). In fact, in a phagocytic killing study, the presence of pooled sera from Sao-immunized pigs did not promote S. suis killing by porcine neutrophils (unpublished observations), suggesting that the antibodies lacked opsonophagocytic function. Host protection against infection caused by S. suis, a highly encapsulated microorganism, is mediated primarily by phagocytosis (46). Therefore, total IgG levels generated in this conventional vaccination model may not adequately reflect the presence of opsonic antibodies that are capable of triggering leukocyte effector functions. To further illustrate the immune response types induced by Sao in the pig vaccination model, IgG isotypes in immunized sera were assessed. IgG1 levels were consistently higher than IgG2 levels, suggesting the induction of predominantly Th2-like responses. Although the concept of a “Th1/Th2” balance is not yet well documented for pigs, recent evidence showed that porcine IgG2 had greater complement-activating ability than did IgG1 (8). Evidence from vaccinations using surface antigens of other gram-positive bacteria has demonstrated that the efficiency of opsonophagocytosis can be dramatically enhanced by using Th1-directing adjuvants, such as CpG and interleukin-12 (4, 30, 33). These adjuvants promote a Th1-like immune response characterized by enhanced production of opsonizing antibodies, especially of the IgG2 isotype. Furthermore, the enhanced antibody-mediated opsonization was clearly reflected in protection (2, 58). These results may provide a promising approach for further evaluation of Sao in a modified vaccination model involving an optimal adjuvant.
In conclusion, Sao is a highly conserved C-terminally anchored surface protein of S. suis, as demonstrated by analyses of its molecular features and electron microscopy as well as by its wide distribution in many S. suis serotypes. Vaccination with the recombinant Sao protein elicits a significant humoral antibody response in piglets, and convalescent-phase swine sera present high titers of antibody against this protein, suggesting that Sao is a potent antigen that is expressed during S. suis infection. However, the potential of Sao as a vaccine candidate remains to be further established since the antibody response was not reflected in protection of pigs in our conventional vaccination model. Further study will evaluate the protection afforded by modification of the immune response, involving an optimal adjuvant, different immunization routes, and different challenge strains.
Acknowledgments
We thank M. Mourez for helpful discussions and comments on the manuscript.
This work was supported by Valorisation Recherche Quebec (VRQ 2201-141) and the NSERC Canadian Research Network on Bacterial Pathogens of Swine (225155-00).
Editor: J. D. Clements
REFERENCES
- 1.Arends, J. P., and H. C. Zanen. 1988. Meningitis caused by Streptococcus suis in humans. Rev. Infect. Dis. 10:131-137. [DOI] [PubMed] [Google Scholar]
- 2.Arulanandam, B. P., J. M. Lynch, D. E. Briles, S. Hollingshead, and D. W. Metzger. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berthelot-Herault, F., R. Cariolet, A. Labbe, M. Gottschalk, J. Y. Cardinal, and M. Kobisch. 2001. Experimental infection of specific pathogen free piglets with French strains of Streptococcus suis capsular type 2. Can. J. Vet. Res. 65:196-200. [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan, R. M., D. E. Briles, B. P. Arulanandam, M. A. Westerink, R. H. Raeder, and D. W. Metzger. 2001. IL-12-mediated increases in protection elicited by pneumococcal and meningococcal conjugate vaccines. Vaccine 19:2020-2028. [DOI] [PubMed] [Google Scholar]
- 5.Burne, R. A., and J. E. Penders. 1992. Characterization of the Streptococcus mutans GS-5 fruA gene encoding exo-beta-d-fructosidase. Infect. Immun. 60:4621-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnette, W. N. 1981. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112:195-203. [DOI] [PubMed] [Google Scholar]
- 7.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawley, A., and B. N. Wilkie. 2003. Porcine Ig isotypes: function and molecular characteristics. Vaccine 21:2911-2922. [DOI] [PubMed] [Google Scholar]
