Abstract
Apoptosis plays a major role in the cytopathic effect induced by reovirus following infection of cultured cells and newborn mice. Strain-specific differences in the capacity of reovirus to induce apoptosis segregate with the S1 and M2 gene segments, which encode attachment protein σ1 and membrane penetration protein μ1, respectively. Virus strains that bind to both junctional adhesion molecule-A (JAM-A) and sialic acid are the most potent inducers of apoptosis. In addition to receptor binding, events in reovirus replication that occur during or after viral disassembly but prior to initiation of viral RNA synthesis also are required for reovirus-induced apoptosis. To determine whether reovirus infection initiated in the absence of JAM-A and sialic acid results in apoptosis, Chinese hamster ovary (CHO) cells engineered to express Fc receptors were infected with reovirus using antibodies directed against viral outer-capsid proteins. Fc-mediated infection of CHO cells induced apoptosis in a σ1-independent manner. Apoptosis following this uptake mechanism requires acid-dependent proteolytic disassembly, since treatment of cells with the weak base ammonium chloride diminished the apoptotic response. Analysis of T1L × T3D reassortant viruses revealed that the μ1-encoding M2 gene segment is the only viral determinant of the apoptosis-inducing capacity of reovirus when infection is initiated via Fc receptors. Additionally, a temperature-sensitive, membrane penetration-defective M2 mutant, tsA279.64, is an inefficient inducer of apoptosis. These data suggest that signaling pathways activated by binding of σ1 to JAM-A and sialic acid are dispensable for reovirus-mediated apoptosis and that the μ1 protein plays an essential role in stimulating proapoptotic signaling.
The mammalian reoviruses are prototype members of the Orthoreovirus genus within the Reoviridae family (70). These viruses are composed of two concentric icosahedral capsids enclosing a segmented, double-stranded RNA genome (46). Reoviruses are highly virulent in newborn mice and injure a variety of organs, including the brain, heart, and liver (70). Apoptosis induced as a consequence of reovirus infection plays an important role in the pathogenesis of both reovirus-induced encephalitis (48, 50, 57) and myocarditis (20, 21, 50). Reoviruses also induce apoptosis of cultured cells (19, 59, 71).
Reovirus infection is initiated by the attachment of virions to cell surface receptors via the σ1 protein (37, 74). The σ1 protein of all three reovirus serotypes engages junctional adhesion molecule-A (JAM-A) (5, 11, 27, 53). The σ1 protein also binds to cell surface carbohydrate; however, the type of carbohydrate bound varies with serotype (1, 23, 51, 52). After receptor binding, virions are internalized into cells by receptor-mediated endocytosis (7, 26). Virions undergo acid-dependent proteolytic disassembly within cellular endosomes, leading to the formation of infectious subvirion particles (ISVPs) (2, 8, 15, 24, 63, 66). ISVPs are characterized by the loss of outer-capsid protein σ3, a conformational change in attachment protein σ1, and cleavage of outer-capsid protein μ1 to form particle-associated fragments δ and φ (13, 44, 47). Subsequent to ISVP formation the σ1 protein is shed, and the μ1 cleavage fragments undergo conformational rearrangement, yielding ISVP*s (12, 14). ISVP*s are putative entry intermediates that penetrate endosomes and deliver transcriptionally active cores into the cytoplasm (45, 49).
Clues about mechanisms by which reoviruses induce apoptosis first emerged from studies of strain-specific differences in the efficiency of apoptosis induction. Reovirus strain type 3 Dearing (T3D) induces apoptosis in cultured cells more efficiently than strain type 1 Lang (T1L) (17, 59, 71). Studies using T1L × T3D reassortant viruses demonstrated that these strain-specific effects are determined by the viral S1 and M2 gene segments (59, 71, 72). The S1 gene encodes the attachment protein σ1 (37, 74), and the M2 gene encodes membrane penetration protein μ1 (38, 44, 49). Thus, these genetic experiments suggest critical functions for the σ1 and μ1 proteins in apoptosis induction by reovirus.
Analysis of reovirus strains T3/C44-SA− (T3SA−) and T3/C44MA-SA+ (T3SA+), which are isogenic at all loci except for a single amino acid polymorphism in σ1 (4), has pointed to an important role for sialic acid binding in reovirus-induced apoptosis (17). Sialic-acid-binding strain T3SA+ induces apoptosis significantly more efficiently than non-sialic-acid-binding strain T3SA−. Concordantly, removal of cell surface sialic acid with neuraminidase or blockade of virus binding to cell surface sialic acid using a soluble competitor, sialyllactose, abolishes the capacity of T3SA+ to induce apoptosis (17). However, engagement of sialic acid is not sufficient to induce apoptosis. Blockade of σ1 binding to JAM-A using either σ1- or JAM-A-specific monoclonal antibodies (MAbs) also diminishes the apoptosis-inducing capacity of sialic-acid-binding reoviruses (5, 71). Collectively, these data demonstrate that reovirus strains that bind to both JAM-A and sialic acid are the most potent inducers of apoptosis.
In addition to receptor binding, postattachment events also are required for reovirus-mediated apoptosis induction (18). Inhibition of viral disassembly using ammonium chloride (AC), a weak base that increases vacuolar pH (43), or E64, an inhibitor of cysteine proteases such as those contained in the endocytic compartment (3), abolishes reovirus-induced apoptosis. On the other hand, interference with steps in viral replication subsequent to ISVP formation and membrane penetration using ribavirin, an inhibitor of viral RNA synthesis (55), does not perturb apoptosis induced by reovirus (18). Thus, in addition to sialic acid- and JAM-A-mediated attachment of reovirus to cells, replication steps during or after viral disassembly that occur before the cytoplasmically delivered core becomes transcriptionally active also contribute to reovirus-induced apoptosis. Since the M2-encoded μ1 protein functions in virus-induced endosomal membrane penetration following disassembly but prior to viral RNA synthesis (38, 44, 49), the deleterious effects of reovirus disassembly inhibitors on apoptosis induction suggest a functional link between the M2 gene segment and differences in the efficiency of apoptosis exhibited by different reovirus strains (59, 71, 72).
In this study, we determined whether reovirus is capable of inducing apoptosis independent of JAM-A and sialic acid binding. We found that antibody-mediated uptake of reovirus into JAM-A-negative, Fc-receptor-expressing cells results in productive infection and leads to apoptosis in a σ1-independent fashion. Moreover, apoptosis induced following this uptake pathway also is dependent on viral disassembly. Analysis of reassortant viruses and an M2 mutant virus demonstrates that the μ1 protein influences the strength of proapoptotic signaling following reovirus infection. These data suggest that signaling induced as a result of σ1 interactions with JAM-A and sialic acid are not necessary for apoptosis induced by reovirus and that the μ1 protein is the viral factor that stimulates the cellular apoptotic machinery.
MATERIALS AND METHODS
Cells, viruses, and antibodies.
HeLa cells were maintained in Dulbecco's modified Eagle medium supplemented to contain 10% fetal bovine serum (FBS), 50 U per ml of penicillin, and 50 μg per ml streptomycin (Invitrogen, Carlsbad, CA). Chinese hamster ovary (CHO) cells were maintained in HAM's F12 medium supplemented to contain 10% FBS, 50 U per ml of penicillin, and 50 μg per ml streptomycin. For maintenance of stably transfected CHO cells, the medium was additionally supplemented to contain 1 mg per ml G418 (Invitrogen). CHO-B1 cells, which express the B1 isoform of murine Fc receptor II, were obtained from Ira Mellman (Yale University). Cells were maintained in MEM Alpha supplemented to contain 10% FBS, 50 U per ml of penicillin, 50 μg per ml streptomycin, and 10 μM methotrexate (EMD Biosciences, San Diego, CA) as previously described (34).
