Abstract
Sequencing studies showed that the γ-glutamylcysteine synthetase (γ-GCS) heavy chain genes from sodium stibogluconate (SSG)-resistant (SSG-R) and SSG-susceptible (SSG-S) Leishmania donovani strains were identical, indicating that SSG resistance was related to quantitative differences in γ-GCS expression rather than gene interstrain polymorphisms. In vitro infection of murine macrophages with the SSG-R strain, but not the SSG-S strain, down regulated expression of host γ-GCS, which would result in a reduction in intramacrophage glutathione (GSH) levels and promote an oxidative intramacrophage environment. This would inhibit, or minimize, the reduction of SSG pentavalent antimony to its more toxic trivalent form. Macrophage studies showed that the SSG-R strain expressed higher levels of γ-GCS compared to the SSG-S strain, which would result in higher GSH levels, giving increased protection against oxidative stress and facilitating SSG efflux. However a similar differential effect on host and parasite γ-GCS expression was not obtained when using tissues from infected mice. In this case γ-GCS expression was organ and strain dependent for both the host and the parasite, indicating that environmental conditions have a profound effect on γ-GCS expression. Consistent with the proposed mechanism from in vitro studies, increasing tissue GSH levels in the presence of SSG by cotreatment of L. donovani-infected mice with SSG solution and GSH incorporated into nonionic surfactant vesicles was more effective in reducing liver, spleen, and bone marrow parasite burdens than monotherapy with SSG. Together, these results indicate that SSG resistance is associated with manipulation of both host and parasite GSH levels by L. donovani.
Glutathione (l-gamma-glutamyl-l-cysteinylglycine, GSH) is important to cell survival since it is used to maintain intracellular redox balance and to protect against potential toxic agents which cause chemical or oxidative stress (19). Inhibition of GSH production is toxic to cells and has been used to increase the susceptibility of pathogens or cancer cells to certain drugs or to modulate the immune response (15). γ-Glutamylcysteine synthetase (l-glutamate:l-cysteine γ-ligase, γ-GCS) catalyzes the rate-limiting step of GSH biosynthesis, and its activity is controlled by nonallosteric feedback by GSH, the availability of cysteine, and factors which control the transcription and posttranslational processing of the enzyme (27). Specific, irreversible inhibition of γ-GCS caused by treatment with the inhibitor l-buthionine [S,R]-sulfoximine (BSO) has been shown to protect against Trypanosoma brucei (2), Plasmodium falciparum (28), and Leishmania donovani infections (6).
Ever since their discovery about 60 years ago, pentavalent antimonials (Sbv) have remained the worldwide first-line treatment of choice for leishmaniasis. We have shown that combined treatment with BSO and sodium stibogluconate (SSG) is more effective than treatment with either alone against L. donovani. Furthermore this combination is able to overcome SSG resistance of a naturally occurring SSG-resistant (SSG-R) strain of L. donovani (6). Administration of GSH has been shown to be protective against a number of infective agents, e.g., influenza virus (4) and AIDS virus (11). Treatment with N-acetylcysteine, which has been used to increase tissue GSH levels (1), protects against influenza virus (12), Leishmania major (32), and Trypanosoma cruzi (17). Thiols such as GSH are also important in mediating resistance to drugs in a number of pathogens (16, 33) including L. donovani (6) and also tumor cells (22). Therefore in this study mRNA levels of host and L. donovani γ-GCS were assessed using real-time PCR (RT-PCR) since γ-GCS expression is a good indicator of GSH production (10). Two strains of L. donovani with different susceptibilities to SSG were used to determine whether differences in resistance to SSG correlated with a difference in γ-GCS expression in the host and/or the parasite. In addition, the effect of exogenous GSH treatment on the survival of L. donovani in the presence and absence of SSG was determined.
MATERIALS AND METHODS
Reagents.
SSG (31.7%, wt/wt, Sbv) was provided by Glaxo-Wellcome Ltd. Potassium antimony tartrate hydrate (37.47%, wt/wt, SbIII) and BSO (used within 6 months of purchase) were obtained from Sigma-Aldrich (Poole, United Kingdom). The nonionic surfactant tetraethylene glycol mono-n-hexadecylether was purchased from Chesham Chemicals Ltd. (Harrow, United Kingdom). Capture and detection anti-gamma interferon (IFN-γ) antibodies, IFN-γ standard, and alkaline phosphatase conjugate were obtained from PharMingen and supplied by Insight Biotechnology (Wembley, United Kingdom). All other reagents were of analytical grade.
Animals and parasites.
Age- and sex-matched BALB/c mice (20 to 25 g, males or females) in-house bred at Strathclyde University were used in this study. Commercially obtained Golden Syrian hamsters (Mesocricetus auratus; Harlan Olac, Bicester, United Kingdom) were used for maintenance of the two L. donovani parasite strains used (strains 200011, SSG-R, and 200016, SSG susceptible [SSG-S]) (7). Mice were infected by intravenous injection (tail vein, no anesthetic) with 1 × 107 to 2 × 107 L. donovani amastigotes on day 0 (5), and studies were carried out in accordance with United Kingdom Home Office regulations.
Vesicle formulations.
Six hundred-micromoles of vesicle constituents, consisting of a 3:3:1 molar ratio of mono-n-hexadecyl ether tetraethylene glycol, cholesterol, and dicetyl phosphate, were melted by heating at 130°C for 5 min. The molten mixture was cooled to 70°C and hydrated with 5 ml of preheated (70°C) aqueous BSO (1.54 mg/ml) to form BSO nonionic surfactant vesicles (BSO-NIV) or GSH (10 mg/ml) to form GSH-NIV. Vesicular formulations were homogenized at 8,000 ± 100 rpm for 15 min at 70°C using a model L4R SU (Silverson Machines) mixer, fitted with a 5/8-in. tubular work head. Vesicle suspensions were stored at room temperature until used, usually on the day of manufacture.
In vitro studies.
