Abstract
The human cytomegalovirus tegument protein pp71 is the product of the UL82 gene. Roles for pp71 in stimulating gene transcription, increasing infectivity of viral DNA, and the degradation of retinoblastoma family proteins have been described. Here we report a novel function for pp71 in limiting accumulation of cell surface major histocompatibility complex (MHC) class I complexes. MHC molecules were analyzed in glioblastoma cells exposed to a replication-defective adenovirus expressing UL82 (Adpp71) or after transient transfection of the UL82 gene. Accumulation of cell surface MHC class I levels diminished in a specific and dose-dependent manner after exposure to Adpp71 but not after exposure to an adenovirus expressing β-galactosidase (Adβgal). UL82 expression did not interfere with accumulation of either MHC class I heavy-chain transcript or protein, nor did UL82 expression correlate with markers of apoptosis. Rather, UL82 expression correlated with an increased proportion of MHC class I molecules exhibiting sensitivity to endoglycosidase H treatment. Finally, we show that, in cells infected with recombinant virus strain missing all of the unique short region MHC class I evasion genes, disruption of UL82 expression by short, interfering RNAs led to increased accumulation of cell surface MHC class I complexes. These findings support a novel role for HCMV pp71 in disruption of the MHC class I antigen presentation pathway.
Human cytomegalovirus(HCMV) infections are prevalent in human populations. Although infections in immunocompetent adults are usually benign, considerable morbidity, mortality, and sequelae are observed in infants infected congenitally and to a lesser extent those infected perinatally. HCMV also causes variable diseases and complications in immunocompromised patient populations (reviewed in reference 36).
The prevalence and pathogenesis of HCMV infections relate to the ability of this virus to establish lifelong infections in its hosts, a property common to viruses in the family Herpesviridae. HCMV encodes a myriad of gene products that interfere with or modulate human immune responses (reviewed in references 30, 32, and 33). Characterization of these viral immune modulation strategies will impact our understanding of how this virus persists in human populations and the development of rationally designed vaccine candidates.
Curiously, both HCMV and murine cytomegalovirus (MCMV) encode multiple gene products that disrupt major histocompatibility complex (MHC) class I-mediated presentation of viral peptides to CD8+ T lymphocytes (reviewed in references 5, 14, and 25). Typically, viral peptides derived from proteasome-mediated degradation of viral gene products are loaded on MHC class I complexes in the endoplasmic reticulum (ER). Assembled MHC class I complexes are subsequently transported to the cell surface, where they can be recognized by cognate T-cell receptors (TCR) on the surfaces of CD8+ T lymphocytes. Engagement of the TCR by the viral peptide-MHC class I complex is a prerequisite for activation of the CD8+ T cells and subsequent cytolysis or control of HCMV-infected cells.
The diversity of strategies employed by the cytomegaloviruses to evade control by antiviral CD8+ cytotoxic T lymphocytes (CTL) may be related to the remarkable efficacy of these immune cells in controlling virus replication and associated clinical disease. For example, in irradiated mice, it was estimated that as few as 10 adoptively transferred virus-specific Lyt-2+ T lymphocytes were sufficient to reduce virus replication upon challenge with MCMV (38). Moreover, in humans, several studies have indicated a link between CTL responses to HCMV and recovery from clinical manifestations of disease (37, 40, 46).
Within the HCMV genome, the MHC class I viral evasion gene products (also referred to as viral evasins) are encoded by a single gene family in the unique short (US) region and are expressed with immediate early (IE) (US3) or early (US2, US6, and US11) kinetics (1, 2, 13, 19, 20, 21, 28, 48). Recently, two additional members of this gene family, US8 and US10, also have been shown to interact with MHC class I molecules (10, 44).
Here we report that the HCMV protein pp71, the product of the UL82 gene, is also capable of interfering with cell surface expression of MHC class I complexes. We found that ectopic UL82 expression in human glioblastoma cells causes a dose-dependent decrease in the accumulation of cell surface MHC class I complexes. UL82 expression did not interfere with accumulation of either MHC class I heavy-chain transcript or protein. Rather, we found that pp71 delayed transport of MHC class I complexes from the ER or cis-Golgi apparatus. Moreover, we show that, in cells infected with recombinant virus RV7186, a mutant strain missing all of the US region MHC class I evasion genes, disruption of UL82 expression by short, interfering RNAs (RNAi) led to increased accumulation of cell surface MHC class I complexes. This is the first report of an HCMV gene expressed outside of the US region and at late times after infection that can interfere with the transport and cell surface expression of MHC class I complexes.
MATERIALS AND METHODS
Cells and cytomegaloviruses. Experiments were performed with U373 MG human glioblastoma cells and U373 MG cells stably transfected with the CIITA gene (designated U373:CIITA) as described previously (8). U373 MG cell lines were maintained in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. Medium for U373:CIITA cells also was supplemented with 400 μg/ml of Geneticin (Gibco). HCMV strain AD169 was purchased from the American Type Culture Collection (ATCC). Recombinant virus RV7186, harboring a deletion spanning the IRS1-US12 genes, was a kind gift from Tom Jones (21). AD169 and RV7186 strains were propagated in MRC-5 cells maintained in modified Eagle's medium supplemented with 10% fetal calf serum, 1.7 mM sodium bicarbonate, 1.4 mM sodium chloride, essential and nonessential amino acids, vitamins, and sodium pyruvate at manufacturer-recommended concentrations (Sigma). Virus titers were determined in MRC-5 cells by standard plaque assay (47).
Recombinant adenoviruses.
Defective recombinant adenoviruses were propagated in HEK 293 cells. Adenovirus containing the β-galactosidase gene (Adβgal) was purchased from OD260 Inc. Recombinant adenovirus containing a UL82 gene (Adpp71) fused with the sequences specifying a FLAG epitope was generated by OD260 Inc. by insertion of the UL82 gene sequences into the E1 region of the Ad5 genome. The UL82 gene sequence utilized for the recombinant adenovirus was derived from plasmid pcDNApp71tag (kind gift from Bodo Plachter [15]). UL82 flanked by a CMV promoter and poly(A) sequences was excised from pcDNApp71tag by MluI and SphI restriction enzymes and inserted into the HindII site of pZAP2.1. This cassette in the left arm of the Ad5 genome was ligated to the right arm of the Ad 5 genome. In this laboratory, adenovirus stocks were prepared on 293 HEK cells maintained in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. Titers of recombinant adenovirus stocks were determined by the Adeno-X rapid titer kit (BD Biosciences) in 293 HEK cells. Adenovirus stocks were also evaluated for expression by exposing U373:CIITA cells to increasing doses (between 0.01 and 1,000 PFU per cell) of Adβgal or Adpp71. At 48 h after infection, Adβgal-infected cells were fixed with 2.5% glutaraldehyde followed by incubation with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (Invitrogen). Adpp71-infected cells were methanol fixed, blocked in 2% bovine serum albumin in phosphate-buffered saline (PBS), and incubated with primary antibody directed against pp71 (kind gift from Tom Shenk). Cells were rinsed and exposed to anti-mouse secondary antibody conjugated to horseradish peroxidase (Santa Cruz) and developed with VIP substrate (Vector Labs). β-Galactosidase activity and pp71 immunoreactivity were visualized by light microscopy. We observed variability in the number of PFU of recombinant adenovirus per cell required to achieve expression of the gene of interest in greater than 95% of the transduced cell population. This variability appeared to be related to the passage history of the recombinant adenovirus stocks. Therefore, in the experiments reported here, the PFU of recombinant adenoviruses per cell are reported using titers determined on HEK cells and were adjusted to insure that greater than 95% of U373:CIITA cells exhibited β-galactosidase activity or pp71 immunoreactivity.
Antibodies.
The anti-MHC class I antibody w6/32 (Dako) was used for immunoprecipitation (4), and the anti-MHC class I antibody HC10 (gift from Hidde Ploegh [43]) was used for immunoblot analysis of MHC class I heavy chains. Anti-HLA-A, -B, and -C (G46-2.6) conjugated to fluorescein isothiocyanate (FITC) or phycoerythrin (PE) was used for flow-cytometric analyses (BD PharMingen). The pp71-specific mouse monoclonal antibody was a generous gift from Tom Shenk. The anti-FLAG (M-2) antibody was purchased from Sigma. Other antibodies include the anti-caspase 3 antibody (E-8; Santa Cruz), antiactin (Chemicon), anti-glyceraldehyde dehydrogenase (GAPDH) (Chemicon), anti-annexin V (BD PharMingen), PE-conjugated anti-HLA-DR (G46-6, BD PharMingen), PE-conjugated anti-CD71 (BD PharMingen), and alkaline phosphatase-conjugated antidigoxigenin (DIG) Fab fragment (Roche).
Flow-cytometric analyses of cell surface proteins.
U373:CIITA cells were cultured in 24-well or 6-well plates and were exposed to 15 or 30 PFU of the AD169 strain of CMV per cell or various doses of defective recombinant adenoviruses Adβgal and Adpp71 as indicated in the figure legends. At times indicated in Results, cells were dislodged from the plastic surface by rinsing in edetate sodium (Versene) and very brief trypsinization, chilled to 4°C, and rinsed one time in Dulbecco's modified Eagle's medium supplemented with 10% serum and two times in cold PBS. Cells were left unstained or reacted with FITC- or PE-conjugated anti-HLA-A, -B, and -C; anti-CD71; or anti-HLA-DR antibodies according to the manufacturer's recommendation (BD PharMingen). Cells were fixed in 4% paraformaldehyde and analyzed using a FACSCalibur (Becton Dickinson). The data were analyzed with the aid of CellQuest software (Becton Dickinson). In double-stain experiments, single-stained controls were used to appropriately adjust fluorochrome compensation levels.
Preparation of cell lysates and immunoblotting.
Uninfected U373:CIITA cells and cells exposed to CMV or recombinant adenoviruses were rinsed in PBS and solubilized in lysis buffer containing 1% Triton X-100, 50 mM Tris, 150 mM NaCl, and 1% (vol/vol) protease inhibitor cocktail (Sigma). Lysates were incubated at 4°C for 30 min, and insoluble material was pelleted by centrifugation. Equivalent amounts of protein from each lysate were separated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to nitrocellulose sheets (Amersham). Sheets were reacted with the primary antibodies indicated in Results, and subsequently to a horseradish peroxidase-conjugated secondary antibody (Santa Cruz). Bound antibodies were visualized using a chemiluminescent detection system (ECL; Amersham) and exposure to film.