T1L and T3D are laboratory stocks. Isolation and characterization of T3SA− and T3SA+ have been previously described (4). Reovirus temperature-sensitive mutant tsA279.64 was obtained from Kevin Coombs (University of Manitoba) (30). Purified reovirus virions were generated by using second- or third-passage L-cell lysate stocks of twice-plaque-purified reovirus as previously described (28). Viral particles were freon-extracted from infected cell lysates, layered onto 1.2- to 1.4-g/cm3 CsCl gradients, and centrifuged at 62,000 × g for 18 h. Bands corresponding to virions (1.36 g/cm3) were collected and dialyzed in virion storage buffer (150 mM NaCl, 15 mM MgCl2, 10 mM Tris-HCl [pH 7.4]). Concentrations of reovirus virions in purified preparations were determined from an equivalence where an optical density at 260 nm of 1 equaled 2.1 × 1012 virions (64).
Reovirus T3D virions were inactivated using a UV cross-linker (Ultra-lum-UVC-508; Marsh Biomedical, Rochester, NY). Virus at a concentration of 109 PFU per ml was irradiated by using short-wave (254 nm) UV on ice at a distance of 10 cm for 5 min at 1,20,000 μJ/cm2 in a 6-cm tissue culture dish (Costar, Cambridge, MA). These conditions are sufficient to reduce viral infectivity to levels that are undetectable by fluorescent focus assays using confluent L929 cell monolayers (data not shown).
Generation and characterization of MAbs 5C6, 9BG5, and 7F4, which are specific for the type 1 σ1, type 3 σ1, and λ2 proteins, respectively, have been previously described (10, 73). The immunoglobulin G (IgG) fractions of polyclonal rabbit antisera raised against T1L and T3D (76) were purified by using protein A-Sepharose as previously described (4). A mixture of these sera was capable of recognizing all strains of reovirus used in this study. Protein A-purified, JAM-A-specific MAb J10.4 was obtained from Charles Parkos (Emory University) (40). Human coxsackievirus and adenovirus receptor (hCAR)-specific MAb RmcB was provided by Jeffrey Bergelson (University of Pennsylvania). Anti-Myc MAb 9E10 and rat anti-Fc MAb were obtained from BD Biosciences (San Jose, CA). Fluorescent dye-conjugated secondary antibodies were obtained from Molecular Probes (Invitrogen).
Generation of CHO cells stably expressing JAM-A or JAM-AΔCT.
Human JAM-A was subcloned into expression plasmid pcDNA3.1 (Invitrogen). Truncation mutant JAM-AΔCT was generated by PCR using full-length JAM-A cDNA as template. Sequences encoding amino acids 1 to 260 (Δ261 to 299) were cloned and appended with a stop codon using T7 primer and 5′-TACGGGATCCTCAGGCAAACCAGATGCC-3′ as forward and reverse primers, respectively. The gene-specific primer encompasses nucleotides 981 to 995 of the JAM-A cDNA. The PCR product was digested with BamHI and introduced into pcDNA3.1 using complementary restriction sites. Fidelity of cloning was confirmed by automated sequencing. CHO cells stably expressing empty vector alone, JAM-A, and JAM-AΔCT were generated by transfection of CHO-K1 cells with empty pcDNA3.1 vector, pcDNA3.1 encoding JAM-A, or pcDNA3.1 encoding JAM-AΔCT. Cells were grown to 50% confluence in 6-cm dishes and transfected with 4 μg of each plasmid by using Lipofectamine Plus reagent (Invitrogen) according to the manufacturer's instructions. Transfected cells were selected by growth in the presence of 1 mg per ml G418. After the 10th passage in the presence of G418, cells expressing high levels of JAM-A as assessed by JAM-A-specific MAb J10.4 staining (mean fluorescence intensity, >5,000) were separated using a BD FACsort cell sorter (Becton-Dickinson, Palo Alto, CA).
Immunoblot for JAM-A.
Cell extracts were prepared from CHO cells (1 × 106) transfected with empty vector, JAM-A, or JAM-AΔCT by sonication in phosphate-buffered saline (PBS) supplemented to contain protease inhibitor cocktail (Roche, Indianapolis, IN). Extracts were resolved by electrophoresis in 10% polyacrylamide gels and transferred to nitrocellulose membranes. Immunoblots were performed by using JAM-A-specific MAb J10.4 followed by horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Amersham Pharmacia Biotech, Piscataway, NJ), each diluted 1:1,000 in PBS containing 0.1% Tween 20 and 5% low-fat dry milk.
Flow cytometric analysis for JAM-A surface expression.
CHO cells stably transfected with empty vector, JAM-A, or JAM-AΔCT were detached from plates using PBS containing 10 mM EDTA. Cells were washed twice with chilled PBS prior to incubation with 10 μg per ml of either JAM-A specific MAb J10.4 or control MAb (hCAR-specific MAb) at 4°C for 1 h. Cells were stained with a 1:1,000 dilution of phycoerythrin (PE)-labeled anti-mouse IgG at 4°C for 1 h and analyzed using a FACScan flow cytometer (Becton-Dickinson).
Assessment of viral infectivity by indirect immunofluorescence.
Monolayers of cells (2 × 105) in 24-well plates (Costar) were adsorbed with the indicated multiplicity of infection (MOI) of reovirus at room temperature for 1 h. Following removal of the inoculum, cells were washed with PBS and incubated in complete medium at 37°C for 18 h to permit completion of a single cycle of viral replication. Monolayers were fixed with 1 ml of methanol at −20°C for a minimum of 30 min, washed twice with PBS, blocked with 2.5% Ig-free bovine serum albumin (Sigma-Aldrich, St. Louis, MO) in PBS, and incubated at room temperature for 1 h with polyclonal rabbit anti-reovirus serum at a 1:1,000 dilution in PBS-0.5% Triton X-100. Monolayers were washed twice with PBS-0.5% Triton X-100 and incubated with a 1:1,000 dilution of Alexa546-labeled anti-rabbit IgG. Monolayers were washed with PBS, and infected cells were visualized by indirect immunofluorescence using an Axiovert 200 fluorescence microscope (Carl Zeiss, New York, NY). Infected cells were identified by the presence of intense cytoplasmic fluorescence that was excluded from the nucleus. No background staining of uninfected control monolayers was noted. Reovirus antigen-positive cells were quantified by counting fluorescent cells in at least three random fields of view in duplicate wells at a primary magnification of 20×.
Antibody-mediated infections.
Reovirus virions (1 × 1011) were incubated at 4°C overnight with various concentrations of MAbs in 1 ml PBS. Cells were adsorbed with virus at an MOI of either 100 or 500 PFU per ml at room temperature for 1 h. Following removal of the inoculum, cells were washed with PBS and incubated in complete medium at 37°C for various intervals. For inhibition of viral disassembly and RNA synthesis, cells were maintained in 20 mM AC or 200 μM ribavirin, respectively, throughout the course of infection. For inhibition of disassembly, cells also were pretreated for 1 h with medium containing 20 mM AC.
Quantitation of apoptosis by acridine orange (AO) staining.
Cells (2 × 105) grown in 24-well tissue culture plates were adsorbed with reovirus virions or MAb-virion complexes at various MOIs. Following incubation at 37°C for 48 h, the percentage of apoptotic cells was determined by using AO staining as previously described (71). For each experiment, 200 to 300 cells were counted in three independent wells, and the percentage of cells exhibiting condensed chromatin was determined by epi-illumination fluorescence microscopy using a fluorescein filter set (Carl Zeiss).
Antibody-mediated cross-linking of Fc receptors.
Cells (2 × 105) were chilled to 4°C and incubated with 1 μg per ml of rat anti-Fc MAb 2.4G2 at 4°C for 30 min. Unbound antibody was removed by washing with chilled PBS, and cells were incubated with 5 μg per ml of IgM specific for rat IgG at 4°C for 30 min. Following incubation at 37°C for 48 h, cells were scored for apoptosis.
Statistical analysis.
Mean values obtained in infectivity and apoptosis assays were compared using the unpaired Student's t test as applied with Microsoft Excel software. P values of less than 0.05 were considered statistically significant. The contribution of each of the reovirus gene segments to apoptosis induction was assessed by using the nonparametric Mann-Whitney test without adjusting for multiple comparisons and a panel of T1L × T3D reassortant viruses as previously described (59).
RESULTS
A JAM-A truncation mutant lacking the cytoplasmic tail is expressed at the cell surface.