Macrophages studies were carried out as described by Carter et al. (7). L. donovani-infected cells were treated with complete medium (controls) or complete medium containing GSH (2.5 to 20 mM), SSG (0.575 mM Sbv), GSH and SSG (2.5 mM GSH, 0.575 mM Sbv), GSH alone (0.05 to 15 mM), IFN-γ-lipopolysaccharide (LPS) alone (0.01 to 100 U IFN-γ/ml, 0.01 to 100 ng/ml LPS), or GSH and IFN-γ-LPS (50 to 500 μM GSH, 0.01 to 100 U IFN-γ/ml, 0.01 to 100 ng/ml LPS). The mean percentage of cells infected was assessed as before (7), and cell supernatants were transferred to individual microcentrifuge tubes and stored at −20°C until nitrite or cytokine levels were determined.
In vivo efficacy of drug formulations.
Groups of infected mice (n = 4 or 5/treatment) were treated intravenously on day 7 with 0.2 ml of one of the following formulations: phosphate-buffered saline (PBS; controls), free SSG (equivalent to a final dose of 70 to 300 mg Sbv/kg of body weight), BSO-NIV mixed 1:1 (vol/vol) with water (13.7 mg/kg) or with SSG solution (equivalent to a final dose of 70 mg Sbv/kg and 13.7 mg/kg BSO; BSO-NIV/SSG treatment), GSH solution (89 mg/kg), or GSH-NIV mixed 1:1 (vol/vol) with water (89 mg/kg) or with SSG solution (equivalent to a final dose of 70 mg Sbv/kg and 89 mg/kg GSH; GSH-NIV/SSG treatment). Parasite burdens were determined on day 7 posttreatment.
Flow cytometry.
The percentages of CD3+, CD45R/B220+, and F4/80+ cells present in the spleens of control and treated mice were determined by flow cytometry as described by Carter et al. (7). Cells were analyzed using a FACS Canto (BD Systems), gating on forward and side scatter, and the FACsDiva software was used to analyze data.
In vitro proliferation assays.
Single-cell suspensions, prepared from the spleens of uninfected and infected mice, were used in proliferation assays and incubated with medium alone (unstimulated controls) or concanavalin A (5 μg/ml; stimulated controls) as described by Carter et al. (7). Cell supernatants were stored at −20°C until nitrite and cytokine determination could be performed. Nitrite and IFN-γ concentrations in cell supernatants were determined as described by Carter et al. (7).
Nucleic acid extraction and cDNA preparation.
RNA isolation from both L. donovani strains (200011 and 200016) was performed using the Trizol reagent (Invitrogen, Paisley, United Kingdom) based on the single-step acid guanidinium thiocyanate-phenol-chloroform protocol (8). cDNA was produced using a 20-μl reaction volume containing 1 μg of denatured total RNA, 10 μM of random hexamers (Promega, Southampton, United Kingdom), 0.5 mM deoxynucleoside triphosphate mixture, 10 units of RNAsin RNase inhibitor (Promega), 2 μl of 10× first-strand buffer, and 4 units of Omniscript reverse transcriptase (QIAGEN, Crawley, United Kingdom) and stored at −20°C until required. Genomic DNA was extracted from 1 × 107 to 5 × 107 promastigotes of both strains of L. donovani, produced by in vitro culture of infected hamster spleen, using a protocol previously described by Johnson et al. (21) for Toxoplasma gondii.
PCR.
PCR amplification reactions were performed from a 1-μl aliquot of cDNA in a 25-μl reaction using 12.5 μl of 2× ReddyMix PCR master mixture [1.25 units Thermoprime Plus DNA polymerase, 75 mM Tris-HCl (pH 8.8 at 25°C), 20 mM (NH4)2SO4, 1.5 mM MgCl2, 0.01% (vol/vol) Tween 20, 10 mM deoxynucleoside triphosphates; Abgene, Epsom, United Kingdom] and 25 pmol of forward and reverse primers. Reactions consisted of an initial denaturation at 94°C for 3 min followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing for 45 seconds at 55°C, and extension at 72°C for 1 min. A final extension was carried out at 72°C for 10 min.
Amplification, cloning, and sequencing of L. donovani heavy-chain γ-GCS.
Two portions of the heavy-chain γ-GCS gene were initially obtained through degenerate PCR. The separate fragments were bridged by PCR, and this contig was extended by rapid amplification of cDNA ends (RACE) using the 5′/3′ RACE kit according to the manufacturer's instructions (Roche, Lewis, United Kingdom) and genome walking using genomic DNA (gDNA) as described by Min and Powell (29). The complete full-length sequence of γ-GCS was confirmed by PCR from both cDNA and gDNA. PCR products, separated on a 1.5% agarose gel and purified using the QIAquick gel purification kit (QIAGEN), were ligated into pDRIVE using the QIAGEN PCR cloning kit. The ligation reaction was used to transform DH5α bacterial cells using the heat shock transformation method of Cohen et al. (9). Automated sequencing of all cloned products was performed by MRC geneservice Ltd., and sequences were assembled using Sequencher (Gene Codes).
Real-time PCR studies.