Transient transfection of cells.
Cultures of U373 cells in six-well plates were mock transfected, transfected with 1 μg/well of pEGFPN3 (Clontech), or transfected with 1 μg/well of pcDNApp71tag (gift from B. Plachter) using the GenJammer reagent (Strategene). The total amount of DNA was adjusted to 2 μg/well with pcDNA3.1 plasmid (Invitrogen). Forty-eight hours after transfection, cells were dislodged from the plates by brief exposure to trypsin, rinsed, reacted with PE-conjugated anti-human HLA-A, -B, and -C (PharMingen), and analyzed for MHC class I expression by flow cytometry as described above.
Caspase 3 activity and annexin V analyses.
Cultures of U373:CIITA cells in 75-cm2 flasks were mock infected or exposed to CMV or adenoviruses as described in the figure legends. Note that in this series of experiments the numbers of PFU of Adβgal and Adpp71 per cell in the inoculum were adjusted to insure that more than 95% of cells expressed the gene of interest. In parallel, cultures of uninfected U373:CIITA cells were exposed to 0.4 μM of the apoptosis inducer staurosporine. At 48 h after infection or at 0, 0.5, 3, and 6 h after exposure to staurosporine, cell lysates were prepared and equivalent amounts of protein from each lysate were separated by SDS-PAGE, transferred to nitrocellulose sheets, and subjected to immunoblot analysis using anti-caspase 3 antibody. A second experiment was performed essentially as described above except that cells were exposed to staurosporine for 0, 3, 6, or 16 h. Subsequently, cells were dislodged from the flasks and analyzed for caspase activity using a fluorometric caspase 3 activity assay (Calbiochem). In a third experiment, replicate cultures of U373:CIITA cells in 24-well plates were mock infected and exposed the AD169 strain of CMV or exposed to Adβgal or Adpp71. In parallel, cultures of U373:CIITA cells in 24-well plates were exposed to 0.2 μM of the apoptosis inducer staurosporine. At the times indicated in Results, cells were dislodged from the plates and analyzed for cell surface annexin V expression by flow cytometry.
Northern blotting.
RNA was isolated from U373:CIITA cells cultured in six-well plates at the time points indicated in Results by guanidinium isothiocyanate extraction and centrifugation through cesium chloride. Ten micrograms of each RNA sample was subjected to electrophoresis in 3% formaldehyde-agarose gels and transferred to nylon membranes by positive pressure. RNA blots were incubated at 42°C overnight with a heat-denatured [α-32P]CTP-labeled probe generated from HLA-B7 cDNA (ATCC) by nick translation (Amersham) or a digoxigenin-labeled probe generated from GAPDH cDNA (ATCC) also by nick translation (Roche). Nylon membranes exposed to radiolabeled probes were rinsed and exposed to film. Sheets exposed to the DIG-labeled probe were rinsed, exposed to anti-DIG antibody, developed with a chemiluminescent CSPD substrate (Roche), and exposed to film.
[35S]methionine labeling, immunoprecipitation, and endo H treatment.
Cultures of U373:CIITA cells grown in 75-cm2 flasks were mock infected or exposed to 10 PFU of Adβgal per cell or 100 PFU of Adpp71 per cell. At 48 or 72 h after infection, cells were rinsed twice in methionine-free media (Sigma), dislodged from the plates, and incubated in media supplemented with [35S]methionine (Amersham) for 30 min to radiolabel proteins (200 μCi per 2 × 106 cells). Cultures were washed and/or further incubated with unlabeled media and harvested at various times ranging from 0 to 300 min after the radiolabel pulse period. In other experiments U373:CIITA cells grown in 25-cm2 flasks were mock infected or exposed to 10 PFU per cell of Ad169 or RV7186. At 48 after infection, cells were rinsed in methionine-free media (Sigma) and incubated in media supplemented with [35S]methionine (Amersham) for 30 min to radiolabel proteins (180 μCi per 1 × 106 cells). Cells were rinsed and solubilized immediately or after further incubation with unlabeled media for 0, 15, or 60 min. Cells were solubilized in 1% Triton X-100, 50 mM Tris, 150 mM NaCl, and 1% (vol/vol) protease inhibitor cocktail (Sigma), and MHC class I proteins were immunoprecipitated with w6/32 anti-MHC class I antibody. One-half of the isolated proteins were incubated with endoglycosidase H (endo H) (Sigma or New England Biolabs) for 2 h according to the manufacturer's recommendations. Proteins were separated in 10 or 12% denaturing gels, which were fixed in a solution of 20% methanol and 10% acetic acid and dried. Radiolabeled proteins were visualized by autoradiography.
Immunofluorescence microscopy.
Cultures of U373:CIITA cells grown on 12-mm glass coverslips in six-well plates were mock infected or exposed to 50 PFU/cell of the AD169 strain of CMV per cell or to 100 PFU/cell of Adβgal or Adpp71 recombinant adenoviruses. At 48 and 72 h after infection cells were rinsed with PBS, fixed in 4% paraformaldehyde for 30 min at room temperature, rinsed three times with PBS, air dried, and stored at 4°C until staining. After being blocked with PBS containing 1% goat serum and 0.1% Triton X-100 (staining buffer), cells were incubated with a 1:100 dilution of a rabbit polyclonal anti-pp71 antibody, rinsed extensively in staining buffer, and incubated with 1:200 dilution of rhodamine red-conjugated goat anti-rabbit F(ab′)2 fragment. Cells were rinsed five times with staining buffer, incubated with a 1:50 dilution of FITC-conjugated mouse monoclonal anticalnexin (BD Transduction Laboratories), and washed in PBS. Alternatively, cells were reacted with FITC-conjugated anti-MHC class I (clone w6/32; Sigma). Finally, cells were incubated for 5 min with a 1:2,000 dilution of the nuclear stain DRAQ5 (Biostatus Limited, Leicestershire, United Kingdom), followed by five quick washes with PBS. Cells were mounted on glass slides with Prolong antifade kit (Molecular Probes, Eugene, Oregon). Microscopy was performed using a Zeiss LSM 510 Meta microscope. Each fluorescent dye was scanned separately to avoid any spectral overlap. Images were prepared using Zeiss LSM510 software.
RNA interference experiments.
Chemically synthesized RNAi duplexes were purchased from Dharmacon Research, Inc. RNAi duplexes were designed for the following targets: lamin a/c, AACUGGACUUCCAGAAGAACA; pp71 (71-3), AAUCUCCGCACGACACCGUAG; pp71 (71-5), AAGCGGUAACAACGGGGCUCU. All experiments were performed in cultures of U373 or U373:CIITA cells grown in the absence of antibiotics in 24-well plates. Twenty-four hours after plating, cells were transfected with 0.84 μg of RNAi duplex per well using Oligofectamine reagent (Invitrogen). At 24 h after transfection, cells were exposed to AD169 or RV7186 as indicated in the figure legends. Flow cytometry and Western analyses were performed as described above 48 h after infection.
Statistical analysis.
A linear mixed model was fit to the flow-cytometric mean fluorescence data for both MHC class I and CD71. The model included random effects and their variance components for date of experiment, hour within experiment, and residual error. Separate hypothesis tests compared differences between Adpp71 infection and Adβgal infection on MHC class I and CD71 cell surface expression levels. For comparison, CMV infection versus mock infection was also analyzed for MHC class I and CD71 cell surface expression. An interaction contrast test was also performed to compare the difference between Adpp71 infection and Adβgal infection on MHC class I expression to the difference between Adpp71 infection and Adβgal infection on CD71 expression. For this interaction contrast, the data were standardized to standard deviation units for MHC class I and CD71. This was done to show that the Adpp71 effect on MHC class I expression was larger than the Adpp71 effect on CD71 expression. For the dose-response experiments, a linear mixed model was fit to the MHC class I mean fluorescence measurements. The model included random effects and variance components for date of experiment and residual error. A significance test for the contrast of the dose-response curves for Adpp71 versus Adβgal was then performed. For the RNAi experiments, MHC class I mean fluorescence means were compared for RV7186-infected cells treated with RNAi specific for pp71 and cells treated with RNAi specific for lamin.
RESULTS
Decreased accumulation of cell surface MHC class I in cells expressing pp71 protein.
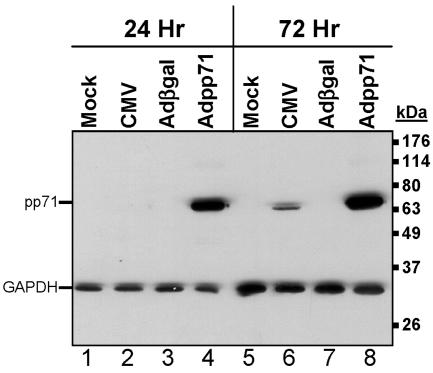
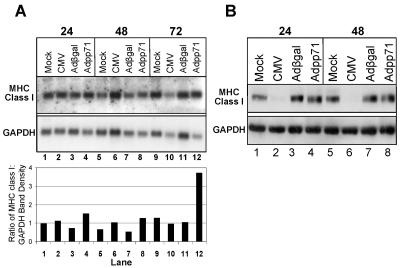
To develop a system to test whether the CMV tegument protein pp71 could modulate or interfere with cellular processes related to the generation of immune responses, we generated a defective recombinant adenovirus containing the UL82 gene. To confirm expression of the UL82 gene product, U373 MG cells stably expressing CIITA, the major MHC class II transactivator, were exposed to 100 PFU/cell of recombinant adenovirus containing the gene sequences for UL82 (Adpp71) or β-galactosidase (Adβgal). In parallel, cells were either mock infected or exposed to 30 PFU/cell of the AD169 strain of CMV. At 24 and 72 h after infection, cells were harvested and solubilized and protein lysates were separated by SDS-PAGE and subjected to immunoblotting using antibody specific to pp71 or, as an additional loading control, antibody specific for GAPDH. As shown in Fig. 1, a protein of the expected molecular mass was observed in cells transduced with Adpp71. Although pp71 could not be detected 24 h after infection with CMV, high levels of pp71 could be detected in cells exposed to Adpp71 at this time (compare lanes 2 and 4). At 72 h after infection, pp71 was detected in both CMV- and Adpp71-infected cells, though again higher levels were observed in cells exposed to Adpp71 (compare lanes 6 and 8).