- 9.Elliott, S. D., F. Clifton-Hadley, and J. Tai. 1980. Streptococcal infection in young pigs. V. An immunogenic polysaccharide from Streptococcus suis type 2 with particular reference to vaccination against streptococcal meningitis in pigs. J. Hyg. (London) 85:275-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischetti, V. A. 2000. Surface proteins on gram-positive bacteria, p. 11-24. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 11.Fischetti, V. A., V. Pancholi, and O. Schneewind. 1990. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol. Microbiol. 4:1603-1605. [DOI] [PubMed] [Google Scholar]
- 12.Freiberg, C., R. Fellay, A. Bairoch, W. J. Broughton, A. Rosenthal, and X. Perret. 1997. Molecular basis of symbiosis between Rhizobium and legumes. Nature 387:394-401. [DOI] [PubMed] [Google Scholar]
- 13.Galina, L., U. Vecht, H. J. Wisselink, and C. Pijoan. 1996. Prevalence of various phenotypes of Streptococcus suis isolated from swine in the USA based on the presence of muraminidase-released protein and extracellular factor. Can. J. Vet. Res. 60:72-74. [PMC free article] [PubMed] [Google Scholar]
- 14.Gill, D. R., and G. P. Salmond. 1990. The identification of the Escherichia coli ftsY gene product: an unusual protein. Mol. Microbiol. 4:575-583. [DOI] [PubMed] [Google Scholar]
- 15.Gottschalk, M., R. Higgins, M. Jacques, M. Beaudoin, and J. Henrichsen. 1991. Characterization of six new capsular types (23 through 28) of Streptococcus suis. J. Clin. Microbiol. 29:2590-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottschalk, M., R. Higgins, M. Jacques, M. Beaudoin, and J. Henrichsen. 1991. Isolation and characterization of Streptococcus suis capsular types 9-22. J. Vet. Diagn. Investig. 3:60-65. [DOI] [PubMed] [Google Scholar]
- 17.Gottschalk, M., R. Higgins, M. Jacques, K. R. Mittal, and J. Henrichsen. 1989. Description of 14 new capsular types of Streptococcus suis. J. Clin. Microbiol. 27:2633-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottschalk, M., A. Lebrun, H. Wisselink, J. D. Dubreuil, H. Smith, and U. Vecht. 1998. Production of virulence-related proteins by Canadian strains of Streptococcus suis capsular type 2. Can. J. Vet. Res. 62:75-79. [PMC free article] [PubMed] [Google Scholar]
- 19.Gottschalk, M., and M. Segura. 2000. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet. Microbiol. 76:259-272. [DOI] [PubMed] [Google Scholar]
- 20.Hardt, W. D., and J. E. Galan. 1997. A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc. Natl. Acad. Sci. USA 94:9887-9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins, R., and M. Gottschalk. 1998. Distribution of Streptococcus suis capsular types in 1997. Can. Vet. J. 39:299-300. [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins, R., M. Gottschalk, M. Boudreau, A. Lebrun, and J. Henrichsen. 1995. Description of six new capsular types (29-34) of Streptococcus suis. J. Vet. Diagn. Investig. 7:405-406. [DOI] [PubMed] [Google Scholar]
- 23.Higgins, R., and M. Gottschalk. 2005. Streptococcal diseases, p. 769-783. In B. E. Straw, S. D'Allaire, W. L. Mengeling, and D. J. Taylor (ed.), Diseases of swine, 9th ed. Iowa State University Press, Ames, Iowa.
- 24.Hill, J. E., M. Gottschalk, R. Brousseau, J. Harel, S. M. Hemmingsen, and S. H. Goh. 2005. Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet. Microbiol. 107:63-69. [DOI] [PubMed] [Google Scholar]
- 25.Holt, M. E., M. R. Enright, and T. J. Alexander. 1988. Immunisation of pigs with live cultures of Streptococcus suis type 2. Res. Vet. Sci. 45:349-352. [PubMed] [Google Scholar]
- 26.Ioannou, X. P., P. Griebel, R. Hecker, L. A. Babiuk, and S. van Drunen Littel-van den Hurk. 2002. The immunogenicity and protective efficacy of bovine herpesvirus 1 glycoprotein D plus Emulsigen are increased by formulation with CpG oligodeoxynucleotides. J. Virol. 76:9002-9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs, A. A., A. J. van den Berg, and P. L. Loeffen. 1996. Protection of experimentally infected pigs by suilysin, the thiol-activated haemolysin of Streptococcus suis. Vet. Rec. 139:225-228. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 29.Leach, J. E., and F. F. White. 1996. Bacterial avirulence genes. Annu. Rev. Phytopathol. 34:153-179. [DOI] [PubMed] [Google Scholar]