To assess expression of γ-GCS in the host and in the parasite, primers were designed to amplify a portion of the L. donovani heavy-chain γ-GCS gene (-GCSldfor, 5′-TATCAAGTCTCGCTACGACT-3′; -GCSldrev, 5′-CGGAGTCCTTCAGAAGTT-3′), the L. donovani alpha-tubulin housekeeping gene (Ldatubfor, 5′-ACATCACGAACTCGGTGTTT-3′; Ldatubrev, 5′-TTCGTCTTGATCGTCGCAAT-3′), the mouse heavy-chain γ-GCS gene (γ-GCSmmfor, 5′-AGAACAATCGCTTTAGGATCA-3′; γ-GCSmmrev, 5′-AGAAGATGATCGATGCCTTC-3′), and the mouse GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene (MmGAPDHfor, 5′-AGA TTG TTG CCA TCA ACG AC 3′; MmGAPDHrev, 5′-ATG ACA AGC TTC CCA TTC TC-3′). Samples were run on an Mx3000P real-time PCR machine (Stratagene) using 96-well plates (Abgene). One microliter of cDNA was used in a 25-μl reaction mixture consisting of 8.33 μl of 2× SYBR green master mixture (Stratagene or Abgene), 25 pmol of a forward primer and a reverse primer, and 14.67 μl molecular-grade water (Sigma). Reactions consisted of an initial denaturation at 94°C for 10 min (Stratagene master mixture) or 15 min (Abgene master mixture) followed by up to 45 cycles of denaturation at 94°C for 30 seconds, annealing for 30 seconds at 55°C, and extension at 72°C for 1 min. Melting point analysis was performed between 55°C and 95°C to ensure the expected product was amplified in the absence of nonspecific products or primer dimers. The cycle threshold values for the samples were determined by the Mx3000P system software and used to assess their relative expression compared with the housekeeping genes (GAPDH gene, host; alpha-tubulin gene, L. donovani), which were used to normalize results. Quantification of the level of gene expression was carried out using the mean level in the uninfected control or the SSG-S-infected control as the “comparator” when determining expression of mouse γ-GCS and data for either strain when determining expression of Leishmania γ-GCS.
Statistical analysis of data.
Parasite data from in vivo experiments were analyzed using a one-way analysis of variance (using log10-transformed parasite burden for the spleen and liver data). Differences between treatments were then analyzed using a Fisher's protected least significant difference test using the Statview version 5.0.1 software package. Cytokine, flow cytometry, nitrite, and RT-PCR data were analyzed using the nonparametric Mann-Whitney U test.
Nucleotide sequence accession numbers.
The full-length γ-GCS heavy-chain genes from the SSG-S and SSG-R strains of L. donovani have been assigned GenBank accession numbers AAQ73826 and AAR 24259, respectively.
RESULTS
γ-GCS sequencing.
The full-length γ-GCS heavy-chain genes from both the SSG-S and SSG-R strains of L. donovani were obtained. The sequences were identical and 2,064 bp in length.
In vitro expression of γ-GCS in L. donovani and mouse macrophages.
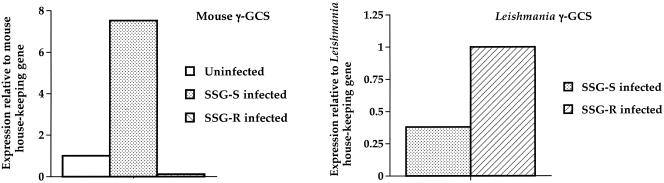
The host and the L. donovani γ-GCS mRNA levels in vitro in macrophages were determined. L. donovani had a strain-dependent effect on the level of murine macrophage γ-GCS expression (Fig. 1, left). Indeed, infection with the SSG-S strain of L. donovani was associated with an increase in the mouse γ-GCS mRNA levels compared to uninfected controls. In marked contrast, infection of macrophages with the SSG-R strain was associated with a down-regulation in mouse γ-GCS mRNA expression (Fig. 1, left). When parasite γ-GCS expression was determined, L. donovani exhibited a strain-dependent effect, with the SSG-R strain having higher levels of γ-GCS transcripts compared to the SSG-S strain (Fig. 1, right).
FIG. 1.
γ-GCS levels in uninfected macrophages and macrophages infected with the SSG-S or SSG-R strain of L. donovani. The amount of mouse γ-GCS (left) or Leishmania γ-GCS (right) present was quantified using RT-PCR. The data are representative of three separate experiments, which show the same differences between treatments indicated.
In vivo expression of γ-GCS in L. donovani and mouse tissues.
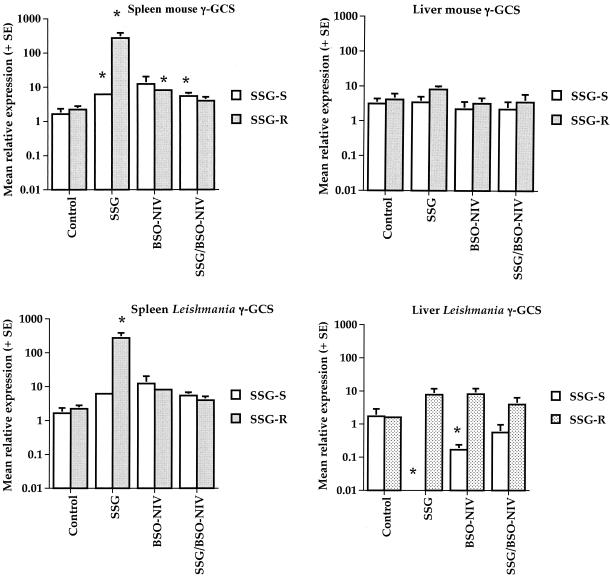
The mean relative levels of expression of mouse γ-GCS mRNA in the spleen (Fig. 2, top left) and the liver (Fig. 2, top right) were not consistently different in two separate experiments. Similarly, there was no significant difference in the expression of L. donovani γ-GCS mRNA in the spleens (Fig. 2, bottom left) or livers (Fig. 2, bottom right) of mice infected with either L. donovani strain. Treatment with SSG, BSO-NIV, or BSO-NIV/SSG had no significant effect on the expression of mouse γ-GCS mRNA in the livers of mice infected with either strain of L. donovani (Fig. 2, top right). However treatment with SSG caused a significant increase in splenic mouse γ-GCS mRNA levels relative to the housekeeping gene in mice infected with either strain (P < 0.05; Fig. 2, top left). BSO-NIV treatment significantly increased splenic mouse γ-GCS mRNA levels relative to the housekeeping gene in mice infected with the SSG-R strain but not the SSG-S strain; BSO-NIV/SSG treatment significantly increased splenic mouse γ-GCS mRNA levels relative to the housekeeping gene in mice infected with the SSG-S strain but not the SSG-R strain (Fig. 2, top left). Only treatment with SSG resulted in a significant increase in splenic Leishmania γ-GCS mRNA levels relative to the housekeeping gene in mice infected with the SSG-R strain (P < 0.05; Fig. 2, bottom left). None of the treatments had a significant effect on splenic Leishmania γ-GCS mRNA levels compared to control values for samples from mice infected with the SSG-S strain (Fig. 2, bottom left). Treatment with SSG or BSO-NIV resulted in a significant decrease in liver Leishmania γ-GCS mRNA levels relative to the housekeeping gene in mice infected with the SSG-S strain (P < 0.05; Fig. 2, bottom right), whereas similar treatment had no effect on liver Leishmania γ-GCS mRNA levels in mice infected with the SSG-R strain (Fig. 2, bottom right). BSO-NIV/SSG treatment had no significant effect on liver Leishmania γ-GCS levels compared to control values for samples from mice infected with either strain (Fig. 2, bottom right).