FIG. 1.
Accumulation of pp71 protein in cells infected with recombinant adenovirus containing the UL82 gene compared to CMV-infected cells. Shown is a film image of electrophoretically separated cell lysates reacted with antibody to pp71. Cultures of U373:CIITA cells in six-well plates were either mock infected or exposed to 30 PFU/cell of the AD169 strain of human cytomegalovirus or 100 PFU/cell of Adβgal or Adpp71. Cells were harvested 24 h and 72 h after exposure to virus and solubilized, and 30 μg of protein from each lysate was subjected to electrophoresis in a denaturing polyacrylamide gel. Proteins were transferred to nitrocellulose sheets and reacted with mouse monoclonal antibody to pp71 and, as an additional loading control, mouse monoclonal antibody to GAPDH, as described in Materials and Methods.
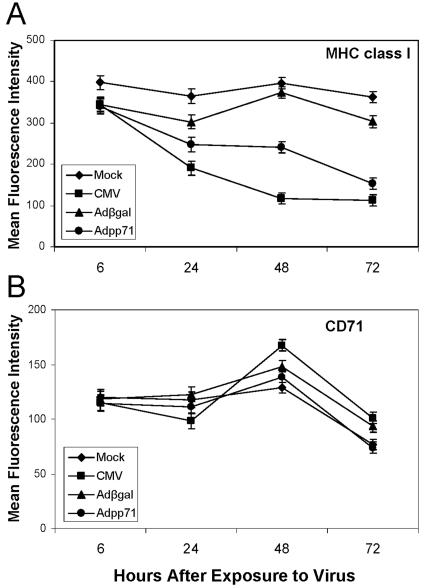
The recombinant adenoviruses were next employed to test whether expression of the pp71 protein altered accumulation of cell surface MHC class I or CD71. Cultures of U373:CIITA cells in 24-well plates were mock infected, exposed to 15 or 30 PFU/cell of the AD169 strain of human cytomegalovirus per cell, or exposed to 100 PFU of Adβgal or Adpp71. Cells were dislodged from the plates at the times after infection indicated in Fig. 2 and analyzed for cell surface MHC class I, CD71, and MHC class II proteins by flow cytometry as described in Materials and Methods. A linear mixed model was fit to the mean fluorescence data for both MHC class I and CD71 values derived from three independent experiments (Fig. 2). The results were as follows. Relative to uninfected cells, cells infected with CMV exhibited a 1.9-fold reduction in MHC class I cell surface expression beginning at 24 h (Fig. 2A). By 72 h after infection, the levels of MHC class I expression were 3.2-fold lower than that observed in uninfected cells. In a pattern similar to that for CMV-infected cells, cell surface MHC class I levels also decreased in Adpp71-infected cells. Relative to Adβgal-infected cells, 1.5-fold- and 1.9-fold-lower values were derived from the 48- and 72-h time points, respectively. In contrast, there was no change in (48 h) or 1.2-fold-lower values (72 h) for CD71 cell surface expression in Adpp71-infected cells compared to Adβgal-infected cells (Fig. 2B).
FIG. 2.
Accumulation of cell surface molecules in cells infected with CMV or recombinant adenoviruses expressing β galactosidase, or pp71. Shown are cell surface MHC class I and CD71 levels in cells expressing pp71. Cultures of U373:CIITA cells in 24-well plates were either mock infected or exposed to 15 or 30 PFU/cell of the AD169 strain of human cytomegalovirus or 100 PFU/cell of defective adenovirus Adβgal or Adpp71. Cells were dislodged from the plates at the indicated times after infection, reacted with FITC-conjugated anti-MHC class I (A) and PE-conjugated anti-CD71 antibodies (B), and analyzed by flow cytometry. Data points represent least-square mean fluorescence intensities and standard errors of the means for between 6 and 12 replicate samples for each infection condition at each time point, which were obtained from three independent experiments.
The statistical modeling ascertained the significance of these differences over the 24- to 72-h time period. The test of the interaction contrast revealed that the effect of Adpp71 infection on MHC class I surface expression was significantly greater than on CD71 surface expression (P = 0.0044). As expected, MHC class I expression was significantly lower in CMV-infected cells compared to mock-infected cells (P < 0.0001). Also as expected, MHC class I expression was significantly lower in Adpp71-infected cells compared to Adβgal-infected cells (P < 0.0001). The mixed linear model employed also detected a less robust but nevertheless significant difference (P = 0.0069) between Adpp71 infection and Adβgal infection with respect to CD71 surface expression. The difference between Adpp71 and Adβgal was 2.0 standard deviation units for MHC class I expression and 0.8 standard deviation unit for CD71 expression. As confirmed by the interaction contrast test, this difference in effects was significantly larger for MHC class I expression.
In other experiments we also evaluated the effect of ectopic pp71 expression on MHC class II cell surface expression. The findings (data not shown) were similar to the CD71 pattern in that cell surface MHC class II mean fluorescence values in cells exposed to either Adβgal or Adpp71 were similar to that observed in uninfected cells. This series of studies suggests that pp71 has little or no effect on the general accumulation of cell surface proteins. Rather, the effect of pp71 is specific to MHC class I proteins or to proteins that utilize the same transport pathway as MHC class I complexes.
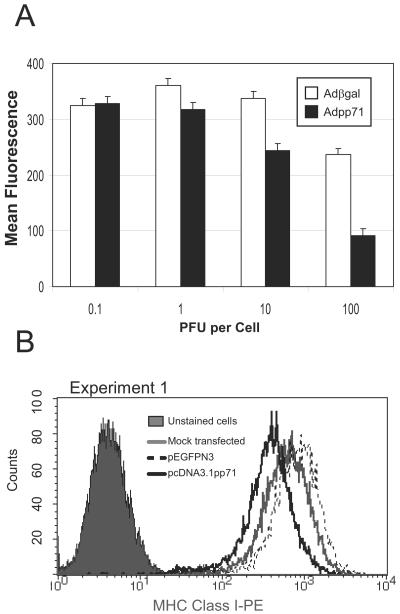
We observed that, in cells infected with Adβgal, MHC class I cell surface levels were typically somewhat lower than in uninfected cells. To determine if the effect of Adpp71 infection on MHC class I cell surface levels was due to adenovirus gene products (e.g., E3/19kD) or nonspecific effects of adenovirus infection or to an unusual characteristic of the U373:CIITA cell line, two additional experiments were performed. In the first experiment (Fig. 3A), replicate cultures of U373:CIITA cells in 24-well plates cells were exposed to increasing doses of Adβgal or Adpp71. At 24 and 48 h after infection, cells were dislodged from the plates and analyzed for MHC class I cell surface expression by flow cytometry. Parallel concurrent studies indicated that 100% of cells exposed to 100 PFU of Adβgal per cell expressed β-galactosidase as determined in an in situ activity assay, and 100% of cells exposed to 100 PFU of Adpp71 per cell expressed pp71, as determined by an immunohistochemical approach (data not shown). Little or no change in MHC class I cell surface levels was noted in cells exposed to low multiplicities of infection of recombinant adenoviruses (0.01 and 0.1 PFU per cell). In cells exposed to 1 PFU per cell, MHC class I levels were slightly decreased in cells expressing pp71 compared to cells expressing β-galactosidase. At 10 PFU per cell, this difference was magnified such that MHC class I levels were 1.4-fold lower in cells expressing pp71. At 100 PFU per cell, MHC class I levels were 2.5-fold lower in Adpp71-infected cells compared to cells exposed to an equivalent dose of Adβgal. A similar pp71-specific dose-dependent decrease in MHC class I levels was noted at 24 h after infection (data not shown). Statistical analysis of the overall dose-response curve indicated that the MHC class I mean fluorescence values were significantly less in Adpp71-infected cells compared to cells exposed to Adβgal (P = 0.0039). At the highest dose of 100 PFU per cell, MHC class I levels decreased somewhat in cells exposed to Adβgal (27% lower than in cells exposed to 10 PFU of Adβgal per cell). These data confirm that pp71 expression influences cell surface MHC class I levels in a dose-dependent manner and that potential adenovirus-specific effects on MHC class I cell surface levels were modest.
FIG. 3.
Effects of pp71 on accumulation cell surface MHC class I cannot be attributed to nonspecific effects of exposure to recombinant adenoviruses. (A) MHC class I cell surface levels in cells exposed to increasing doses of defective recombinant adenoviruses. Cultures of U373:CIITA cells in 24-well plates were exposed to defective adenovirus Adβgal or Adpp71 at increasing multiplicities of infection. Cells were dislodged from the plates 48 h after infection, reacted with FITC-conjugated anti-MHC class I antibodies, and analyzed by flow cytometry. Shown are least-square mean fluorescence values and standard errors of means from five replicate samples derived from two independent experiments. (B) Histogram of MHC class I cell surface levels in transfected cells. Cultures of U373 cells in six-well plates were mock transfected or transfected with plasmid pcDNA3.1 containing the UL82 gene or plasmid pEGFPN3. Histograms are shown for cells reacted with FITC-conjugated anti-MHC class I antibodies compared to unstained cells.
In the second series of experiments, cultures of U373 MG cells in six-well plates were transfected with plasmid pcDNA3.1 containing the UL82 gene or a plasmid containing the gene for an enhanced green fluorescent protein (GFP). At 48 h after infection, cells were dislodged from the plates and analyzed for MHC class I cell surface expression by flow cytometry. One of several experiments is shown in Fig. 3B. Cells expressing pp71 exhibited a decrease of 2.1-fold in the mean fluorescence of MHC class I cell surface molecules compared to pEGFPN3-transfected cells. Taken together, these studies indicate that expression of pp71 results in a selective and specific decrease of cell surface MHC class I molecules in U373 cells and in U373:CIITA cells.
Decreased cell surface MHC class I in cells expressing pp71 protein could not be attributed to induction of apoptosis.