- 30.Lefeber, D. J., B. Benaissa-Trouw, J. F. Vliegenthart, J. P. Kamerling, W. T. Jansen, K. Kraaijeveld, and H. Snippe. 2003. Th1-directing adjuvants increase the immunogenicity of oligosaccharide-protein conjugate vaccines related to Streptococcus pneumoniae type 3. Infect. Immun. 71:6915-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lofthouse, S. A., A. E. Andrews, A. D. Nash, and V. M. Bowles. 1995. Humoral and cellular responses induced by intradermally administered cytokine and conventional adjuvants. Vaccine 13:1131-1137. [DOI] [PubMed] [Google Scholar]
- 32.Lun, S., J. Perez-Casal, W. Connor, and P. J. Willson. 2003. Role of suilysin in pathogenesis of Streptococcus suis capsular serotype 2. Microb. Pathog. 34:27-37. [DOI] [PubMed] [Google Scholar]
- 33.Lynch, J. M., D. E. Briles, and D. W. Metzger. 2003. Increased protection against pneumococcal disease by mucosal administration of conjugate vaccine plus interleukin-12. Infect. Immun. 71:4780-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marques, M. B., D. L. Kasper, A. Shroff, F. Michon, H. J. Jennings, and M. R. Wessels. 1994. Functional activity of antibodies to the group B polysaccharide of group B streptococci elicited by a polysaccharide-protein conjugate vaccine. Infect. Immun. 62:1593-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McArthur, J., E. Medina, A. Mueller, J. Chin, B. J. Currie, K. S. Sriprakash, S. R. Talay, G. S. Chhatwal, and M. J. Walker. 2004. Intranasal vaccination with streptococcal fibronectin binding protein Sfb1 fails to prevent growth and dissemination of Streptococcus pyogenes in a murine skin infection model. Infect. Immun. 72:7342-7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMillan, D. J., M. R. Batzloff, C. L. Browning, M. R. Davies, M. F. Good, K. S. Sriprakash, R. Janulczyk, and M. Rasmussen. 2004. Identification and assessment of new vaccine candidates for group A streptococcal infections. Vaccine 22:2783-2790. [DOI] [PubMed] [Google Scholar]
- 37.Miyaji, E. N., D. M. Ferreira, A. P. Lopes, M. C. Brandileone, W. O. Dias, and L. C. Leite. 2002. Analysis of serum cross-reactivity and cross-protection elicited by immunization with DNA vaccines against Streptococcus pneumoniae expressing PspA fragments from different clades. Infect. Immun. 70:5086-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray, M. T., D. L. Schiller, and W. W. Franke. 1992. Sequence analysis of cytoplasmic mRNA-binding proteins of Xenopus oocytes identifies a family of RNA-binding proteins. Proc. Natl. Acad. Sci. USA 89:11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichani, A. K., R. S. Kaushik, A. Mena, Y. Popowych, D. Dent, H. G. Townsend, G. Mutwiri, R. Hecker, L. A. Babiuk, and P. J. Griebel. 2004. CpG oligodeoxynucleotide induction of antiviral effector molecules in sheep. Cell Immunol. 227:24-37. [DOI] [PubMed] [Google Scholar]
- 40.Okumura, K., H. I. Kawsar, T. Shimizu, T. Ohta, and H. Hayashi. 2005. Identification and characterization of a cell-wall anchored DNase gene in Clostridium perfringens. FEMS Microbiol. Lett. 242:281-285. [DOI] [PubMed] [Google Scholar]
- 41.Okwumabua, O., O. Abdelmagid, and M. M. Chengappa. 1999. Hybridization analysis of the gene encoding a hemolysin (suilysin) of Streptococcus suis type 2: evidence for the absence of the gene in some isolates. FEMS Microbiol. Lett. 181:113-121. [DOI] [PubMed] [Google Scholar]
- 42.Pallares, F. J., C. S. Schmitt, J. A. Roth, R. B. Evans, J. M. Kinyon, and P. G. Halbur. 2004. Evaluation of a ceftiofur-washed whole cell Streptococcus suis bacterin in pigs. Can. J. Vet. Res. 68:236-240. [PMC free article] [PubMed] [Google Scholar]