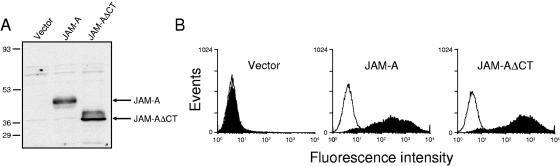
Anti-JAM-A MAb J10.4, which inhibits reovirus binding to JAM-A, also blocks reovirus-induced apoptosis (5). These findings suggest that JAM-A binding is essential for reovirus-induced apoptosis. The JAM-A cytoplasmic tail is approximately 45 amino acids in length, contains 13 potential phosphorylation sites, and interacts with several PDZ domain-containing proteins, suggesting a role in ligand-induced cell signaling (6, 25). To determine whether the cytoplasmic tail of JAM-A contributes to reovirus-induced apoptosis by evoking proapoptotic signaling events, CHO cells were stably transfected with empty vector or vector encoding full-length JAM-A or a C-terminally truncated form of JAM-A that lacks the cytoplasmic tail (JAM-AΔCT). CHO cells were selected for these experiments, since they are poorly permissive to reovirus infection (27); yields of reovirus following infection of CHO cells are 100- to 1,000-fold higher following ectopic expression of JAM-A (11, 27). Whole-cell extracts from stably expressing cells were analyzed for expression of JAM-A by immunoblotting (Fig. 1A). While no JAM-A-specific band was detected in the vector-transfected cells, both full-length JAM-A and the faster migrating JAM-AΔCT proteins were expressed to high levels in the cell lines tested. The surface expression of both JAM-A and JAM-AΔCT was assessed by flow cytometry using JAM-A-specific MAb J10.4 (Fig. 1B). Both wild-type and mutant JAM-A proteins displayed approximately equivalent surface expression, suggesting that removal of the C-terminal domain of JAM-A does not prevent transport of JAM-A to the cell surface. These stably transfected cells are therefore suitable for analysis of infection and apoptosis induction by reovirus.
FIG. 1.
Stable expression of JAM-A and JAM-AΔCT in CHO cells. (A) Whole-cell lysates (1 × 105 cell equivalents) were prepared from CHO cells stably transfected with empty vector, JAM-A, or JAM-AΔCT; resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis; transferred to nitrocellulose; and immunoblotted using anti-JAM-A MAb J10.4. The positions of full-length JAM-A and truncated JAM-AΔCT are shown on the right. The positions of molecular mass standards (in kilodaltons) are shown on the left. (B) Stably transfected CHO cells (1 × 106) were incubated with either anti-JAM MAb J10.4 (filled histograms) or an isotype-matched control (anti-hCAR) MAb (open histograms) at 10 μg per ml, followed by incubation with PE-labeled anti-mouse Ig secondary antibody. The results are presented as fluorescence intensity.
Reovirus induces equivalent levels of apoptosis in CHO cells that express JAM-A and JAM-AΔCT.
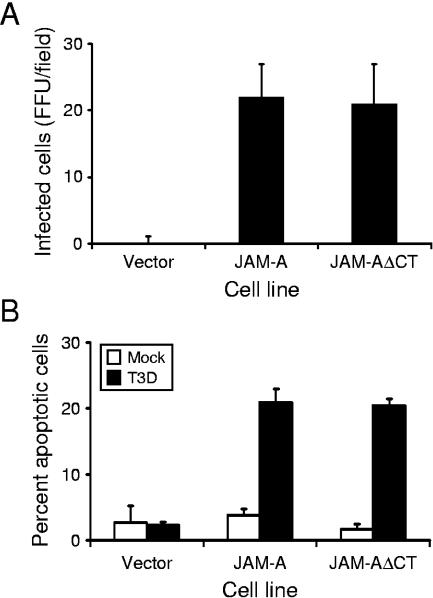
To determine whether CHO cells stably expressing JAM-AΔCT can support reovirus infection, cells were adsorbed with reovirus strain T3D at an MOI of 10 PFU per cell, and viral infectivity was assessed by using indirect immunofluorescence (Fig. 2A). CHO cells transfected with either empty vector or those engineered to stably express full-length JAM-A were used as controls. In contrast to vector-transfected cells, cells expressing either JAM-A or JAM-AΔCT were equivalently capable of supporting infection by T3D. These data indicate that although JAM-A expression is required for efficient infection of CHO cells, the JAM-A cytoplasmic tail is dispensable.
FIG. 2.
Infection and apoptosis of JAM-A- and JAM-AΔCT-expressing CHO cells. (A) Cells were adsorbed with T3D at an MOI of 10 PFU per cell. After incubation at 37°C for 18 h, cells were fixed using methanol. Infected cells were visualized by immunostaining with polyclonal rabbit anti-reovirus sera, followed by incubation with Alexa546-labeled anti-rabbit IgG. Reovirus-infected cells were quantified by counting fluorescent cells. The results are presented as mean fluorescent focus units (FFU) per field. Error bars indicate standard deviations. (B) Cells were adsorbed with either PBS (mock) or T3D at an MOI of 100 PFU per cell. Cells were harvested at 48 h after infection and stained with AO. The results are expressed as the mean percentage of cells undergoing apoptosis for three independent experiments. Error bars indicate standard deviations.
To determine whether the JAM-A cytoplasmic tail is required for apoptosis, stably transfected CHO cell lines were adsorbed with T3D at an MOI of 100 PFU per cell, and apoptosis was assessed by using AO staining (Fig. 2B). None of the cell lines tested showed significant apoptosis following mock infection (<5%). T3D infection of cells transfected with vector alone induced levels of apoptosis equivalent to those following mock infection of cells. In contrast, T3D infection of either the JAM-A- or JAM-AΔCT-expressing cell lines induced an equivalent percentage of cells to undergo apoptosis (∼22%). Therefore, analogous to our findings in the infectivity assays, the cytoplasmic tail of JAM-A is not required for reovirus-induced apoptosis.
Antibody-mediated uptake of reovirus into Fc receptor-expressing cells leads to infection and apoptosis.
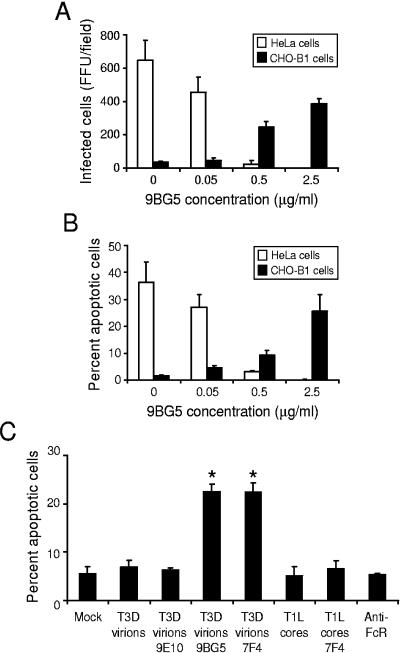
To determine whether the requirement for JAM-A during reovirus infection and apoptosis can be bypassed, we utilized antibody-mediated infection of Fc receptor-expressing CHO (CHO-B1) cells. These cells stably express the B1 isoform of the mouse Fc receptor II (34). Therefore, in the absence of JAM-A, these cells should allow internalization of antibody-reovirus complexes into cells via Fc receptors, resulting in efficient infection of normally nonpermissive cells. For these experiments, T3D virions were incubated with increasing concentrations of σ1-specific, neutralizing MAb 9BG5 (10) prior to infection of CHO-B1 cells. HeLa cells were used in parallel to confirm the neutralizing efficacy of MAb 9BG5 at the concentrations tested. For each cell line, the number of infected cells was assessed 18 h postinfection by indirect immunofluorescence. As anticipated, the efficiency of reovirus infection of HeLa cells decreased in proportion to antibody concentration, with little infection detected in the presence of 2.5 μg per ml of 9BG5 (Fig. 3A). We conclude that 9BG5 interferes with σ1-JAM-A interactions, which are critical for reovirus infection of HeLa cells. In contrast, the efficiency of infection of CHO-B1 cells by T3D increased in proportion to 9BG5 concentration, with maximal infection observed in the presence of a completely neutralizing concentration of 9BG5, 2.5 μg per ml (Fig. 3A). These findings demonstrate that reovirus infection can be established in a JAM-A-independent manner if an alternative high-affinity binding moiety is provided. These data corroborate a previous report of antibody-mediated enhancement of reovirus infection of a murine macrophage-like cell line (9).