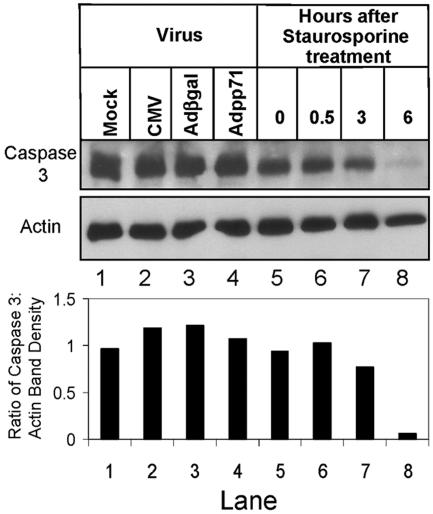
One possibility to account for the decrease in MHC class I cell surface levels is that toxicity related to expression of the CMV pp71 protein causes apoptosis of cells infected with Adpp71. To test this possibility, full-length caspase 3 protein levels were examined in cells infected with CMV, Adβgal, or Adpp71 (Fig. 4). Cultures of U373:CIITA cells in six-well plates were mock infected, exposed to 30 PFU/cell of the AD169 strain of CMV, or exposed to 15 PFU/cell of Adβgal or 100 PFU of Adpp71. In parallel, cultures of U373:CIITA cells in six-well plates were exposed to 0.2 μM of the apoptosis inducer staurosporine. Cells exposed to virus were harvested and solubilized 48 h after infection, and cells exposed to staurosporine were harvested at the times indicated in Fig. 4. Equivalent amounts of protein from each lysate were separated by SDS-PAGE, transferred to nitrocellulose sheets, and reacted with anti-caspase 3 antibody and, subsequently, antiactin antibody. Levels of full-length caspase 3 did not change relative to actin levels in cells infected with CMV, Adβgal, or Adpp71 (lanes 1 to 4). In contrast, exposure of cells to staurosporine led to a loss of full-length caspase 3 protein relative to actin levels within 6 h (lanes 5 to 8), consistent with cleavage of caspase 3 protein associated with apoptosis. These findings mirrored caspase 3 activity levels as determined by cleavage of fluorescent caspase 3 substrate (data not shown).
FIG. 4.
Effects of pp71 on accumulation cell surface MHC class I cannot be attributed to induction of apoptosis. Film image of electrophoretically separated cell lysates reacted with antibody to caspase 3 or actin. Cultures of U373:CIITA cells in six-well plates were mock infected, exposed to 30 PFU of the AD169 strain of CMV per cell, or exposed to15 PFU of Adβgal or 100 PFU of Adpp71 per cell. In parallel, cultures of U373:CIITA cells in six-well plates were exposed to 0.2 μM staurosporine. Cells exposed to virus were harvested and solubilized 48 h after infection, and cells exposed to staurosporine were harvested at the times indicated. One hundred micrograms of protein from each lysate was subjected to electrophoresis in a denaturing polyacrylamide gel. Proteins were transferred to nitrocellulose sheets and reacted with anti-caspase 3 antibody. Nitrocellulose sheets were then stripped and reprobed with antibody to actin as described in Materials and Methods. Integrated optical density determinations were made for each relevant band visualized on the film images, and the ratio of caspase 3 band density to actin band density is indicated for each lane below the film image.
In a second experiment, annexin V reactivity was evaluated as a measure of apoptosis in cells expressing UL82 (data not shown). Staurosporine-treated cells exhibited a decrease in MHC class I levels that correlated with increased annexin V reactivity. However, in cells infected with CMV and Adpp71, the decrease in MHC class I cell surface levels did not correlate with increased annexin V reactivity. From these experiments we conclude that the decrease in MHC class I cell surface levels in Adpp71-infected cells cannot be attributed to induction of apoptosis related to toxicity of adenovirus infection or expression of pp71.
Expression of pp71 protein does not alter MHC class I alpha chain transcript or protein levels.
Because pp71 is known to affect transcription of cellular genes and interacts or colocalizes with proteins demonstrated to repress transcription of cellular genes (e.g., DAXX, promyelocytic leukemia protein), we reasoned that pp71 may cause a decrease in accumulation of cell surface MHC class I proteins, either directly or indirectly, by negatively influencing transcription of MHC class I genes. To test this hypothesis, MHC class I α chain mRNA levels were analyzed (Fig. 5A). Replicate cultures of U373:CIITA cells in six-well plates were mock infected, exposed to 15 PFU of the AD169 strain of CMV per cell, or exposed to 100 PFU of Adβgal or Adpp71 per cell. Cells exposed to virus were harvested at 24, 48, and 72 h after infection, and total RNA was isolated and subjected to electrophoresis in duplicate formaldehyde-agarose gels. RNA was transferred to nylon membranes and incubated with a [32P]CTP-labeled riboprobe generated from HLA-B7 cDNA and the duplicate blot with a digoxigenin-labeled probe specific for GAPDH. Integrated optical density quantitation of RNA bands was performed, and the ratios of MHC class I transcripts to GAPDH transcripts are shown below the image of the Northern blots. At 24 h, 48 h, and 72 h after infection, the quantities of MHC class I transcript detected relative to GAPDH in mock- and CMV-infected cells were similar (compare lanes 1 and 2, 5 and 6, and 9 and 10). The quantity of MHC class I transcript detected relative to GAPDH was slightly higher in cells infected with Adpp71 compared to the quantity detected in cells infected with Adβgal at 24 and 48 h (compare lanes 3 and 4 and 7 and 8). At 72 h, however, almost fourfold-more class I transcript was observed in Adpp71-infected cells compared to mock-, CMV-, and Adβgal-infected cells (compare lanes 9 to 11 to 12). From this experiment we conclude that pp71-associated effects on transcription of MHC class I genes are not responsible for the decrease in accumulation of cell surface MHC class I molecules.
FIG. 5.
MHC class I transcript and protein levels are not altered in cells expressing pp71. (A) Autoradiographic and film images of electrophoretically separated RNA hybridized to HLA-B7-specific and GAPDH-specific probes. Replicate cultures of U373:CIITA cells in six-well plates were mock infected, exposed to 15 PFU/cell of the AD169 strain of CMV, or exposed to 100 PFU/cell of recombinant adenoviruses containing either the β-galactosidase gene or UL82. Cells exposed to virus were harvested at the times indicated. The cells of one well for each infection were solubilized, total RNA was isolated, and equivalent amounts of RNA were subjected to electrophoresis in formaldehyde-agarose gels in duplicate. RNA was transferred to nylon membranes by positive pressure. One nylon blot was incubated with 32P-labeled cDNA for HLA-B7 and the duplicate blot with a digoxigenin-labeled cDNA probe for GAPDH as described in Materials and Methods. 32P-labeled probes hybridized to MHC class I transcripts were visualized by autoradiography. Digoxigenin-labeled probes hybridized to GAPDH transcripts were reacted with anti-DIG antibody and visualized by chemiluminescence. Integrated optical density determinations were made for each relevant transcript, and the ratio of MHC class I to GAPDH transcript density is indicated for each lane below the autoradiographic and film images. (B) Film image of electrophoretically separated cell lysates reacted with antibody to MHC class I and GAPDH. To assess MHC class I protein levels, cells from the second well for each infection were harvested and solubilized and equivalent amounts of proteins were subjected to electrophoresis in a denaturing acrylamide gel. Proteins were transferred to nitrocellulose sheets and reacted with antibodies to MHC class I and GAPDH as described in Materials and Methods.
Because pp71 has been shown known to facilitate the degradation of cellular retinoblastoma family proteins, we tested whether pp71 could facilitate degradation of MHC class I proteins. To accomplish this, we determined if total protein levels of the MHC class I heavy chain were altered in cells expressing pp71. Cells grown in six-well plates were solubilized at 24 and 48 h after exposure to 15 PFU/cell of the AD169 strain of CMV or 100 PFU/cell of Adβgal or Adpp71. Equivalent amounts of protein from each lysate were separated by SDS-PAGE, transferred to nitrocellulose sheets, and reacted with HC10 antibody, which recognizes free heavy chains, and with antiactin antibody. As shown in Fig. 5B, MHC class I heavy chain levels were dramatically reduced in CMV-infected cells, consistent with the activities of the HCMV US2 and US11 gene products in targeting MHC class I molecules for degradation. In contrast, MHC class I heavy chain levels were similar in uninfected and Adβgal- and Adpp71-infected cells. These results indicate that the decreased accumulation of cell surface MHC class I levels cannot be attributed to pp71-mediated degradation of MHC class I heavy chains.
MHC class I molecules are delayed in acquiring endo H resistance in cells expressing pp71.
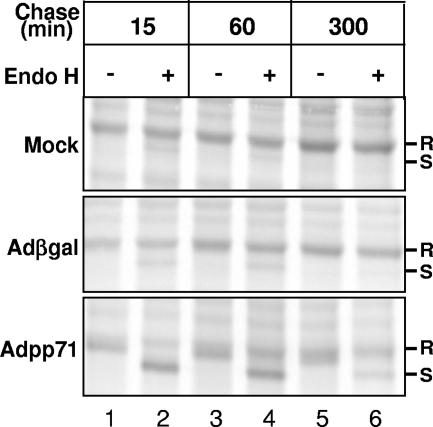
Because the biosynthesis and accumulation of total cellular MHC class I proteins appeared unaffected by pp71 expression, we tested whether transport of MHC class I complexes was compromised in pp71-expressing cells by examining the acquisition of endo H resistance of MHC class I heavy chains. Cultures of U373:CIITA cells in 75-cm2 flasks were mock infected or exposed to 10 PFU/cell of Adβgal or 100 PFU/cell of Adpp71. Forty-eight hours after infection, cells were incubated in media supplemented with [35S]methionine for 30 min to radiolabel proteins. Cultures were washed and/or further incubated with unlabeled media and harvested at 15, 60, and 300 min after the radiolabel pulse period. Cells were harvested and solubilized, and MHC class I proteins were isolated using w6/32 anti-MHC class I antibody. One-half of the isolated proteins were incubated with endo H. Proteins were separated by SDS-PAGE, and radiolabeled proteins were visualized by autoradiography (Fig. 6). After a 15-min chase period, MHC class I molecules were predominantly in endo H-resistant form in uninfected cells and in cells expressing β-galactosidase (lanes 1 and 2, top two panels). A similar profile was observed after a 60-min chase period, and essentially all MHC class I complexes were endo H resistant after a 300-min chase period (compare lane 3 to 4 and 5 to 6 in the top two panels). In dramatic contrast, a predominance of heavy chains remained endo H sensitive at both 15 min and 60 min after the pulse period in Adpp71-infected cells (lanes 1 and 2 and 3 and 4, respectively, in the bottom panel compared to the two top panels). Surprisingly, even after a 5-h chase period, a fraction of MHC class I molecules remain in endo H-sensitive form in cells expressing pp71 (lanes 5 and 6, bottom panel). These observations are consistent with a role for pp71 in causing a delay in transport or processing of MHC class I complexes prior to transit out of the medial Golgi apparatus.