- 43.Perch, B., K. B. Pedersen, and J. Henrichsen. 1983. Serology of capsulated streptococci pathogenic for pigs: six new serotypes of Streptococcus suis. J. Clin. Microbiol. 17:993-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reid, S. D., N. M. Green, G. L. Sylva, J. M. Voyich, E. T. Stenseth, F. R. DeLeo, T. Palzkill, D. E. Low, H. R. Hill, and J. M. Musser. 2002. Postgenomic analysis of four novel antigens of group A streptococcus: growth phase-dependent gene transcription and human serologic response. J. Bacteriol. 184:6316-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneewind, O., P. Model, and V. A. Fischetti. 1992. Sorting of protein A to the staphylococcal cell wall. Cell 70:267-281. [DOI] [PubMed] [Google Scholar]
- 46.Segura, M., M. Gottschalk, and M. Olivier. 2004. Encapsulated Streptococcus suis inhibits activation of signaling pathways involved in phagocytosis. Infect. Immun. 72:5322-5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serhir, B., D. Dubreuil, R. Higgins, and M. Jacques. 1995. Purification and characterization of a 52-kilodalton immunoglobulin G-binding protein from Streptococcus suis capsular type 2. J. Bacteriol. 177:3830-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serhir, B., D. Dugourd, M. Jacques, R. Higgins, and J. Harel. 1997. Cloning and characterization of a dextranase gene (dexS) from Streptococcus suis. Gene 190:257-261. [DOI] [PubMed] [Google Scholar]
- 49.Sheoran, A. S., S. Artiushin, and J. F. Timoney. 2002. Nasal mucosal immunogenicity for the horse of a SeM peptide of Streptococcus equi genetically coupled to cholera toxin. Vaccine 20:1653-1659. [DOI] [PubMed] [Google Scholar]
- 50.Smith, H. E., F. H. Reek, U. Vecht, A. L. Gielkens, and M. A. Smits. 1993. Repeats in an extracellular protein of weakly pathogenic strains of Streptococcus suis type 2 are absent in pathogenic strains. Infect. Immun. 61:3318-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith, H. E., U. Vecht, A. L. Gielkens, and M. A. Smits. 1992. Cloning and nucleotide sequence of the gene encoding the 136-kilodalton surface protein (muramidase-released protein) of Streptococcus suis type 2. Infect. Immun. 60:2361-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torremorell, M., C. Pijoan, and S. Dee. 1999. Experimental exposure of young pigs using a pathogenic strain of Streptococcus suis serotype 2 and evaluation of this method for disease prevention. Can. J. Vet. Res. 63:269-275. [PMC free article] [PubMed] [Google Scholar]
- 53.Trottier, S., R. Higgins, G. Brochu, and M. Gottschalk. 1991. A case of human endocarditis due to Streptococcus suis in North America. Rev. Infect. Dis. 13:1251-1252. [DOI] [PubMed] [Google Scholar]
- 54.Wanda, S. Y., and R. Curtiss III. 1994. Purification and characterization of Streptococcus sobrinus dextranase produced in recombinant Escherichia coli and sequence analysis of the dextranase gene. J. Bacteriol. 176:3839-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willson, P. J., A. Rossi-Campos, and A. A. Potter. 1995. Tissue reaction and immunity in swine immunized with Actinobacillus pleuropneumoniae vaccines. Can. J. Vet. Res. 59:299-305. [PMC free article] [PubMed] [Google Scholar]
- 56.Wisselink, H. J., N. Stockhofe-Zurwieden, L. A. Hilgers, and H. E. Smith. 2002. Assessment of protective efficacy of live and killed vaccines based on a non-encapsulated mutant of Streptococcus suis serotype 2. Vet. Microbiol. 84:155-168. [DOI] [PubMed] [Google Scholar]
- 57.Wisselink, H. J., U. Vecht, N. Stockhofe-Zurwieden, and H. E. Smith. 2001. Protection of pigs against challenge with virulent Streptococcus suis serotype 2 strains by a muramidase-released protein and extracellular factor vaccine. Vet. Rec. 148:473-477. [DOI] [PubMed] [Google Scholar]
- 58.Wortham, C., L. Grinberg, D. C. Kaslow, D. E. Briles, L. S. McDaniel, A. Lees, M. Flora, C. M. Snapper, and J. J. Mond. 1998. Enhanced protective antibody responses to PspA after intranasal or subcutaneous injections of PspA genetically fused to granulocyte-macrophage colony-stimulating factor or interleukin-2. Infect. Immun. 66:1513-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang, B., W. Zhu, L. B. Johnson, and F. F. White. 2000. The virulence factor AvrXa7 of Xanthomonas oryzae pv. oryzae is a type III secretion pathway-dependent nuclear-localized double-stranded DNA-binding protein. Proc. Natl. Acad. Sci. USA 97:9807-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]