FIG. 2.
Comparison of mouse (spleen, top left; liver, top right) and Leishmania (spleen, bottom left; liver, bottom right) γ-GCS levels in the livers and spleens of L. donovani-infected mice. Spleen and liver samples were obtained from mice infected with the SSG-S or SSG-R strain of L. donovani on day 14 postinfection. Mice were treated with PBS alone (controls), free SSG (SSG final dose, 70 mg Sbv/kg), BSO-NIV (final BSO dose, 34.7 mg/kg) mixed 1:1 (vol/vol) with water, or BSO-NIV (final BSO dose, 34.7 mg/kg) mixed 1:1 (vol/vol) with SSG solution (SSG final dose, 70 mg Sbv/kg; BSO-NIV/SSG treatment). The mean relative expression of the mouse γ-GCS plus SE and the Leishmania γ-GCS plus SE was determined using the mean value for the SSG-S strain as the normalizer. The data are representative of two separate experiments which show the same significant differences between treatments indicated. *, P < 0.05 compared to the relevant control.
No correlation between changes in host or parasite γ-GCS mRNA expression and in vivo treatment efficacy was apparent (Table 1). For example, the most effective antileishmanial treatment against either strain (BSO-NIV/SSG), which caused >98% suppression in liver parasite burdens for both strains compared to controls (Table 1), had no significant effect on host or parasite Leishmania γ-GCS mRNA levels in the liver compared to corresponding control values (Fig. 2, top right and bottom right).
TABLE 1.
Effect of different treatments on the spleen, liver, and bone marrow parasite burdens of mice infected with the SSG-S or SSG-R strain of L. donovani on day 14 postinfectiona
| Strain and treatment | Mean parasite suppression (%) ± SE in:
|
||
|---|---|---|---|
| Spleen | Liver | Bone marrow | |
| SSG-S | |||
| SSG | 0 ± 0 | 42 ± 11** | 0 ± 0 |
| BSO-NIV | 0 ± 0 | 44 ± 3** | 0 ± 0 |
| BSO-NIV/SSG | 98 ± 1*** | 99 ± 1*** | 97 ± 4** |
| SSG-R | |||
| SSG | 27 ± 14 | 4 ± 4 | 25 ± 14 |
| BSO-NIV | 36 ± 3 | 23 ± 14 | 37 ± 16 |
| BSO-NIV/SSG | 47 ± 18 | 98 ± 1*** | 50 ± 14 |
Mice infected with the SSG-S or SSG-R strain of L. donovani were treated on day 7 postinfection with PBS alone (control), free SSG (equivalent to a final dose of 70 mg Sbv/kg), BSO-NIV (final BSO dose, 34.7 mg/kg) mixed 1:1 (vol/vol) with water, or BSO-NIV (final BSO dose, 34.7 mg/kg) mixed 1:1 (vol/vol) with SSG solution (SSG final dose of 70 mg Sbv/kg; BSO-NIV / SSG treatment). **, P < 0.001; ***, P < 0.0001, compared to the relevant control.
In vivo treatment with exogenous GSH has a strain-dependent antileishmanial effect and increases the efficacy of SSG treatment.
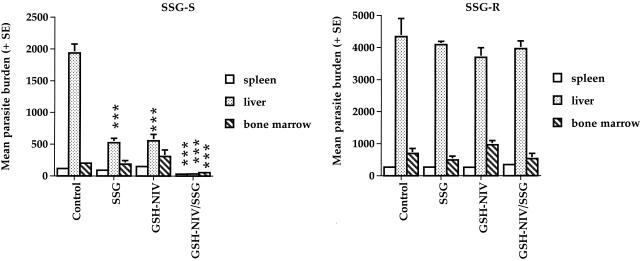
Preliminary experiments showed that treatment with GSH solution had no effect on L. donovani parasite burdens in infected mice (data not shown). However using NIV to target a greater proportion of the GSH dose to infected tissues resulted in a significant reduction in liver parasite burdens compared to corresponding controls (P < 0.0001) in mice infected with the SSG-S strain of L. donovani (Fig. 3, left). This effect was not evident in mice infected with the SSG-R strain (Fig. 3, right). Combined treatment with SSG and GSH-NIV (GSH-NIV/SSG) was more effective than treatment with SSG or GSH-NIV alone against the SSG-S strain (Fig. 3, left) but was inactive against the SSG-R strain (Fig. 3, right). Thus, GSH-NIV/SSG treatment caused a significant reduction in parasite burdens in all three sites surveyed (P < 0.0001) in SSG-S-infected mice and a greater reduction in liver parasite burdens compared with SSG or GSH-NIV treatment alone (Fig. 3, left).
FIG. 3.