FIG. 6.
Delay of acquisition of endo H resistance in cells expressing pp71 protein. Shown is an autoradiographic image of isolated MHC class I complexes from cells expressing pp71 or β-galactosidase with or without endoglycosidase H treatment. Cultures of U373:CIITA cells grown in 75-cm2 flasks were mock infected or exposed to 10 PFU of Adβgal or 100 PFU of Adpp71 per cell. Seventy-two hours after infection, cells were incubated with [35S]methionine for 30 min to radiolabel proteins. Cultures were washed with unlabeled media and further incubated for 0, 60, and 300 min after the pulse period. Cells were then solubilized, and MHC class I proteins were isolated with W6/32 anti-MHC class I antibody and protein A-agarose beads. One-half of the isolated proteins were incubated with endo H for 2 h as indicated in Materials and Methods. Proteins were separated in a 12% denaturing gel and visualized by autoradiography. Plus and minus signs indicate whether or not samples were treated with endo H. endo H-resistant and -sensitive forms of MHC class I molecules are denoted by R and S, respectively. Three experiments were performed. The experiment shown included the longest chase period (5 h) of the three experiments.
Localization of pp71 in cytosolic compartments.
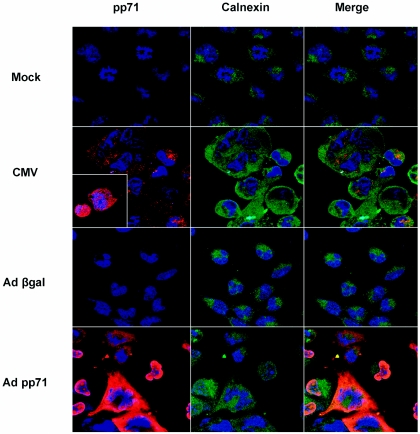
The previous experiments predict that pp71 functions directly or indirectly in a premedial Golgi compartment to limit accumulation of MHC class I complexes. To test if pp71 resides in such a compartment, immunofluorescence microscopy was performed using antibodies specific for pp71 and the endoplasmic reticulum resident protein calnexin. Cultures of U373:CIITA cells cultured on glass coverslips were mock infected or exposed to 50 PFU of the AD169 strain of CMV per cell or to 100 PFU of Adβgal or Adpp71 recombinant adenoviruses per cell. At 48 h after infection cells were fixed, permeabilized, and stained with antibody to pp71, followed by rhodamine-conjugated secondary antibody, and subsequently stained with FITC-conjugated anticalnexin antibody. As shown in Fig. 7, pp71 reactivity was observed in the cytoplasm in CMV-infected cells, though both nuclear and cytoplasmic staining was noted at this time (inset shows an image of the field of CMV-infected cells exhibiting nuclear pp71 reactivity). As expected, in Adpp71-infected cells, pp71 reactivity was substantially stronger and also was observed in the nucleus and cytoplasm. Merged images show that the pp71 and calnexin immunofluorescence signals partially overlap in both CMV- and Adpp71-infected cells. A small proportion of the pp71 immunofluorescence signal also partially overlapped with the MHC class I signal in cytoplasmic compartments in both Adpp71- and CMV-infected cells (data not shown). Thus, by 48 h after infection, at least a proportion of pp71 proteins expressed in infected cells localize to cytoplasmic compartments that contain MHC class I complexes, including the endoplasmic reticulum. We could not convincingly demonstrate a direct interaction between MHC class I heavy chains and pp71 by coimmunoprecipitation studies (data not shown). Therefore, we conclude that pp71 either transiently interacts with MHC class I complexes or functions indirectly to delay transport or processing of MHC class I complexes.
FIG. 7.
Nuclear and cytosolic localization of pp71. Immunofluorescence images of pp71 and calnexin in infected cells. Cultures of U373:CIITA cells cultured on glass coverslips in six-well plates were mock infected or exposed to 50 PFU of the AD169 strain of CMV per cell or to 100 PFU of Adβgal or Adpp71 recombinant adenoviruses per cell. At 48 h after infection cells were fixed and stained with antibody to pp71 followed by rhodamine-conjugated secondary antibody, and subsequently stained with FITC-conjugated anticalnexin antibody. Nuclei were visualized with DRAQ 5. Images of pp71 reactivity are shown in the left column, images of calnexin reactivity are in the middle column, and merged images are shown in the right column. The inset image of CMV-infected cells shows pp71 reactivity in nuclear and cytosolic compartments at this time point.
RNAi-mediated knockdown of pp71 expression impacts MHC class I cell surface accumulation during infection.
The previous studies suggest that pp71 may function in infected cells to limit accumulation of cell surface MHC class I molecules. To test this hypothesis, we devised an experimental system based on the following observations and assumptions: (i) newly synthesized, rather than virion-associated, pp71 molecules are relevant to the disruption MHC class I cell surface accumulation; (ii) an experimental system in which the virion-associated gene transactivation function of pp71 was disrupted would confound data interpretation due alterations in the virus replication program; and (iii) if the virion-associated pp71 protein does function to limit cell surface accumulation of MHC class I molecules, this activity would be obscured if the MHC class I evasin functions of viral immediate early (US3) and early (US2, US6, and US11) gene products are dominant. Therefore, we set out to limit expression of newly synthesized pp71 using short, interfering RNAs during infection with recombinant virus RV7186, which lacks all of the known CMV MHC class I evasin genes.
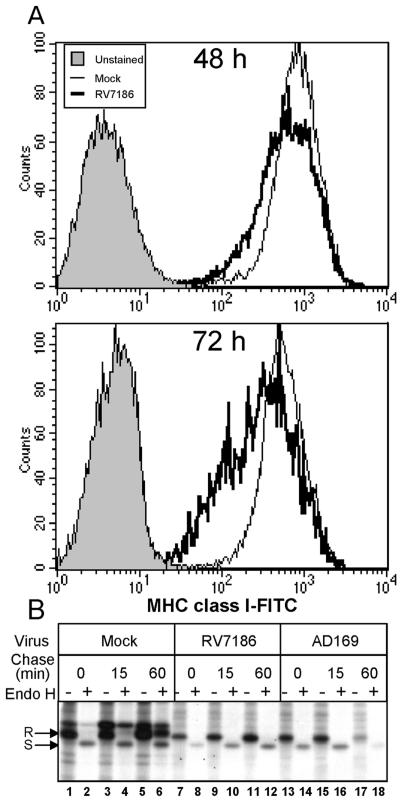
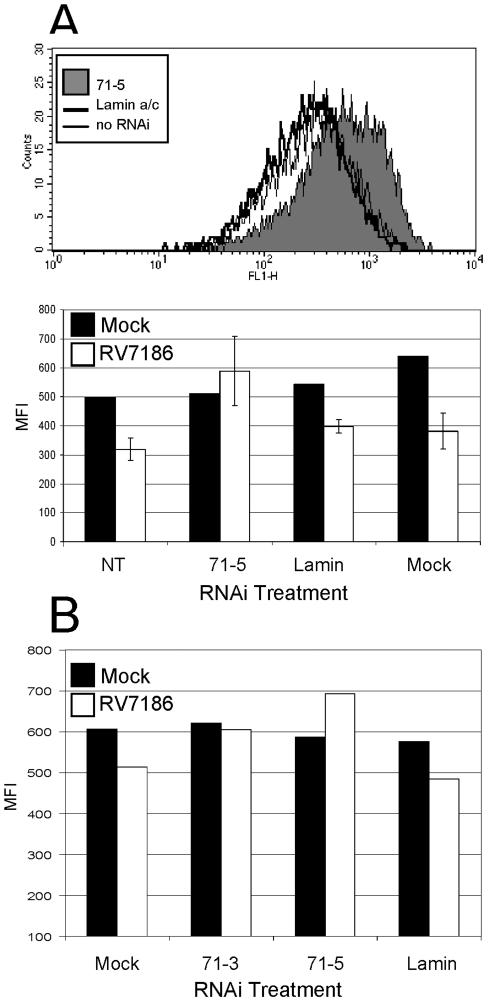
We first tested the assumptions of this model. We predicted that MHC class I cell surface levels in cells infected with RV7186 would be decreased relative to uninfected cells. To test this, U373:CIITA cells were exposed to 2 PFU of RV7186 per cell or were left uninfected. At 48 and 72 h after infection, cells were reacted with FITC-conjugated anti-MHC class I antibodies and analyzed for cell surface MHC class I levels by flow cytometry. Representative histograms shown in Fig. 8A show a modest (1.3-fold) but highly reproducible shift toward decreased MHC class I fluorescence in RV7186-infected cells relative to uninfected cells. The magnitude of the downshift in MHC class I cell surface fluorescence increases at the 72-h time point such that the geometric mean fluorescence is 2.4-fold lower in RV7186-infected cells.
FIG. 8.
Delayed transport and cell surface accumulation of MHC class I complexes in RV7186-infected cells. (A) Histograms of MHC class I levels in mock- and RV7186-infected cells. U373:CIITA cells cultured in six-well plates were exposed to 2 PFU of RV7186 per cell or were left uninfected. Cells were dislodged from the plates at 48 and 72 h after infection, reacted with FITC-conjugated anti-MHC class I antibodies, and analyzed for cell surface MHC class I levels by flow cytometry. Histograms are shown for unstained cells, mock-infected cells, and RV7186-infected cells. (B) Autoradiographic image of MHC class I molecules from mock-, AD169-, and RV7186-infected cells with or without endoglycosidase H treatment. Triplicate cultures of U373:CIITA cells grown in 25-cm2 flasks were exposed to 2 PFU of the AD169 strain of CMV per cell or 2 PFU of mutant strain RV7186 per cell or were left uninfected. At 68 h after infection, cells were incubated with [35S]methionine for 30 min to radiolabel proteins. Cultures were washed with unlabeled media and further incubated for 0, 15, and 60 min after the pulse period. Cells were then solubilized, and MHC class I proteins were isolated with W6/32 anti-MHC class I antibody and protein A-agarose beads. One-half of the isolated proteins were incubated with endo H for 2 h as indicated in Materials and Methods. Proteins were separated in a 10% denaturing gel and visualized by autoradiography. Plus and minus signs indicate whether or not samples were treated with endo H. endo H-resistant and -sensitive forms of MHC class I molecules are denoted by R and S, respectively.