FIG. 3.
Infection and apoptosis of HeLa cells and CHO-B1 cells in the presence of MAb 9BG5. (A) Reovirus T3D particles were incubated overnight with the indicated concentration of MAb 9BG5 and adsorbed to either HeLa cells or CHO-B1 cells at an MOI of 100 PFU per cell. After incubation at 37°C for 18 h, cells were fixed using methanol. Infected cells were visualized by immunostaining with polyclonal rabbit anti-reovirus sera, followed by Alexa546-labeled anti-rabbit IgG. Reovirus-infected cells were quantified by counting fluorescent cells. The results are presented as mean fluorescent focus units (FFU) per field. Error bars indicate standard deviations. (B) HeLa cells or CHO-B1 cells were adsorbed with 100 PFU per cell of either virus or virus-antibody complex, harvested at 48 h after infection, and stained with AO. (C) CHO-B1 cells were mock-infected, infected with T3D virions with or without 2.5 μg per ml of Myc-specific MAb 9E10 (antibody control), σ1-specific MAb 9BG5, or λ2-specific MAb 7F4 at an MOI of 100 PFU per cell or were infected with T1L core particles with or without 2.5 μg per ml of MAb 7F4 at an MOI of 104 particles per cell. CHO-B1 cells also were incubated with 1 μg per ml of anti-Fc receptor rat IgG MAb 2.4G2, followed by incubation with 5 μg per ml of IgM specific for rat IgG (anti-FcR). Cells were harvested at 48 h after infection and stained with AO. The results are expressed as the mean percentage of cells undergoing apoptosis for three independent experiments. Error bars indicate standard deviations. *, P < 0.05 as determined by Student's t test in comparison to T3D incubated with control MAb 9E10.
To determine whether infection initiated in a JAM-A-independent manner also triggers apoptosis, the capacity of reovirus-9BG5 complexes to induce apoptotic cell death of both HeLa cells and CHO-B1 cells was assessed by using AO staining (Fig. 3B). Approximately 35% of HeLa cells showed apoptotic nuclei at 48 h postinfection in the absence of antibody treatment of virions. Consistent with the decrease in the capacity of reovirus to infect HeLa cells in the presence of 9BG5, the percentage of cells undergoing apoptosis also decreased with increasing 9BG5 concentrations. However, in the absence of 9BG5, T3D induced minimal apoptosis in CHO-B1 cells in comparison to mock-infected cells. In concordance with the infectivity data, the percentage of apoptotic CHO-B1 cells increased with increasing concentrations of 9BG5, with maximal apoptosis seen following pretreatment with 2.5 μg per ml MAb (∼25%). These findings demonstrate that reovirus infection initiated in the absence of JAM-A binding also leads to apoptosis. Therefore, σ1-JAM-A interactions or signaling pathways induced as a consequence of these interactions are dispensable for apoptosis induction by reovirus.
To exclude the possibility that binding of MAb to the Fc receptor contributes to apoptosis induction, we tested whether incubation of reovirus with irrelevant anti-Myc MAb 9E10 was capable of inducing apoptosis (Fig. 3C). While incubation of T3D with two different MAbs directed against the reovirus outer-capsid, 7F4 (λ2) and 9BG5 (σ1), induced 21% and 22% apoptosis, respectively, following infection of CHO-B1 cells, incubation of T3D with MAb 9E10 failed to induce apoptosis at levels higher than those of mock-infected cells. These findings suggest that only antibodies directed against reovirus outer-capsid proteins allow virus attachment to CHO-B1 cells and subsequent induction of apoptosis. To exclude the involvement of signaling induced as a result of Fc receptor cross-linking due to binding of reovirus-antibody complexes, Fc receptors were cross-linked using 2.4G2 (a rat anti-Fc receptor MAb) and an IgM antibody directed against rat IgG. This treatment did not induce apoptosis in CHO-B1 cells in comparison to mock-treated cells. As an additional control for the effect of cross-linking Fc receptors, T1L core particles, which lack outer-capsid proteins σ1, σ3, and μ1, were incubated with MAb 7F4 and added to CHO-B1 cells. Neither untreated cores nor 7F4-core complexes induced apoptosis of these cells. These data suggest that apoptosis does not result from proapoptotic signaling induced as a consequence of Fc-receptor cross-linking but requires binding of reovirus particles containing outer-capsid proteins.
Viral disassembly is required for apoptosis induced by Fc-mediated uptake of reovirus.
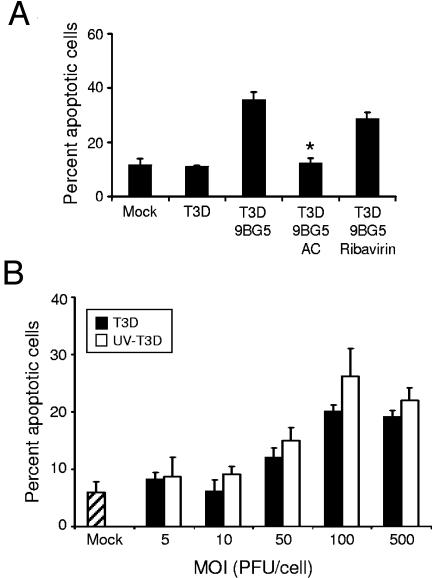
To determine whether viral disassembly in cellular endosomes is required for apoptosis following Fc-mediated delivery of reovirus, CHO-B1 cells were treated with AC prior to infection by MAb-treated T3D virions. Treatment of cells with 20 mM AC, a concentration sufficient to block reovirus disassembly (66, 77), abolished the capacity of T3D to induce apoptosis (Fig. 4A). Thus, acid-dependent proteolytic disassembly is required for apoptosis induction by this uptake mechanism. To determine whether the apoptosis-inhibitory effect of AC is due to blockade of viral RNA synthesis, we tested ribavirin, an inhibitor of viral RNA synthesis (55), for the capacity to diminish apoptosis. In keeping with our previously published results (18), apoptosis induced by reovirus infection via Fc-mediated uptake was unaffected by ribavirin (Fig. 4A). To corroborate these findings, we tested the apoptosis-inducing capacity of UV-inactivated reovirus virions, which are incapable of establishing productive infection (60 and data not shown). We found that UV-inactivated reovirus induced apoptosis following Fc receptor-mediated uptake of a high MOI of virus (Fig. 4B), consistent with our previously published observations (71). Collectively, these results demonstrate that steps in reovirus replication that occur after attachment but before transcription are required for induction of apoptosis, regardless of the type of receptor used to initiate infection (18). In addition, these results suggest that death signaling during reovirus infection may occur independently of receptor engagement.
FIG. 4.
Apoptosis following Fc receptor-mediated uptake of viable and UV-inactivated reovirus virions. (A) T3D alone or preincubated with 2.5 μg per ml of MAb 9BG5 was adsorbed to CHO-B1 cells at an MOI of 500 PFU per cell. (B) UV-inactivated T3D preincubated with 2.5 μg per ml of MAb 9BG5 was adsorbed to CHO-B1 cells at the indicated MOIs. After incubation at 37°C for 48 h in untreated medium or medium containing either 20 mM AC or 200 μM ribavirin, cells were stained with AO. The results are expressed as the mean percentage of cells undergoing apoptosis for three independent experiments. Error bars indicate standard deviations. *, P < 0.05 as determined by Student's t test in comparison to T3D incubated with MAb 9BG5.
Fc receptor-dependent infection abolishes σ1-related differences in the apoptosis-inducing capacity of reovirus.