Effect of different treatments on the spleen, liver, and bone marrow parasite burdens of mice infected with the SSG-S (left) or SSG-R (right) strains of L. donovani on day 14 postinfection. Mice infected with the SSG-S or SSG-R strain of L. donovani were treated with PBS alone (control), free SSG (SSG final dose, 70 mg Sbv/kg), GSH-NIV (final GSH dose, 89 mg/kg) mixed 1:1 (vol/vol) with water, or GSH-NIV (final GSH dose 89 mg/kg) mixed 1:1 (vol/vol) with SSG solution (SSG final dose, 70 mg Sbv/kg; GSH-NIV/SSG treatment). ***, P < 0.001 compared to the relevant control. The data are representative of three separate experiments, which show the same significant differences between the treatments indicated.
In vivo treatment with exogenous GSH and host immunity.
Combined GSH-NIV/SSG treatment was not associated with an increase in spleen weights in treated mice (data not shown). GSH-NIV/SSG treatment was associated with a significant suppression in IFN-γ (mean production ± standard error [SE] for SSG-S strain: control, 3.1 ± 0.6 ng/ml; GSH-NIV/SSG treatment, 1.9 ± 0.8 ng/ml; P < 0.05) and nitrite production (mean production ± SE for SSG-S strain: control, 9.1 ± 3.8; GSH-NIV/SSG treatment, 0.4 ± 0.4; P < 0.05) by unstimulated splenocytes compared to relevant controls from mice infected with the SSG-S, but not the SSG-R strain. Similar levels of nitrite and IFN-γ were produced by concanavalin A-stimulated cells from control and SSG-, GSH-NIV-, or GSH-NIV/SSG-treated mice infected with either strain of L. donovani (data not shown). GSH-NIV/SSG treatment had no significant effect on the percentage of F4/80+, CD3+, or B220+ cells present in the spleen compared to control values (data not shown).
In vitro treatment with exogenous GSH is not antiparasitic.
The above studies indicated that GSH was leishmanicidal against the SSG-S strain of L. donovani but did not indicate whether this was due to a direct antiparasitic effect or whether GSH acted indirectly. Treatment with GSH at concentrations that occur within cells, i.e., up to 10 mM (23), had no significant effect on parasite survival within macrophages (mean percentages of infection [± SE] for the SSG-S strain: control, 78% ± 5%; 10 mM GSH treatment, 81% ± 6%; mean percentages for the SSG-R strain: control, 88% ± 4.3%; 10 mM GSH treatment, 87% ± 0.9%). Increasing GSH to concentrations above 20 mM was toxic to infected macrophages, with cells infected with L. donovani promastigotes being more susceptible to cytotoxicity than cells infected with amastigotes (data not shown). Joint treatment with 20 mM GSH and IFN-γ-LPS (various doses) was associated with a reduction in nitrite production (for 20 U IFN-γ/ml and 10 ng/ml LPS, the mean nitrite level for the SSG-S strain [± SE] was 30.5 ± 2.5 μM; for 20 U IFN-γ/ml, 10 ng/ml LPS, and 20 mM GSH, the mean nitrite level was 19.6 ± 2.8 μM [P < 0.01]; corresponding values for the SSG-R strain were 45.9 ± 1.0 μM and 21.0 ± 0.2 μM, respectively) by cells infected by either strain of L. donovani compared to treatment with IFN-γ-LPS alone, which may reflect the inherent toxicity of GSH. However, the presence of GSH did not influence the ability of IFN-γ-LPS to stimulate parasite killing (mean percent suppression of parasite numbers compared to relevant controls [± SE] for the SSG-S strain: treatment with IFN-γ-LPS, 96% ± 5.5%; treatment with IFN-γ-LPS plus GSH, 95% ± 0.8%; corresponding values for the SSG-R strain were 89% ± 5.5% and 89% ± 5.5%, respectively). Treatment with GSH at or below 10 mM had no effect on the nitrite production of IFN-γ-LPS-stimulated macrophages infected with either strain of L. donovani and did not affect the efficacy of IFN-γ-LPS against either strain of L. donovani (data not shown).
In vitro treatment with exogenous GSH did not enhance the efficacy of SSG.
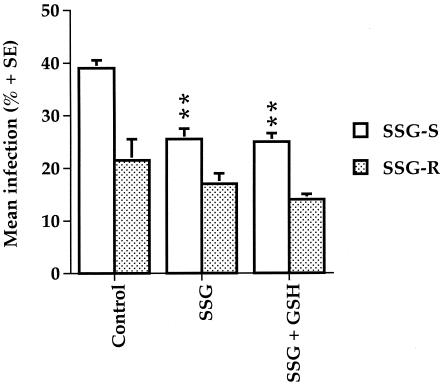
As expected, treatment with SSG (70 μg Sbv/ml) alone resulted in a significant reduction in the percentage of cells infected with the SSG-S strain, but not the SSG-R strain, of L. donovani (Fig. 4). Joint treatment with exogenous GSH (2.5 mM) did not enhance the efficacy of SSG treatment, nor did it have any visible effect on cell viability.
FIG. 4.
Effect of GSH treatment on the efficacy of SSG treatment in macrophages infected with the SSG-S or SSG-R strains of L. donovani. Macrophages, infected with SSG-S or SSG-R strain of L. donovani promastigotes, were treated with medium alone (control), SSG alone (0.575 mM Sbv), or SSG (0.575 mM Sbv) and GSH (2.5 mM), and the mean percent infection was determined. **, P < 0.01 compared to relevant control The data are representative of three separate experiments, which show the same significant differences between the treatments indicated.
DISCUSSION
The mechanism of SSG resistance was investigated using two clinical strains of L. donovani with different inherent susceptibilities to SSG. Previous studies have demonstrated that parasite or host γ-GCS has a role in mediating drug resistance since SSG resistance could be modulated by coadministration of the γ-GCS-specific inhibitor BSO with SSG. Cloning and sequencing of the γ-GCS heavy-chain genes, which code for the catalytic portion of the enzyme (27), from the SSG-S and SSG-R strains demonstrated that their coding regions were identical, indicating that differences in inherent enzyme activity were not responsible for variation in SSG susceptibility. However it was still possible that differences in γ-GCS activity could be mediated by variation in the transcriptional or posttranscriptional (e.g., mRNA stabilization/destabilization or modification) regulation of the heavy or light chains of γ-GCS, or both (27). Therefore in this study RT-PCR was used to compare the relative levels of expression of the host and the parasite heavy-chain γ-GCS to determine whether changes in their relative expression levels correlated with in vitro and in vivo differences in SSG susceptibility.