The decrease in MHC class I cell surface levels correlated with delayed acquisition of endo H resistance of MHC class I complexes in RV7186-infected cells. Cultures of U373:CIITA cells in 25-cm2 flasks were mock infected or exposed to 2 PFU of AD169 or RV7186 per cell. At 68 h after infection, cells were incubated in media supplemented with [35S]methionine for 30 min to radiolabel proteins. Cultures were washed and further incubated with unlabeled media for 0, 15, and 60 min after the radiolabel pulse period. Cells were harvested and solubilized, and MHC class I proteins were isolated using W6/32 anti-MHC class I antibody. One-half of the isolated proteins from each sample were treated with endo H. Proteins were separated by SDS-PAGE, and radiolabeled proteins were visualized by autoradiography (Fig. 8B). In uninfected and infected cells, 100% of the isolated complexes were sensitive to endo H after the radiolabeling period (0-min chase; compare lanes 1 and 2 to 7 and 8 and to 13 and 14). After a 60-min chase period, the majority of MHC class I complexes in uninfected cells acquired resistance to endo H (lanes 5 and 6). In contrast, MHC class I molecules remained endo H sensitive after the 60-min chase period in RV7186-infected cells (lanes 11 and 12). As expected, after a 60-min chase period, the abundance of MHC class I molecules in AD169 was greatly diminished relative to that in RV7186-infected cells (compare lanes 17 and 18 to lanes 11 and 12), consistent with the activities of the US2 and US11 proteins in promoting the degradation of these complexes. From this series of experiments we conclude that, at least in U373-derived cell lines, infection with RV7186 leads to a delay in maturation or transport of MHC class I complexes and decreased accumulation of MHC class I molecules at the cell surface and that this activity is independent of genes contained within the IRS1-US12 region of the HCMV genome.
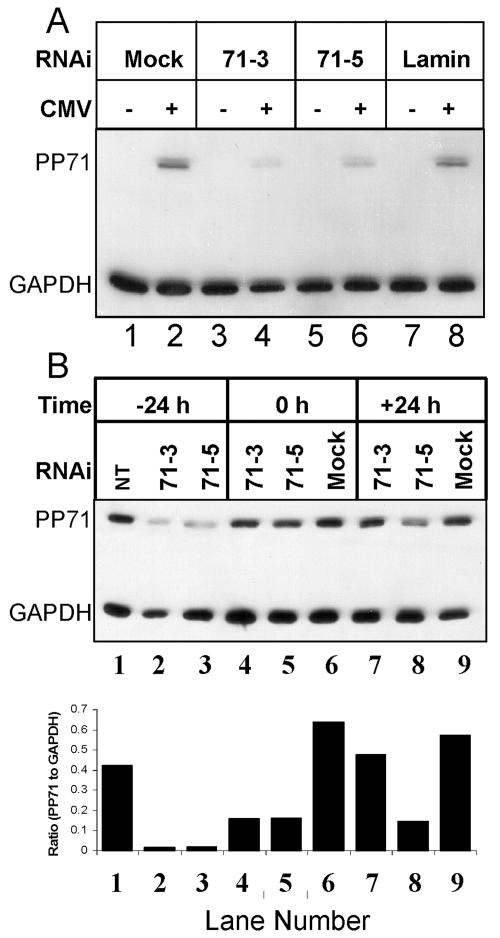
We next set out to determine if targeted duplex RNAi would function to knock down pp71 protein accumulation (Fig. 9A). U373:CIITA cells were transfected with one of two RNAi duplexes specific for pp71 or an RNAi duplex specific for lamin a/c or were left untreated. Cells were mock infected or exposed to 15 PFU of AD169 per cell. At 48 h after infection, cells were solubilized, and equivalent amounts of proteins were subjected to electrophoresis in a denaturing acrylamide gel. Proteins were transferred to nitrocellulose sheets and reacted with antibodies to MHC class I and GAPDH. Both pp71-specific RNAi duplexes could knock down pp71 accumulation in AD169-infected cells (71-3 and 71-5; lanes 3 and 4 and 5 and 6, respectively). Accumulation of lamin a/c protein was also diminished by transfection with RNAi targeted to this gene but not other RNAi (data not shown). pp71 expression was unaffected or only slightly affected by transfection with lamin-specific RNAi duplexes compared to mock-transfected cells (compare lanes 2 and 8). We concluded that transfection of cells with RNAi duplexes prior to infection with strain AD169 resulted in the selective knockdown in accumulation of both viral and cellular target proteins. In a second experiment (Fig. 9B), cells were transfected with RNAi duplexes specific for pp71 (71-3 and 71-5) 24 h prior to infection, immediately after removal of the virus inoculum (0 h), or 24 h after infection and pp71 levels were analyzed at 48 h after infection. Integrated optical density quantitation of protein bands was performed, and the ratios of pp71 to GAPDH transcripts are shown below the image of the immunoblot. We observed the most pronounced decrease in pp71 protein levels when cells were treated with RNAi duplexes 24 h before infection (compare lanes 2 and 3 to lanes 5 and 6 and lanes 8 and 9). This study also suggested that the 71-5 RNAi duplex was more robust than the 71-3 duplex in limiting pp71 expression inasmuch as the 7-5 RNAi was effective even when delivered 24 h after infection (compare lanes 7 and 8).
FIG. 9.
RNAi-mediated knockdown of pp71 protein accumulation. Film image of electrophoretically separated cell lysates reacted with antibody to pp71 or GAPDH. (A) U373:CIITA cells cultured in 24-well plates were transfected with 0.84 μg of the indicated RNAi duplex per well. Twenty-four hours after transfection, cells were mock infected or exposed to 15 PFU of AD169 per cell. Cells were harvested 48 h after infection and solubilized, and equivalent amounts of proteins were subjected to electrophoresis in a denaturing acrylamide gel. Proteins were transferred to nitrocellulose sheets and reacted with antibodies to MHC class I and GAPDH as described in Materials and Methods. (B) Cells were left untreated, exposed to transfection reagent only (mock), or transfected with 0.84 μg of 71-3 or 71-5 RNAi duplex 24 h before infection (−24 h), immediately after removal of the virus inoculum (0 h), or 24 h after infection (+24 h). Protein lysates were generated and analyzed as described for panel A. Integrated optical density determinations were made for pp71 and GAPDH signals, and the ratio of pp71 to GAPDH protein density is indicated for each lane below the film image.
To ascertain if knockdown of pp71 expression would influence accumulation of MHC class I complexes at the cell surface, this experiment was repeated with RV7186-infected U373:CIITA cells (Fig. 10A) and RV7186-infected parental U373 cells (Fig. 10B). At 48 h after infection, cells were dislodged from the plates and analyzed for cell surface MHC class I levels by flow cytometry by reaction with FITC-conjugated anti-MHC class I antibody. Representative histograms from the first experiment are shown in the upper panel of Fig. 10A. These histograms show that cells treated with pp71-specific RNAi duplexes (71-5) exhibited a shift toward increased MHC class I cell surface fluorescence compared to RV7186-infected cells that were mock transfected or transfected with a lamin a/c-specific RNAi duplex. The results of this experiment compared to uninfected cells are shown in the bar graph in Fig. 10A and can be summarized as follows: (i) transfection of either pp71-specific or lamin a/c-specific RNAi duplexes did not appreciably alter cell surface MHC class I levels in uninfected cells, and mock transfection resulted in only slightly higher MHC class I levels; (ii) in untransfected U373:CIITA cells, the mean fluorescence intensities for MHC class I cell surface molecules were on average 36% lower in RV7186-infected cells compared to uninfected cells; (iii) the mean fluorescence intensities for MHC class I molecules were on average 26% lower in RV7186-infected cells pretreated with lamin a/c-specific RNAi compared to similarly treated uninfected cells; and (iv) the decrease in MHC class I cell surface levels in RV7186-infected cells was not observed if cells were previously transfected with of pp71-specific RNAi. Statistical analysis showed that MHC class I levels were significantly lower in cells treated with RNAi specific to lamin compared to cells treated with the pp71-specific RNAi (P = 0.0106). Exactly the same pattern was observed when this experiment was performed with parental U373 cells (Fig. 10B), though the magnitude of the decrease in MHC class I cell surface expression upon RV7186 infection was smaller than in U373:CIITA cells. In a subset of our studies we observed that MHC class I cell surface fluorescence was sometimes slightly higher in RV7186-infected cells treated with the 71-5 and 71-3 RNAi relative to mock-infected cells. Although this could be due to nonspecific effects of these RNAi treatments, we also observed decreasing MHC class I levels with increasing cell density. Thus, it is possible that this relates to reduced cell density of RV7186-infected cells relative to uninfected cells at the time of analysis. From this series of experiments we conclude that (i) MHC class I cell surface levels are modestly decreased upon infection of glioblastoma cells with a recombinant human cytomegalovirus that lacks all known MHC class I evasin genes, (ii) transfection of cells with RNAi duplexes prior to infection can effectively diminish accumulation of newly synthesized pp71 protein, and (iii) MHC class I cell surface levels are not diminished in RV7186-infected cells when de novo pp71 expression is limited by pp71-specific RNAi treatment.
FIG. 10.