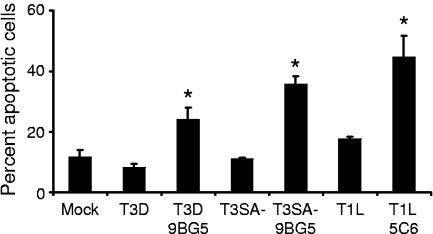
Differences in the capacity of some reovirus strains to induce apoptosis are linked to differences in the affinity for sialic acid (17). To determine whether reovirus strains that are incapable of binding to sialic acid can induce apoptosis when infection is initiated via Fc-dependent uptake, strains T1L and T3SA−, neither of which is capable of binding to sialic acid (4, 16, 23), were incubated with σ1-specific antibodies and adsorbed to CHO-B1 cells. Incubation of T1L virions with type 1 σ1-specific MAb 5C6 (73) and incubation of T3SA− virions with type 3 σ1-specific 9BG5 (10) resulted in significantly higher levels of apoptosis in CHO-B1 cells in comparison to levels observed following infection with untreated T1L and T3SA− virions (Fig. 5). The percentage of cells undergoing apoptosis following antibody-mediated infection of non-sialic-acid-binding reovirus strains was at least equal to that observed following infection with T3D. Therefore, σ1-related differences in apoptosis efficiency are overcome when infection is initiated via Fc-mediated uptake. These results make it unlikely that the σ1 protein is the viral effector of apoptosis induction.
FIG. 5.
Apoptosis induction in CHO-B1 cells following Fc-mediated infection by T1L and T3SA−. Cells were adsorbed with T3D, T3SA−, or T1L with or without 2.5 μg per ml of MAb 9BG5 (for type 3 strains) or MAb 5C6 (for T1L) at an MOI of 100 PFU per cell. After incubation at 37°C for 48 h, cells were harvested and stained with AO. The results are expressed as the mean percentage of cells undergoing apoptosis for three independent experiments. Error bars indicate standard deviations. *, P < 0.05 as determined by Student's t test in comparison to the same strain incubated without antibody.
Differences in apoptosis induction following Fc receptor-dependent infection of T1L × T3D reassortant viruses are linked to the M2 gene segment.
We have demonstrated previously that the viral S1 and M2 gene segments segregate with differences in the apoptosis-inducing capacity of T1L and T3D (59, 71). To ascertain whether any strain-specific differences exist when the effect of the S1 gene segment is circumvented by Fc receptor-dependent uptake, CHO-B1 cells were adsorbed with T1L × T3D reassortant viruses after incubation with σ1-specific antibodies. Type 1 and type 3 strains were incubated with σ1-specific MAbs 5C6 and 9BG5, respectively, prior to infection. Each of the reassortant viruses tested produced a similar number of infected cells as assessed by indirect immunofluorescence (data not shown), suggesting equivalent efficiency of antibody-mediated uptake and infection. The percentage of cells undergoing apoptosis as a result of antibody-mediated infection of cells was assessed at 48 h after infection. The reassortant viruses were ranked from highest to lowest by apoptosis-inducing capacity (Table 1). Although the reassortants did not cluster distinctly into two groups with high and low apoptotic potential, six of the seven strains with the highest levels of apoptosis had an M2 gene segment derived from T3D. Conversely, seven of the eight strains with the lowest levels of apoptosis had an M2 gene segment derived from T1L. Analysis of the data using the Mann-Whitney test showed that only the M2 gene segregated at a statistically significant level with the capacity of these strains to induce apoptosis (P = 0.02). There was not a statistically significant association between apoptosis and the S1 gene (P = 0.28), which is the primary apoptosis determinant following infection of JAM-A-expressing cells (17, 59, 71, 72), when infection was initiated in an Fc-dependent fashion. These data suggest that the M2-encoding μ1 protein, which functions in penetration of cell membranes (38, 44, 49), is the primary viral determinant of strain-specific differences in apoptosis induction following infection by Fc-mediated uptake.
TABLE 1.
Apoptosis induction by T1L × T3D reassortant viruses in CHO-B1 cells
| Virus isolate | Origin of gene segmentsa
|
% Apoptosisb | SD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | M1 | M2 | M3 | S1 | S2 | S3 | S4 | |||
| Reassortant | ||||||||||||
| EB138 | D | L | L | D | D | L | D | D | L | L | 80.5 | 3.84 |
| KC150 | D | L | L | L | D | L | D | D | L | D | 62.8 | 2.23 |
| KC9 | D | D | L | D | D | D | L | D | D | D | 58.6 | 3.28 |
| EB97 | D | D | L | D | D | D | D | D | D | L | 52.3 | 3.89 |
| EB68 | L | D | L | L | D | L | L | L | D | D | 41.2 | 4.98 |
| 1HA.3 | L | L | L | L | L | L | D | L | L | L | 40.6 | 7.15 |
| EB144 | L | L | L | L | D | D | L | L | D | L | 38.2 | 3.99 |
| EB98 | L | D | L | L | L | L | L | D | L | D | 32.6 | 3.17 |
| EB121 | D | D | L | D | L | D | L | D | D | D | 28.9 | 8.13 |
| EB113 | L | L | L | D | L | L | L | L | D | L | 26.6 | 3.85 |
| G16 | L | L | L | D | L | L | L | D | L | L | 24.4 | 3.62 |
| G2 | L | D | L | L | L | L | D | L | L | L | 23.1 | 4.90 |
| EB143 | D | L | L | L | L | L | D | L | L | L | 23.0 | 4.05 |
| EB145 | D | D | D | D | D | L | L | D | D | D | 16.2 | 3.40 |
| EB120 | D | D | D | L | L | D | D | D | L | L | 14.5 | 3.33 |
| Parental | ||||||||||||
| T3D | D | D | D | D | D | D | D | D | D | D | 23.0 | 1.81 |
| T1L | L | L | L | L | L | L | L | L | L | L | 33.6 | 2.34 |
Parental origin of each gene segment: L, gene segment derived from T1L; D, gene segment derived from T3D.
CHO-B1 cells (2 × 105) were adsorbed with virus strains at an MOI of 100 PFU per cell. After 1 h, the inoculum was removed, fresh medium was added, and cells were incubated at 37°C for 48 h and stained with AO to assess apoptosis. Shown are the mean percentage and standard deviations of cells undergoing apoptosis for three independent experiments. The M2 gene was the only gene associated with the efficiency of apoptosis as determined by using the nonparametric Mann-Whitney test, without adjusting for multiple comparisons (P = 0.02).
Reovirus mutant tsA279 is inefficient in apoptosis induction.
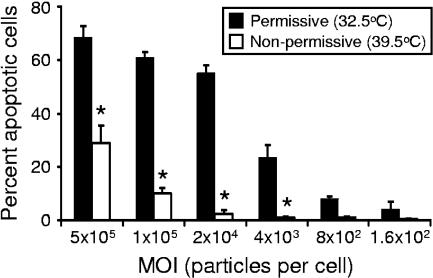
To determine whether a mutant virus with a defective μ1 protein is altered in the capacity to trigger apoptosis, we used reovirus strain tsA279.64, which contains a temperature-sensitive mutation that maps to the M2 gene segment (30). This virus was derived from a coinfection of T1L and tsA279, which contains temperature-sensitive mutations in both the M2 and L2 gene segments. The tsA279.64 virus contains the mutant M2 gene segment but not the mutant L2 gene segment, thus facilitating analysis of the contribution of the M2 gene segment to apoptosis induction. When assembled at nonpermissive temperature, virions containing the mutant M2 gene segment cannot penetrate membranes due to a misfolded μ1 protein (30). To examine whether μ1-mediated membrane penetration is required for apoptosis induction, HeLa cells were adsorbed with increasing MOIs of tsA279.64 virions assembled under permissive and nonpermissive conditions. The percentage of cells with apoptotic nuclei was assessed by using AO staining 48 h after infection at nonpermissive temperature (Fig. 6). At all MOIs tested, particles assembled at nonpermissive temperature induced less apoptosis than particles assembled at permissive temperature. These data demonstrate that virions containing a μ1 protein that is inefficient in membrane penetration are less potent inducers of apoptosis, which further highlights a key role for the μ1 protein in apoptosis induction.
FIG. 6.