In vitro infection with L. donovani had a strain-dependent effect on murine macrophage γ-GCS expression, with the SSG-S strain causing an up-regulation, and the SSG-R strain almost ablating, γ-GCS expression, compared with uninfected controls. Previous studies by other workers have shown that infection with L. donovani can modulate the expression of a range of genes (3), resulting in a down-regulation in most cases. For example, L. donovani infection down-regulates macrophage expression of the transcription factors NF-κB p105 and RelB, which regulate the expression of genes associated with signaling pathways, including control of inflammatory responses. A binding site for the transcription factor NF-κB is in the promoter region of the γGCS heavy-chain gene (27), and recent studies have shown that NF-κB regulates the expression of γ-GCS (20). In macrophages NF-κB regulates the expression of γ-GCS if cells are stimulated with low levels of nitric oxide (23) or exposed to reactive oxygen intermediates (34). Down-regulation of the antioxidant GSH would seem to be a disadvantage to the parasite since it would make its host cell a more hostile environment (i.e., more oxidative) when exposed to oxidative stress (e.g., as part of the host's antileishmanial immune response) and more susceptible to cytotoxic effects, such as apoptosis. However, infection with L. donovani protects against apoptosis (30) and is associated with a reduced expression of the proapoptotic BAD gene (3). SSG is often considered a prodrug that requires reduction of antimony from the pentavelent to the trivalent form to be fully effective (13). Indeed recent studies have indicated that uptake of SbIII by Leishmania parasites is mediated by aquaglyceroporin (14) and that expression of aquaglyceroporin in the amastigote may control antimony resistance by controlling SbIII influx. Therefore, preferential induction of an oxidative environment would reduce SSG efficacy and favor drug resistance. The SSG-R strain had increased levels of γ-GCS compared with the SSG-S strain, which would result in higher GSH levels, and thus give it a higher antioxidant capability and compensate for the oxidative environment produced by down-regulating host γ-GCS. This would also correlate with the higher resistance of the SSG-R strain compared to the SSG-S strain to killing by immunostimulated macrophages or treatment with hydrogen peroxide or potassium antimony tartrate found in previous studies (7). Other workers have also reported that Leishmania drug-resistant strains overexpress genes associated with GSH biosynthesis (γ-GCS and GSH synthetase genes) in the presence or absence of drug. For example, γ-GCS was found to be overexpressed by 2- to 3-fold using microarrays, which would be similar to the levels found in in vitro-infected macrophages in this study, whereas Northern blots indicated that the overexpression was much higher, at 11-fold (18).
Unfortunately, infection with either strain did not have the same differential effect on the host γ-GCS expression in vivo compared to that found in vitro. This may be due to the fact that in vitro studies used just macrophages whereas in vivo studies used whole tissues. Differences in the relative levels of expression are likely to occur only in infected macrophages and thus may be difficult to measure in complex, whole tissues. Furthermore, γ-GCS expression may be affected by the fidelity associated with processing samples where the magnitude in relative expression is relatively small (<10-fold) and by in vivo conditions, including not only the presence of other cell types but also their influence on infected macrophages and endogenous levels of GSH in uninfected cells (25). However RT-PCR studies did highlight that host and parasite γ-GCSs are differentially regulated and that the effects are organ specific. SSG, BSO-NIV, and BSO-NIV/SSG treatments were associated with an increase in host γ-GCS in mice infected with either strain of L. donovani in the spleen but not in the liver. There is some correlation between host γ-GCS expression and total liver GSH levels measured in previous studies (6). For example, SSG treatment had no significant effect on total liver GSH levels measured in mice infected with either L. donovani strain (6) or host γ-GCS expression in this study. However SSG treatment was associated with a significant reduction in γ-GCS expression in the SSG-S, but not the SSG-R, strain compared to controls. Therefore, this study emphasizes the importance of being able to differentiate between host and parasite GSH since simply measuring total GSH did not reveal species-specific effects. Ideally an in situ method is required to monitor GSH levels, e.g., using confocal laser scanning microscopy (35). The greatest increase in host γ-GCS was obtained in spleen samples from mice infected with the SSG-R strain and treated with SSG (>100-fold increase), whereas the greatest increase in Leishmania γ-GCS was obtained in spleen samples from mice infected with the SSG-S strain. Perhaps the high increase in host γ-GCS and thus GSH production protected the SSG-R strain against SSG, by promoting drug efflux or by acting as an antioxidant, and thus negated the requirement for parasite evasive measures such as up-regulation of Leishmania γ-GCS.
This study showed that using NIV to deliver GSH enhanced its antileishmanial activity since similar treatment with GSH solution was inactive. Free GSH has a plasma half-life of 2 min and is rapidly removed from the circulation via the kidneys and lungs (26). We have previously shown that using a NIV formulation can alter drug pharmacokinetics and improve targeting to tissues (6). The antileishmanial activity of GSH was dependent on the environmental conditions and the strain of L. donovani used. GSH did not have any inherent antileishmanial activity in this study, even at cytotoxic doses (20 mM), since similar numbers of parasites were present in control samples and macrophages infected with either strain of L. donovani. This was surprising since Singh et al. (34) imply that GSH would be antileishmanial since treatment of J774.1 cells with 2 mM GSH resulted in a reduction in uptake of L. donovani. In this study, in vivo treatment with GSH-NIV resulted in a significant reduction in liver parasite burdens in mice infected with the SSG-S strain but not the SSG-R strain, indicating that the environmental conditions are important. Previous workers have shown that treatments to increase GSH levels correlate with immunological changes which would favor parasite killing, i.e., an enhanced ability to produce IL-12, which would drive Th1 responses and IFN-γ and nitric oxide production (31). However in this study GSH-NIV treatment alone or combined with SSG had no significant effect on IFN-γ or nitrite, used as an indirect measure of nitric oxide, production, indicating that therapeutic efficacy was not dependent on changes in host immune responses. In contrast, Rocha-Vieria et al. (32) showed that oral treatment with N-acetyl-l-cysteine to increase GSH levels in mice infected with L. major resulted in a reduction in parasite burdens. This effect was associated with an increase in the number of IFN-γ- and tumor necrosis factor alpha-producing cells in the lymph nodes of treated mice. However perhaps the agent used to supplement intracellular GSH levels or the immunostimulant used is important. Murata et al. (31) used GSH diethyl ester, which is more efficiently transported into cells than GSH (24).