Knockdown of pp71 expression impacts MHC class I cell surface accumulation during infection. (A) Shown is a histogram and mean fluorescence intensities of MHC class I cell surface levels in RV7186-infected cells pretreated with RNAi duplexes. Cells cultured in 24-well plates were transfected with the indicated RNAi duplex. At 24 h after transfection, cells were mock infected or exposed to 3 PFU per cell of RV7186. Forty-eight hours after infection, cells were dislodged from the plates, reacted with FITC-conjugated anti-MHC class I antibody, and analyzed for cell surface MHC class I levels by flow cytometry. The upper panel shows representative histograms from of RV7186-infected cells pretreated with no RNAi, RNAi specific for lamin a/c, or RNAi specific for pp71. The lower panel shows the mean fluorescence intensities of mock-infected cells (averages of two replicate wells) and RV7186-infected cells (average of three triplicate wells) left untreated (NT) or transfected with RNAi duplexes specific for pp71 (71-3 or 71-5) or lamin a/c (lamin) or exposed to transfection reagent only (mock). (B) In a second experiment, triplicate wells of U373 cells in 24-well plates were mock infected or exposed to 10 PFU of RV7186 per cell 24 h after transfection with reagent only (mock) or RNAi duplexes specific for pp71 (71-3, 71-5) or lamin a/c. Cells from triplicate samples were dislodged from the plates, pooled, and analyzed for cell surface MHC class I levels by flow cytometry after reacting with FITC-conjugated anti-MHC class I antibody.
DISCUSSION
In this report, we provide data supporting a novel role for HCMV pp71 in disruption of the MHC class I antigen presentation pathway. HCMV pp71, the product of the UL82 gene, is a virion matrix or tegument phosphoprotein (34, 41). Similar to many herpesvirus gene products that have been investigated, pp71 exerts multiple roles during the cytomegalovirus life cycle. It is a transactivator of both viral and cellular genes and can synergize with the transactivating activities of IE2p86 and ppUL35 (6, 17, 27, 29, 42). pp71 also increases the infectivity of viral DNA in a manner independent from its function as a transactivator of the IE1 and IE2 genes (3). Additionally, pp71 accelerates cell cycle progression through the G1 phase in asynchronous cells and promotes G0-to-S phase progression in quiescent cells (23, 24). These cell cycle-regulatory activities are derived from the ability of pp71 to target hypophosphorylated forms of the retinoblastoma family of proteins for degradation in a proteasome-dependent but ubiquitin-independent manner (22).
We now provide data indicating that pp71 is also capable of interfering with cell surface expression of MHC class I complexes. The data supporting this function are as follows: (i) ectopic UL82 expression in human glioblastoma cells did not cause observable toxicity but did cause a dose-dependent decrease in accumulation of cell surface MHC class I complexes; (ii) UL82 expression did not interfere with accumulation of either MHC class I heavy-chain transcript or protein but rather delayed transport of MHC class I complexes from the ER or cis-Golgi apparatus; and (iii) disruption of UL82 expression by RNAi led to increased accumulation of cell surface MHC class I complexes in cells infected with the recombinant strain RV7186, which lacks the US region MHC class I evasion genes. These findings support a novel function for pp71 in retaining MHC class I molecules in the ER/cis-Golgi such that accumulation of cell surface MHC class I complexes is diminished.
While pp71 does not have protein sequence similarity to the other known evasins, it is functionally similar to US3 and US10 of HCMV and the m152/gp40 of MCMV in that all of these proteins prevent the forward transport of MHC class I molecules through the ER and/or Golgi compartments (10, 20, 49). Although pp71 and MHC class I complexes partially overlap in their cytoplasmic localization, pp71 does not appear to form a stable interaction with MHC class I molecules, similar to the activity of m152/gp40 of MCMV (49).
All of the pp71 functions described earlier have been linked (though not necessarily exclusively) to virion-associated pp71. In contrast, several lines of evidence indicate that newly synthesized pp71 is responsible for limiting cell surface accumulation of MHC class I complexes. The first line of evidence is the subcellular localization of pp71. Upon infection, virion-associated pp71 interacts with the cellular protein Daxx and localizes to the nucleus at discrete sites known as nuclear domain 10 (ND10) structures (15, 16, 18, 31). Localization to ND10 structures correlates with the function of pp71 as a transactivator of viral (and cellular) genes (16, 18, 31). As shown here and in studies by other laboratories, pp71 accumulates in both nuclear and cytoplasmic compartments at later times of infection (15). The appearance of newly synthesized pp71 in cytosolic compartments temporally correlates with decreased accumulation of cell surface MHC class I molecules. The strongest line of evidence is derived from the RNAi studies. Under these experimental conditions, functions of pp71 associated with virion-delivered protein remain intact, whereas functions related to newly synthesized pp71 are limited by the activity of short interfering RNAs. These studies support the hypothesis that newly synthesized pp71 is responsible for disrupting MHC class I cell surface accumulation.
These data are consistent with at least two possible scenarios. One scenario is that the MHC class I downmodulation is a consequence of a function for pp71 in altering the trafficking of glycoproteins, possibly relevant to virus maturation. In support of this possibility, it appears that rather substantial levels of pp71 protein must accumulate to observe this effect and that this occurs at times after infection that coincide with readily observable gross morphological changes and, presumably, changes in the membrane-associated subcellular compartments. The second scenario is that the pp71-mediated downmodulation of cell surface MHC class I represents a specific viral immune evasion strategy. In support of this possibility, ectopic expression of pp71 results in delayed acquisition of endo H resistance by MHC class I molecules. Also, the modest decrease in cell surface MHC class levels observed in cells infected with the RV7186 recombinant virus is abrogated by limiting accumulation of pp71 protein using RNAi.
If the first scenario is correct, the implication is that pp71 also functions to influence protein trafficking. We provide evidence suggesting pp71 acts relatively specifically inasmuch as MHC class II and transferrin receptor levels were either not altered or only marginally altered by overexpression of pp71. However, it remains to be determined whether this activity is specific to MHC class I molecules or extends to other cellular or viral proteins that share similar structures and/or trafficking patterns.
With respect to the immune evasion scenario, it is interesting to note that there is precedence for viral matrix or tegument proteins functioning as immunomodulators. For example, pp65 appears to exert pleiotropic activities in HCMV-mediated attenuation of host immune responses, including blocking the presentation of peptides derived from the IE1 viral transcription factor (12), disrupting HLA-DR expression in response to interferon gamma (35) and antagonizing NF-κB- and IRF-mediated accumulation of cellular antiviral gene transcripts (7).
However, if pp71 does act to suppress presentation of viral peptides during natural infections, it raises the question of why so many viral gene products target the same MHC class I pathway. Many researchers in this field have speculated that these gene products are not simply redundant but rather are necessary to optimize attenuation of CD8+ T-cell-mediated surveillance and control of infected cells. For example, multiple viral evasins may be necessary in order be effective in the face of extensive MHC polymorphism. In support of this, US2 targets HLA-A and possibly HLA-B locus products, though HLA-C, HLA-E, and HLA-G proteins appear resistant (11; reviewed in reference 9). Thus, it may be illuminating to ascertain if pp71 selectively interferes with cell surface expression of a subset of HLA locus products or other HLA-related molecules.
Another possibility is that multiple gene products evolved because they exhibit different efficacies in disrupting MHC class I antigen presentation in different cell types, as has been demonstrated for US2 and US11 in human primary peripheral blood mononuclear cell-derived dendritic cells (39). In support of this possibility, it is interesting to note that, while MHC class I levels were unchanged in RV7186-infected human foreskin fibroblast cells compared to uninfected cells (21), our findings indicate a pp71-dependent decrease in MHC class I cell surface levels in RV7186-infected U373 MG glioblastoma cells relative to uninfected cells.
Evidence also has accumulated to suggest that these viral molecules optimize disruption of the MHC class I pathway through synergy, cooperation, or possibly antagonism (2, 26, 45). From this perspective, it is of interest that US region evasin genes are expressed with immediate early or early gene class profiles whereas UL82 gene expression is biphasic, peaking at 12 h and 72 h after infection in human fibroblasts. While it is conceivable that pp71 could act in concert with other MHC class I pathway evasin molecules at relatively early times after infection, it is also possible that pp71 protein that accumulates late in infection acts to limit cell surface expression of MHC class I complexes that escape the activities of the US region gene products. Such an activity could potentially provide a selective advantage through increasing the percentage of cells that escape from CD8+-T-cell-mediated cytolysis during the late stages of infection.
In summary, we have described a novel function for HCMV pp71. This function is capable of limiting the accumulation of MHC class I complexes in a manner that is consistent with a role for pp71 in regulating protein trafficking and/or escape from recognition of virus-specific CD8+ T lymphocytes.
Acknowledgments
We gratefully acknowledge Bodo Plachter for the pcDNApp71DNAtag plasmid, Tom Shenk for the anti-pp71 antibody, Hidde Ploegh for the HC10 antibody, Tom Jones for the RV7186 recombinant virus, and Jeremy Boss for the gift of CIITA cDNA. We also acknowledge Barnabe Assogba, Yan Li, and Mark Kotur for their technical assistance and Daniel Miller for generating the U373:CIITA line and his contributions at the initial stages of this work. Finally, we acknowledge David Jarjoura, Managing Director, The Ohio State University Center for Biostatistics, and Kyle M. Porter, consulting research statistician, The Ohio State University Center for Biostatistics, for statistical support.
This work was supported by grant AI51411-03 from the National Institutes of Health.