Apoptosis induced by μ1 temperature-sensitive mutant tsA279.64. HeLa cells were adsorbed with tsA279.64 grown at permissive or nonpermissive temperatures at the MOIs shown. After incubation at 37°C for 48 h, cells were harvested and stained with AO. The results are expressed as the mean percentage of cells undergoing apoptosis for three independent experiments. Error bars indicate standard deviations. *, P < 0.05 as determined by Student's t test in comparison to virions grown at permissive temperature at an equivalent MOI.
DISCUSSION
We have previously shown that binding of reovirus to JAM-A and sialic acid is required for efficient induction of apoptosis (5, 17). In addition, we have reported that viral disassembly in cellular endosomes is also necessary for apoptosis induction by reovirus (18). However, since receptor binding is a prerequisite for virus disassembly, these findings do not resolve the question of whether reovirus attachment and disassembly provide two distinct signals, both of which are required for apoptosis induction, or whether the viral disassembly events are sufficient for proapoptotic signaling. To address this question, we uncoupled reovirus attachment to JAM-A and sialic acid from viral disassembly by providing an alternative means of viral entry. We report here that antibody-mediated uptake of reovirus into Fc receptor-expressing CHO cells independent of binding to JAM-A and sialic acid leads to productive infection (9) and apoptosis. Furthermore, we demonstrate that antibody-directed binding of reovirus to Fc receptors expressed on CHO cells is not sufficient for reovirus-induced apoptosis. Analogous to JAM-A- and sialic acid-dependent infection, viral replication steps during or after disassembly in endosomes but prior to RNA synthesis also are required for reovirus-induced apoptosis when infection is initiated by Fc-mediated uptake. Analysis of apoptosis induction by T1L × T3D reassortant viruses following Fc-mediated uptake showed that differences in the efficiency of apoptosis exhibited by type 1 and type 3 strains segregate with the μ1-encoding M2 gene segment. Neither core particles that lack the μ1 protein and are therefore incapable of penetrating endosomal membranes nor the thermosensitive M2 mutant virus tsA279.64, which contains a misfolded, penetration-defective μ1 protein, can efficiently induce apoptosis. These findings suggest that reovirus membrane penetration protein μ1 induces proapoptotic signaling during or after endosomal membrane penetration.
In our reassortant analysis, the viral M2 gene segment was the only viral gene segment that segregated at a statistically significant level with apoptosis-inducing capacity. Although levels of apoptosis induced by reassortant viruses containing a T3D M2 gene segment were generally higher than those induced by reassortant viruses containing a T1L M2 gene segment, the percentage of cells undergoing apoptosis formed a continuum of values rather than two distinct clusters. Since our data suggest that apoptosis is induced during or after endosomal membrane penetration mediated by the μ1 protein, steps in viral replication preceding membrane penetration, such as attachment, internalization, and disassembly, also may influence the magnitude of the apoptotic response. Thus, viral gene products that mediate these steps also may contribute to the observed differences in levels of apoptosis. Minor strain-specific effects attributed to other viral gene segments also may explain our finding that antibody-mediated uptake of parental strain T1L into Fc receptor-expressing cells induced higher levels of apoptosis than those induced by T3D (Fig. 5, Table 1). Alternatively, the type 1 and type 3 σ1-specific MAbs used in our experiments may differ in either affinity for σ1 or efficiency in internalization via Fc receptors. However, we think that this explanation is unlikely, since no association between the σ1-encoding S1 gene and apoptosis-inducing potential was observed. Therefore, although it is likely that there are multiple viral genetic determinants of reovirus-induced apoptosis, our statistical analysis of T1L × T3D reassortant viruses, in addition to our findings using M2 temperature-sensitive mutant tsA279.64, lead us to conclude that the M2-encoded μ1 protein is the primary mediator of proapoptotic signaling.
In addition to the findings here, three independent lines of evidence support a crucial role for μ1 in reovirus-induced apoptosis. First, differences in apoptosis efficiency displayed by strains T1L and T3D following infection of JAM-A-expressing cells are linked in part to the μ1-encoding M2 gene (59, 71, 72). Second, studies using pharmacologic inhibitors of reovirus replication place the apoptosis-inducing events subsequent to viral disassembly but prior to RNA synthesis (18), which coincides with μ1-mediated membrane penetration (12, 38, 44, 49). Third, transient transfection of a plasmid encoding T3D μ1 is sufficient to induce apoptosis in CHO cells (C. M. Coffey, L. J. Anguish, A. Sheh, I. S. Kim, K. Chandran, M. L. Nibert, and J. S. Parker, Abstr. Am. Soc. Virol. Annu. Meet., abstr. W41-4, 2005). Interestingly, although plasmid-mediated expression of μ1 neither mimics normal delivery of μ1 via endosomal rupture during viral membrane penetration nor de novo expression of μ1 in infected cells (since μ1 is mostly found associated with its protector protein σ3 [61, 62, 67]), it leads to the same consequence.
It is not known how the viral disassembly events culminating in μ1-mediated membrane penetration elicit proapoptotic signaling. We envision two possibilities. First, endosomal disruption by μ1 may lead to release of hydrolytic enzymes such as cathepsins, which in turn damage mitochondria and stimulate death signaling (22, 29, 58). Interestingly, mitochondrial injury has been reported as early as 4 h following reovirus adsorption (35, 36), suggesting the involvement of an early viral replication event. It is also possible that release of these enzymes causes apoptosis via their action on death regulators such as Bid (65). Of note, Bid cleavage has been observed during reovirus infection and has been hypothesized to play a role in apoptosis induction (35). Second, fragments of μ1 produced during proteolytic viral disassembly are known to gain access to the cytoplasm (14). These fragments may activate other cellular sensors of viral infection or directly injure mitochondria to induce apoptosis. Concordantly, the μ1 protein localizes to mitochondria during infection or when expressed from plasmids in transfected cells (C. M. Coffey, L. J. Anguish, A. Sheh, I. S. Kim, K. Chandran, M. L. Nibert, and J. S. Parker, Abstr. Am. Soc. Virol. Annu. Meet., abstr. W41-4, 2005), suggesting a postendosomal site of action. Interestingly, a 30-residue C-terminal fragment of μ1 is sufficient to localize to mitochondria and induce apoptosis (C. M. Coffey, L. J. Anguish, A. Sheh, I. S. Kim, K. Chandran, M. L. Nibert, and J. S. Parker, Abstr. Am. Soc. Virol. Annu. Meet., abstr. W41-4, 2005).
Although our findings point to μ1 as a key viral regulator of proapoptotic signaling, this work does not explain the previously established unequivocal association between the S1-encoded σ1 protein and the efficiency of apoptosis induction by reovirus (59, 71, 72). Strains encoding a σ1 protein capable of binding to JAM-A and sialic acid are the most potent inducers of apoptosis (17). We did not observe efficient infection of CHO-B1 cells in the absence of MAb pretreatment at the MOIs used. Therefore, we think that these cells do not express sufficient quantities of sialic acid or JAM-A on the cell surface to effect productive infection. Thus, infection of these cells appears to be dependent only on the presence of a high-affinity receptor such as the Fc receptor. Since the efficiency of antibody-mediated uptake and delivery of both sialic-acid-binding and non-sialic-acid-binding strains of reovirus via Fc receptors is essentially equivalent in CHO-B1 cells, σ1-related differences are negated. An alternative explanation for our findings is that antibody-mediated attachment of virions to Fc receptors stimulates a signaling cascade in cells that mimics signaling induced as a consequence of σ1 binding to JAM-A and sialic acid and that Fc receptor-mediated signaling acts in concert with the viral disassembly events to elicit apoptosis. However, given the marked differences in functional properties displayed by JAM-A and Fc receptors, this explanation seems less likely. Therefore, our current and previous studies suggest that the linkage of σ1 to apoptosis efficiency is not related to σ1-mediated stimulation of the cellular proapoptotic machinery but rather the capacity of σ1 to efficiently deliver virions into endosomes for disassembly. We hypothesize that, as opposed to viral infection, which requires penetration by just one infectious virion, efficient apoptosis induction by reovirus requires endosomal penetration by multiple virions. Since sialic acid allows more avid binding of virions to cells (4), we think that this entry route may deliver virions more efficiently into endosomal compartments for uncoating and subsequent membrane penetration, leading to higher levels of apoptosis (17).