An alternative physicochemical explanation would explain the ability of GSH-NIV to enhance the activity of SSG treatment. The induction of a reductive environment by cotreatment with GSH-NIV would favor the transformation of antimony in SSG to its more toxic trivalent form and thus explain the increased efficacy of combined GSH-NIV/SSG treatment obtained in vivo. Recent studies have shown that treatment of amastigotes with either pentavalent or trivalent antimony has two adverse effects on the parasites. First, such treatment decreases the thiol buffering capacities of the parasites by inducing rapid efflux of intracellular trypanothione (TSH) and GSH in equimolar amounts, which would make the parasites more susceptible to oxidative stress induced, e.g., by an ongoing immune response. Second, it causes an increase in the accumulation of the disulfide forms of GSH and TSH within the parasites by inhibiting TSH reductase, which would have profound effects on the redox potentials within the parasites. An increase in redox potential of +70 mV can cause a cell to go from proliferation into apoptosis (36). The inability of GSH, at nontoxic doses, to increase the efficacy of SSG in vitro indicates that environmental conditions are important. Perhaps the poor ability of GSH to enter cells (24) would explain the lack of correlation between in vitro and in vivo results, or perhaps an oxidative environment associated with the immune responses induced in vivo is required.
In summary, this study showed that L. donovani parasites with increased resistance to SSG have developed a mechanism to transcriptionally down-regulate host γ-GCS production and augment its resistance to oxidative stress by increasing its own γ-GCS production. It also indicates that L. donovani γ-GCS is a rational target for novel drugs and the potential of selective inhibitors of this enzyme.
Acknowledgments
K. Carter received support from The Royal Society of London to initiate some of the experiments carried out in this study.
REFERENCES
- 1.Arfsten, D., E. Johnson, A. Thitoff, A. Jung, E. Wilfong, S. Lohrke, T. Bausman, J. Eggers, and A. Bobb. 2004. Impact of 30-day oral dosing with N-acetyl-L-cysteine on Sprague-Dawley rat physiology. Int. J. Toxicol. 23:239-247. [DOI] [PubMed] [Google Scholar]
- 2.Arrick, B. A., O. W. Griffith, and A. Cerami. 1981. Inhibition of glutathione synthesis. J. Exp. Med. 153:720-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buates, S., and G. Matlashewski. 2001. General suppression of macrophage gene expression during Leishmania donovani infection. J. Immunol. 166:3416-3422. [DOI] [PubMed] [Google Scholar]
- 4.Cai, J., Y. Chen, S. Seth, S. Furukawa, R. W. Compans, and D. P. Jones. 2003. Inhibition of influenza infection by glutathione. Free Radic. Biol. Med. 34:928-936. [DOI] [PubMed] [Google Scholar]
- 5.Carter, K. C., A. J. Baillie, J. Alexander, and T. F. Dolan. 1988. The therapeutic effect of sodium stibogluconate in the BALB/c mice infected with L. donovani is organ dependent. J. Pharm. Pharmacol. 40:370-373. [DOI] [PubMed] [Google Scholar]
- 6.Carter, K. C., S. Sundar, C. Spickett, O. C. Pereira, and A. B. Mullen. 2003. The in vivo susceptibility of Leishmania donovani strains to sodium stibogluconate is drug specific and can be reversed by inhibiting glutathione biosynthesis. Antimicrob. Agents Chemother. 47:1529-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter, K. C., S. Hutchison, A. Boitelle, H. W. Murray, S. Sundar, and A. B. Mullen. Sodium stibogluconate resistance in Leishmania donovani is associated with greater resistance to macrophage antileishmanial responses and potassium antimony tartrate. Parasitology, in press. [DOI] [PubMed]
- 8.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, S. N., A. C. Chang, and L. Hsu. 1972. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R factor DNA. Proc. Natl. Acad. Sci. USA 69:2110-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin, C. C., M. E. Rosenfeld-Franklin, C. White, T. J. Kavanagh, and N. Fausto. 2003. TGFbeta1-induced suppression of glutathione antioxidant defenses in hepatocytes: caspase-dependent post-translational and caspase-independent transcriptional regulatory mechanisms. FASEB J. 17:1535-1537. [DOI] [PubMed] [Google Scholar]
- 11.Fraternale, A., A. Casabianca, C. Orlandi, A. Cerasi, L. Chiarantini, G. Brandi, and M. Magnani. 2002. Macrophage protection by addition of glutathione (GSH)-loaded erythrocytes to AZT and DDI in a murine AIDS model. Antivir. Res. 56:263-272. [DOI] [PubMed] [Google Scholar]
- 12.Ghezzi, P., and D. Ungheri. 2004. Synergistic combination of N-acetylcysteine and ribavirin to protect from lethal influenza viral infection in a mouse model. Int. J. Immunopathol. Pharmacol. 17:99-102. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin, L. G., and J. E. Page. 1943. A study of the excretion of organic antimonials using polographic procedure. Biochem. J. 37:198-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gourbal, B., N. Sonuc, H. Bhattacharjee, D. Légaré, S. Sundar, M. Ouellette, B. P. Rosen, and R. Mukhopadhyay, R. 2004. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J. Biol. Chem. 279:31010-31017. [DOI] [PubMed] [Google Scholar]