REFERENCES
- 1.Ahn, K., A. Gruhler, B. Galocha, T. R. Jones, E. J. Wiertz, H. L. Ploegh, P. A. Peterson, Y. Yang, and K. Früh. 1997. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6:613-621. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, K. S., A. Angulo, P. Ghazal, P. A. Peterson, Y. Yang, and K. Früh. 1996. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc. Natl. Acad. Sci. USA 93:10990-10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldick, C. J., Jr., A. Marchini, C. E. Patterson, and T. Shenk. 1997. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J. Virol. 71:4400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnstable, C. J., W. F. Bodmer, G. Brown, G. Galfre, C. Milstein, A. F. Williams, and A. Ziegler. 1978. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens: new tools for genetic analysis. Cell 14:9-20. [DOI] [PubMed] [Google Scholar]
- 5.Bauer, D., and R. Tampe. 2002. Herpes viral proteins blocking the transporter associated with antigen processing TAP—from genes to function and structure. Curr. Top. Microbiol. Immunol. 269:87-99. [PubMed] [Google Scholar]
- 6.Bresnahan, W. A., and T. E. Shenk. 2000. UL82 virion protein activates expression of immediate early viral genes in human cytomegalovirus-infected cells. Proc. Natl. Acad. Sci. USA 97:14506-14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browne, E. P., and T. Shenk. 2003. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc. Natl. Acad. Sci. USA 100:11439-11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cebulla, C. M., D. M. Miller, Y. Zhang, B. M. Rahill, P. Zimmerman, J. M. Robinson, and D. D. Sedmak. 2002. Human cytomegalovirus disrupts constitutive MHC class II expression. J. Immunol. 169:167-176. [DOI] [PubMed] [Google Scholar]
- 9.Furman, M. H., H. L. Ploegh, and D. J. Schust. 2000. Can viruses help us to understand and classify the MHC class I molecules at the maternal-fetal interface. Hum. Immunol. 61:1169-1176. [DOI] [PubMed] [Google Scholar]
- 10.Furman, M. H., N. Dey, D. Tortorella, and H. L. Ploegh. 2002. The human cytomegalovirus US10 gene product delays trafficking of major histocompatibility complex class I molecules. J. Virol. 76:11753-11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gewurz, B. E., E. W. Wang, D. Tortorella, D. J. Shcust, and H. L. Ploegh. 2001. Human cytomegalovirus US2 endoplasmic reticulum-luminal domain dictates association with major histocompatibility complex class I in a locus-specific manner. J. Virol. 75:5197-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert, M. J., S. R. Riddell, B. Plachter, and P. D. Greenberg. 1996. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature 383:720-722. [DOI] [PubMed] [Google Scholar]
- 13.Hengel, H., T. Flohr, G. J. Hämmerling, U. H. Koszinowski, and F. Momburg. 1996. Human cytomegalovirus inhibits peptide translocation into the endoplasmic reticulum for MHC class I assembly. J. Gen. Virol. 77:2287-2296. [DOI] [PubMed] [Google Scholar]
- 14.Hengel, H., W. Brune, and U. H. Koszinowski. 1998. Immune evasion by cytomegalovirus—survival strategies of a highly adapted opportunist. Trends Microbiol. 6:190-197. [DOI] [PubMed] [Google Scholar]
- 15.Hensel, G. M., H. H. Meyer, I. Buchmann, D. Pomnmerehne, S. Schmolke, B. Plachter, K. Radsak, and H. F. Kern. 1996. Intracellular localization and expression of the human cytomegalovirus matrix phosphoprotein pp71 (UL82): evidence for its translocation into the nucleus. J. Gen. Virol. 77:3087-3097. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann, H., H. Sindre, and T. Stamminger. 2002. Functional interaction between the pp71 protein of human cytomegalovirus and the PML-interacting protein human Daxx. J. Virol. 76:5769-5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Homer, E. G., A. Rinaldi, M. J. Nicholl, and C. M. Preston. 1999. Activation of herpesvirus gene expression by the human cytomegalovirus protein pp71. J. Virol. 73:8512-8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishov, A. M., O. V. Vladimirova, and G. G. Maul. 2002. Daxx-mediated accumulation of human cytomegalovirus tegument protein pp71 at ND10 facilitates initiation of viral infection at these nuclear domains. J. Virol. 76:7705-7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, T. R., and L. Sun. 1997. Human cytomegalovirus US2 destabilizes major histocompatibility complex class I heavy chains. J. Virol. 71:2970-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, T. R., E. J. Wiertz, L. Sun, K. N. Fish, J. A. Nelson, and H. L. Ploegh. 1996. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc. Natl. Acad. Sci. USA 93:11327-11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones, T. R., L. K. Hanson, L. Sun, J. S. Slater, R. M. Stenberg, and A. E. Campbell. 1995. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression major histocompatibility complex class I heavy chains. J. Virol. 69:4830-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalejta, R. F., and T. Shenk. 2003. Proteasome-dependent, ubiquitin-independent degradation of the Rb family of tumor suppressors by the human cytomegalovirus pp71 protein. Proc. Natl. Acad. Sci. USA 100:3263-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalejta, R. F., and T. Shenk. 2003. The human cytomegalovirus UL82 gene product (pp71) accelerates progression through the G1 phase of the cell cycle. J. Virol. 77:3451-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalejta, R. F., J. T. Bechtel, and T. Shenk. 2003. Human cytomegalovirus pp71 stimulates cell cycle progression by inducing the proteasome-dependent degradation of the retinoblastoma family of tumor suppressors. Mol. Cell. Biol. 23:1885-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kavanagh, D. G., and A. B. Hill. 2001. Evasion of cytotoxic T lymphocytes by murine cytomegalovirus. Semin. Immunol. 13:19-26. [DOI] [PubMed] [Google Scholar]
- 26.Kavanagh, D. G., M. C. Gold, M. Wagner, U. H. Koszinowski, and A. B. Hill. 2001. The multiple immune-evasion genes of murine cytomegalovirus are not redundant: m4 and m152 inhibit antigen presentation in a complementary and cooperative fashion. J. Exp. Med. 194:967-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kronschnabl, M., and T. Stamminger. 2003. Synergistic induction of intercellular adhesion molecule-1 by the human cytomegalovirus transactivators IE2p86 and pp71 is mediated via an Sp1-binding site. J. Gen. Virol. 84:61-73. [DOI] [PubMed] [Google Scholar]
- 28.Lehner, P. J., J. T. Karttunen, G. W. Wilkinson, and P. Cresswell. 1997. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc. Natl. Acad. Sci. USA 94:6904-6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, B., and M. F. Stinski. 1992. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J. Virol. 66:4434-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loenen, W. A., C. A. Bruggeman, and E. J. Wiertz. 2001. Immune evasion by human cytomegalovirus: lessons in immunology and cell biology. Semin. Immunol. 13:41-49. [DOI] [PubMed] [Google Scholar]
- 31.Marshall, K. R., K. V. Rowley, A. Rinaldi, I. P. Nicholson, A. M. Ishov, G. G. Maul, and C. M. Preston. 2002. Activity and intracellular localization of the human cytomegalovirus protein pp71. J. Gen. Virol. 83:1601-1612. [DOI] [PubMed] [Google Scholar]
- 32.Miller, D. M., C. M. Cebulla, and D. D. Sedmak. 2002. Human cytomegalovirus inhibition of major histocompatibility complex transcription and interferon signal transduction. Curr. Top. Microbiol. Immunol. 269:153-170. [DOI] [PubMed] [Google Scholar]
- 33.Mocarski, E. S., Jr. 2002. Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 10:332-339. [DOI] [PubMed] [Google Scholar]
- 34.Nowak, B., C. Sullivan, P. Sarnow, R. Thomas, F. Bricout, J. C. Nicolas, B. Fleckenstein, and A. J. Levine. 1984. Characterization of monoclonal antibodies and polyclonal immune sera directed against human cytomegalovirus virion proteins. Virology 132:325-338. [DOI] [PubMed] [Google Scholar]
- 35.Odeberg, J., B. Plachter, L. Branden, and C. Soderberg-Naucler. 2003. Human cytomegalovirus protein pp65 mediates accumulation of HLA-DR in lysosomes and destruction of the HLA-DR alpha-chain. Blood 101:4870-4877. [DOI] [PubMed] [Google Scholar]
- 36.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2706. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams and Wilkins, Philadelphia, Pa. [Google Scholar]
- 37.Quinnan, G. V., Jr., N. Kirmani, A. H. Rook, J. F. Manischewitz, L. Jackson, G. Moreschi, G. W. Santos, R. Saral, and W. H. Burns. 1982. Cytotoxic T cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N. Engl. J. Med. 307:7-13. [DOI] [PubMed] [Google Scholar]
- 38.Reddehase, M. J., F. Weiland, K. Munch, S. Jonjic, A. Luske, and U. H. Koszinowski. 1985. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J. Virol. 55:264-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rehm, A., A. Engelsberg, D. Tortorella, I. J. Korner, I. Lehman, H. L. Ploegh, and U. E. Hopken. 2002. Human cytomegalovirus gene products US2 and US11 differ in their ability to attack major histocompatibility class I heavy chains in dendritic cells. J. Virol. 76:5043-5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reusser, P., S. R. Riddell, J. D. Meyers, and P. D. Greenberg. 1991. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood 78:1373-1380. [PubMed] [Google Scholar]
- 41.Ruger, B., S. Klages, B. Walla, J. Albrecht, B. Fleckenstein, P. Tomlinson, and B. Barrell. 1987. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J. Virol. 61:446-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schierling, K., T. Stamminger, T. Mertens, and M. Winkler. 2004. Human cytomegalovirus tegument proteins ppUL82 (pp71) and ppUL35 interact and cooperatively activate the major immediate-early enhancer. J. Virol. 78:9512-9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stam, N. J., H. Spits, and H. L. Ploegh. 1986. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J. Immunol. 137:2299-2306. [PubMed] [Google Scholar]
- 44.Tirabassi, R. S., and H. L. Ploegh. 2002. The human cytomegalovirus US8 glycoprotein binds to major histocompatibility complex class I products. J. Virol. 76:6832-6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner, M., A. Gutermann, J. Podlech, M. J. Reddehase, and U. H. Koszinowski. 2002. Major histocompatibility complex class I allele-specific cooperative and competitive interactions between immune evasion proteins of cytomegalovirus. J. Exp. Med. 196:805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walter, E. A., P. D. Greenberg, M. J. Gilbert, R. J. Finch, K. S. Watanabe, E. D. Thomas, and S. R. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038-1044. [DOI] [PubMed] [Google Scholar]
- 47.Wentworth, B. B., and L. French. 1970. Plaque assay of cytomegalovirus strains of human origin. Proc. Soc. Exp. Biol. Med. 135:253-258. [DOI] [PubMed] [Google Scholar]
- 48.Wiertz, E. J., T. R. Jones, L. Sun, M. Bogyo, H. J. Geuze, and H. L. Ploegh. 1996. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84:769-779. [DOI] [PubMed] [Google Scholar]
- 49.Ziegler, H., W. Muranyi, H. C. Burgert, E. Kremmer, and U. H. Koszinowski. 2000. The luminal part of the murine cytomegalovirus glycoprotein gp40 catalyzes the retention of MHC class I molecules. EMBO J. 19:870-881. [DOI] [PMC free article] [PubMed] [Google Scholar]