This study highlights a new role for the viral membrane penetration protein μ1 in apoptosis induction. Other viruses, such as coronavirus, Sindbis virus, and vaccinia virus, also have been reported to require postattachment cell entry events in endosomes to induce apoptosis (33, 39, 54). However, the viral determinants of apoptosis by these viruses are unknown. Interestingly, entry of Sindbis virus into endosomes induces apoptosis through activation of sphingomyelinases and release of the proapoptotic second messenger, ceramide (32). Although we anticipate that interactions between the enveloped Sindbis virus with endosomes differ from those with μ1, it is possible that a lipid second messenger also plays a role in reovirus-induced apoptosis. Collectively, these studies point to cellular endosomes as sites from which proapoptotic signaling events are initiated and imply a conserved mechanism by which host cells detect the presence of invading pathogens. Pathogen detection during the entry phase may allow host cells to more efficiently limit the spread of infection by initiating cell death. Alternatively, induction of apoptosis by a virus early in its replication cycle may prevent or attenuate the development of an inflammatory response, thereby allowing the virus to better evade host defenses. A fascinating similarity exists between the properties of reovirus protein μ1 and several toxins elaborated by bacteria and viruses. Analogous to the capacity of μ1 to mediate membrane permeabilization (12, 31, 41, 69), α toxin of Staphylococcus aureus, lysteriolysin O of Listeria monocytogenes, and killer toxins (K1 and K2) of the yeast L-A virus also can form pores in host cell membranes (42, 68, 75, 78). Interestingly, each of these toxins also has the capacity to induce apoptotic cell death (56, 75). Our ongoing studies are focused on understanding the precise mechanism by which μ1 induces apoptosis during reovirus infection. Through these studies we hope to gain broader insight into events at the pathogen-host interface that evoke death signaling and cause disease.
Acknowledgments
We thank John Parker and members of our laboratory for many helpful discussions and Jim Chappell, Geoff Holm, and Denise Wetzel for careful review of the manuscript. We are grateful to the Nashville Veterans Affairs Hospital Flow Cytometry Facility for assistance and data analysis.
This research was supported by Public Health Service awards T32 CA09385 (J.A.C. and J.C.F.) and R01 AI50080 and the Elizabeth B. Lamb Center for Pediatric Research. Additional support was provided by Public Health Service awards CA68485 for the Vanderbilt-Ingram Cancer Center and DK20593 for the Vanderbilt Diabetes Research and Training Center.
REFERENCES
- 1.Armstrong, G. D., R. W. Paul, and P. W. Lee. 1984. Studies on reovirus receptors of L cells: virus binding characteristics and comparison with reovirus receptors of erythrocytes. Virology 138:37-48. [DOI] [PubMed] [Google Scholar]
- 2.Baer, G. S., D. H. Ebert, C. J. Chung, A. H. Erickson, and T. S. Dermody. 1999. Mutant cells selected during persistent reovirus infection do not express mature cathepsin L and do not support reovirus disassembly. J. Virol. 73:9532-9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett, A. J., A. A. Kembhavi, M. A. Brown, H. Kirschke, C. G. Knight, M. Tamai, and K. Hanada. 1982. L-trans-epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem. J. 201:189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton, E. S., J. L. Connolly, J. C. Forrest, J. D. Chappell, and T. S. Dermody. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276:2200-2211. [DOI] [PubMed] [Google Scholar]
- 5.Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. [DOI] [PubMed] [Google Scholar]
- 6.Bazzoni, G., O. M. Martinez-Estrada, F. Orsenigo, M. Cordenonsi, S. Citi, and E. Dejana. 2000. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J. Biol. Chem. 275:20520-20526. [DOI] [PubMed] [Google Scholar]
- 7.Borsa, J., B. D. Morash, M. D. Sargent, T. P. Copps, P. A. Lievaart, and J. G. Szekely. 1979. Two modes of entry of reovirus particles into L cells. J. Gen. Virol. 45:161-170. [DOI] [PubMed] [Google Scholar]
- 8.Borsa, J., M. D. Sargent, P. A. Lievaart, and T. P. Copps. 1981. Reovirus: evidence for a second step in the intracellular uncoating and transcriptase activation process. Virology 111:191-200. [DOI] [PubMed] [Google Scholar]
- 9.Burstin, S. J., M. W. Brandriss, and J. J. Schlesinger. 1983. Infection of a macrophage-like cell line, P388D1 with reovirus; effects of immune ascitic fluids and monoclonal antibodies on neutralization and on enhancement of viral growth. J. Immunol. 130:2915-2919. [PubMed] [Google Scholar]
- 10.Burstin, S. J., D. R. Spriggs, and B. N. Fields. 1982. Evidence for functional domains on the reovirus type 3 hemagglutinin. Virology 117:146-155. [DOI] [PubMed] [Google Scholar]
- 11.Campbell, J. A., P. Schelling, J. D. Wetzel, E. M. Johnson, J. C. Forrest, G. A. Wilson, M. Aurrand-Lions, B. A. Imhof, T. Stehle, and T. S. Dermody. 2005. Junctional adhesion molecule-A serves as a receptor for prototype and field-isolate strains of mammalian reovirus. J. Virol. 79:7967-7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandran, K., D. L. Farsetta, and M. L. Nibert. 2002. Strategy for nonenveloped virus entry: a hydrophobic conformer of the reovirus membrane penetration protein μ1 mediates membrane disruption. J. Virol. 76:9920-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandran, K., and M. L. Nibert. 2003. Animal cell invasion by a large nonenveloped virus: reovirus delivers the goods. Trends Microbiol. 11:374-382. [DOI] [PubMed] [Google Scholar]
- 14.Chandran, K., J. S. Parker, M. Ehrlich, T. Kirchhausen, and M. L. Nibert. 2003. The delta region of outer-capsid protein μ1 undergoes conformational change and release from reovirus particles during cell entry. J. Virol. 77:13361-13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang, C. T., and H. J. Zweerink. 1971. Fate of parental reovirus in infected cell. Virology 46:544-555. [DOI] [PubMed] [Google Scholar]
- 16.Chappell, J. D., J. L. Duong, B. W. Wright, and T. S. Dermody. 2000. Identification of carbohydrate-binding domains in the attachment proteins of type 1 and type 3 reoviruses. J. Virol. 74:8472-8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connolly, J. L., E. S. Barton, and T. S. Dermody. 2001. Reovirus binding to cell surface sialic acid potentiates virus-induced apoptosis. J. Virol. 75:4029-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connolly, J. L., and T. S. Dermody. 2002. Virion disassembly is required for apoptosis induced by reovirus. J. Virol. 76:1632-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connolly, J. L., S. E. Rodgers, P. Clarke, D. W. Ballard, L. D. Kerr, K. L. Tyler, and T. S. Dermody. 2000. Reovirus-induced apoptosis requires activation of transcription factor NF-κB. J. Virol. 74:2981-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeBiasi, R., C. Edelstein, B. Sherry, and K. Tyler. 2001. Calpain inhibition protects against virus-induced apoptotic myocardial injury. J. Virol. 75:351-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeBiasi, R. L., B. A. Robinson, B. Sherry, R. Bouchard, R. D. Brown, M. Rizeq, C. Long, and K. L. Tyler. 2004. Caspase inhibition protects against reovirus-induced myocardial injury in vitro and in vivo. J. Virol. 78:11040-11050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deiss, L. P., H. Galinka, H. Berissi, O. Cohen, and A. Kimchi. 1996. Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-alpha. EMBO J. 15:3861-3870. [PMC free article] [PubMed] [Google Scholar]