- 15.Griffith, O. W.,. 1999. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 27:922-935. [DOI] [PubMed] [Google Scholar]
- 16.Grondin, K., A. Haimeur, R. Mukhopadhyay, B. P. Rosen, and M. Ouellette. 1997. Co-amplification of the gamma-glutamylcysteine synthetase gene gsh1 and of the ABC transporter gene pgpA in arsenite-resistant Leishmania tarentolae. EMBO J. 16:3057-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guevara, A. G., E. Guilvard, M. M. Borges, M. M., A. Cordeiro da Silva, and A. Ouaissi. 2000. N-acetylcysteine and glutathione modulate the behaviour of Trypanosoma cruzi experimental infection. Immunol. Lett. 71:79-83. [DOI] [PubMed] [Google Scholar]
- 18.Guimond, C., N. Trudel, C. Brochu, N. Marquis, A. El Fadili, R. Peytavi, G. Briand, D. Richard, N. Messier, B. Papadopoulou, J. Corbeil, M. G. Bergeron, D. Legare, and M. Ouellette. 2003. Modulation of gene expression in Leishmania drug resistant mutants as determined by targeted DNA microarrays. Nucleic Acids Res. 31:5886-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddad, J. J.,. 2002. Oxygen-sensing mechanisms and the regulation of redox-responsive transcription factors in development and pathophysiology. Respir. Res. 3:26-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang, J. H., and Y. J. Surh. 2004. Bcl-2 attenuation of oxidative cell death is associated with up-regulation of gamma-glutamylcysteine ligase via constitutive NF-κB activation. J. Biol. Chem. 279:38779-38786. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, A. M., J. P. Dubey, and J. B. Dame. 1986. Purification and characterization of Toxoplasma gondii tachyzoite DNA. Aust. J. Exp. Biol. Med. Sci. 64:351-355. [DOI] [PubMed] [Google Scholar]
- 22.Khynriam, D., and S. B. Prasad. 2003. Cisplatin-induced genotoxic effects and endogenous glutathione levels in mice bearing ascites Dalton's lymphoma. Mutat. Res. 526:9-18. [DOI] [PubMed] [Google Scholar]
- 23.Kurozumi, R., and S. Kojima. 2005. Increase of intracellular glutathione by low-level NO mediated by transcription factor NF-κB in RAW 264.7 cells. Biochim. Biophys. Acta 1744:58-67. [DOI] [PubMed] [Google Scholar]
- 24.Levy, E., M. E. Anderson, and A. Meister. 1993. Transport of glutathione diethyl ester into human cells. Proc. Natl. Acad. Sci. USA 90:9171-9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, S. L., C. M. Chuong, R. B. Widelitz, and S. Y. Ying. 1999. In vivo analysis of cancerous gene expression by RNA-polymerase chain reaction. Nucleic Acids Res. 27:4585-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lomaestro, B. M., and M. Malone. 1995. Glutathione in health and disease pharmacotherapeutic issues. Ann. Pharmacother. 29:1263-1273. [DOI] [PubMed] [Google Scholar]
- 27.Lu, S. C. 2000. Regulation of glutathione synthesis. Curr. Top. Cell. Regul. 36:95-116. [DOI] [PubMed] [Google Scholar]
- 28.Luersen, K., R. D. Walter, and S. Muller. 2002. Plasmodium falciparum-infected red blood cells depend on a functional glutathione de novo synthesis attributable to an enhanced loss of glutathione. Biochem. J. 346:545-552. [PMC free article] [PubMed] [Google Scholar]
- 29.Min, G. S., and J. R. Powell. 1997. Long-distance genome walking using the long and accurate polymerase chain reaction. BioTechniques 24:398-400. [DOI] [PubMed] [Google Scholar]
- 30.Moore, K. J., and G. Matlashewski. 1994. Intracellular infection by. Leishmania donovani inhibits macrophage apoptosis. J. Immunol. 152:2930-2937. [PubMed] [Google Scholar]
- 31.Murata, Y., T. Shimamura, and J. Hamuro. 2002. The polarization of Th1/Th2 balance is dependent on the intracellular thiol redox status of macrophages due to distinctive cytokine production. Int. Immunol. 14:201-212. [DOI] [PubMed] [Google Scholar]
- 32.Rocha-Vieira, E., E. Ferreira, P. Vianna, D. R. De Faria, S. T. Gaze, W. O. Dutra, and K. J. Gollob. 2003. Histopathological outcome of Leishmania major-infected BALB/c mice is improved by oral treatment with N-acetyl-l-cysteine. Immunology 108:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Safeukui, I., F. Mangou, D. Malvy, P. Vincendeau, D. Mossalayi, G. Haumont, R. Vatan, P. Olliaro, and P. Millet, P. 2004. Plasmodium berghei: dehydroepiandrosterone sulfate reverses chloroquino-resistance in experimental malaria infection; correlation with glucose 6-phosphate dehydrogenase and glutathione synthesis pathway. Biochem. Pharmacol. 68:1903-1910. [DOI] [PubMed] [Google Scholar]
- 34.Singh, A. K., S. Balaraman, P. Tewary, and R. Madhubala. 2004. Leishmania donovani activates nuclear transcription factor-κΒ in macrophages through reactive oxygen intermediates. Biochem. Biophys. Res. Commun. 322:1086-1095. [DOI] [PubMed] [Google Scholar]
- 35.Stevenson, D., D. Wokosin, J. Girkin, and M. H. Grant. 2002. Measurement of the intracellular distribution of reduced glutathione in cultured rat hepatocytes using monochlorobimane and confocal laser scanning microscopy. Toxicol. In Vitro 16:609-619. [DOI] [PubMed] [Google Scholar]
- 36.Wyllie, S., M. L. Cunningham, and A. H. Fairlamb. 2004. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J. Biol. Chem. 279:39925-39932. [DOI] [PubMed] [Google Scholar]