- 23.Dermody, T. S., M. L. Nibert, R. Bassel-Duby, and B. N. Fields. 1990. A σ1 region important for hemagglutination by serotype 3 reovirus strains. J. Virol. 64:5173-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebert, D. H., J. Deussing, C. Peters, and T. S. Dermody. 2002. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 277:24609-24617. [DOI] [PubMed] [Google Scholar]
- 25.Ebnet, K., C. U. Schulz, M. K. Meyer Zu Brickwedde, G. G. Pendl, and D. Vestweber. 2000. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J. Biol. Chem. 275:27979-27988. [DOI] [PubMed] [Google Scholar]
- 26.Ehrlich, M., W. Boll, A. Van Oijen, R. Hariharan, K. Chandran, M. L. Nibert, and T. Kirchhausen. 2004. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118:591-605. [DOI] [PubMed] [Google Scholar]
- 27.Forrest, J. C., J. A. Campbell, P. Schelling, T. Stehle, and T. S. Dermody. 2003. Structure-function analysis of reovirus binding to junctional adhesion molecule 1. Implications for the mechanism of reovirus attachment. J. Biol. Chem. 278:48434-48444. [DOI] [PubMed] [Google Scholar]
- 28.Furlong, D. B., M. L. Nibert, and B. N. Fields. 1988. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J. Virol. 62:246-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guicciardi, M. E., J. Deussing, H. Miyoshi, S. F. Bronk, P. A. Svingen, C. Peters, S. H. Kaufmann, and G. J. Gores. 2000. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Investig. 106:1127-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hazelton, P. R., and K. M. Coombs. 1995. The reovirus mutant tsA279 has temperature-sensitive lesions in the M2 and L2 genes: the M2 gene is associated with decreased viral protein production and blockade in transmembrane transport. Virology 207:46-58. [DOI] [PubMed] [Google Scholar]
- 31.Hooper, J. W., and B. N. Fields. 1996. Role of the μ1 protein in reovirus stability and capacity to cause chromium release from host cells. J. Virol. 70:459-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jan, J. T., S. Chatterjee, and D. E. Griffin. 2000. Sindbis virus entry into cells triggers apoptosis by activating sphingomyelinase, leading to the release of ceramide. J. Virol. 74:6425-6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jan, J. T., and D. E. Griffin. 1999. Induction of apoptosis by Sindbis virus occurs at cell entry and does not require viral replication. J. Virol. 73:10296-10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joiner, K. A., S. A. Fuhrman, H. M. Miettinen, L. H. Kasper, and I. Mellman. 1990. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science 249:641-646. [DOI] [PubMed] [Google Scholar]
- 35.Kominsky, D. J., R. J. Bickel, and K. L. Tyler. 2002. Reovirus-induced apoptosis requires both death receptor- and mitochondrial-mediated caspase-dependent pathways of cell death. Cell Death Differ. 9:926-933. [DOI] [PubMed] [Google Scholar]
- 36.Kominsky, D. J., R. J. Bickel, and K. L. Tyler. 2002. Reovirus-induced apoptosis requires mitochondrial release of Smac/DIABLO and involves reduction of cellular inhibitor of apoptosis protein levels. J. Virol. 76:11414-11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, P. W., E. C. Hayes, and W. K. Joklik. 1981. Protein σ1 is the reovirus cell attachment protein. Virology 108:156-163. [DOI] [PubMed] [Google Scholar]
- 38.Liemann, S., K. Chandran, T. S. Baker, M. L. Nibert, and S. C. Harrison. 2002. Structure of the reovirus membrane-penetration protein, μ1, in a complex with its protector protein, σ3. Cell 108:283-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu, Y., Y. Cai, and X. Zhang. 2003. Induction of caspase-dependent apoptosis in cultured rat oligodendrocytes by murine coronavirus is mediated during cell entry and does not require virus replication. J. Virol. 77:11952-11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu, Y., A. Nusrat, F. J. Schnell, T. A. Reaves, S. Walsh, M. Ponchet, and C. A. Parkos. 2000. Human junction adhesion molecule regulates tight junction resealing in epithelia. J. Cell Sci. 113:2363-2374. [DOI] [PubMed] [Google Scholar]
- 41.Lucia-Jandris, P., J. W. Hooper, and B. N. Fields. 1993. Reovirus M2 gene is associated with chromium release from mouse L cells. J. Virol. 67:5339-5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinac, B., H. Zhu, A. Kubalski, X. L. Zhou, M. Culbertson, H. Bussey, and C. Kung. 1990. Yeast K1 killer toxin forms ion channels in sensitive yeast spheroplasts and in artificial liposomes. Proc. Natl. Acad. Sci. USA 87:6228-6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maxfield, F. R. 1982. Weak bases and ionophores rapidly and reversibly raise the pH in endocytic vesicles in cultured mouse fibroblasts. J. Cell Biol. 95:676-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nibert, M. L., and B. N. Fields. 1992. A carboxy-terminal fragment of protein μ1/μ1C is present in infectious subvirion particles of mammalian reoviruses and is proposed to have a role in penetration. J. Virol. 66:6408-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nibert, M. L., A. L. Odegard, M. A. Agosto, K. Chandran, and L. A. Schiff. 2005. Putative autocleavage of reovirus mu1 protein in concert with outer-capsid disassembly and activation for membrane permeabilization. J. Mol. Biol. 345:461-474. [DOI] [PubMed] [Google Scholar]
- 46.Nibert, M. L., and L. A. Schiff. 2001. Reoviruses and their replication, p. 1679-1728. In D. M. Knipe and P. M. Howley (ed.), Fields Virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 47.Nibert, M. L., L. A. Schiff, and B. N. Fields. 1991. Mammalian reoviruses contain a myristoylated structural protein. J. Virol. 65:1960-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oberhaus, S. M., R. L. Smith, G. H. Clayton, T. S. Dermody, and K. L. Tyler. 1997. Reovirus infection and tissue injury in the mouse central nervous system are associated with apoptosis. J. Virol. 71:2100-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Odegard, A. L., K. Chandran, X. Zhang, J. S. Parker, T. S. Baker, and M. L. Nibert. 2004. Putative autocleavage of outer capsid protein μ1, allowing release of myristoylated peptide μ1N during particle uncoating, is critical for cell entry by reovirus. J. Virol. 78:8732-8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Donnell, S. M., M. W. Hansberger, J. L. Connolly, J. D. Chappell, M. J. Watson, J. M. Pierce, J. D. Wetzel, W. Han, E. S. Barton, J. C. Forrest, T. Valyi-Nagy, F. E. Yull, T. S. Blackwell, J. N. Rottman, B. Sherry, and T. S. Dermody. 2005. Organ-specific roles for transcription factor NF-κB in reovirus-induced apoptosis and disease. J. Clin. Investig. 115:2341-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paul, R. W., A. H. Choi, and P. W. K. Lee. 1989. The α-anomeric form of sialic acid is the minimal receptor determinant recognized by reovirus. Virology 172:382-385. [DOI] [PubMed] [Google Scholar]
- 52.Paul, R. W., and P. W. K. Lee. 1987. Glycophorin is the reovirus receptor on human erythrocytes. Virology 159:94-101. [DOI] [PubMed] [Google Scholar]
- 53.Prota, A. E., J. A. Campbell, P. Schelling, J. C. Forrest, T. R. Peters, M. J. Watson, M. Aurrand-Lions, B. Imhof, T. S. Dermody, and T. Stehle. 2003. Crystal structure of human junctional adhesion molecule 1: implications for reovirus binding. Proc. Natl. Acad. Sci. USA 100:5366-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramsey-Ewing, A., and B. Moss. 1998. Apoptosis induced by a postbinding step of vaccinia virus entry into Chinese hamster ovary cells. Virology 242:138-149. [DOI] [PubMed] [Google Scholar]
- 55.Rankin, U. T., Jr., S. B. Eppes, J. B. Antczak, and W. K. Joklik. 1989. Studies on the mechanism of the antiviral activity of ribavirin against reovirus. Virology 168:147-158. [DOI] [PubMed] [Google Scholar]
- 56.Reiter, J., E. Herker, F. Madeo, and M. J. Schmitt. 2005. Viral killer toxins induce caspase-mediated apoptosis in yeast. J. Cell Biol. 168:353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richardson-Burns, S. M., D. J. Kominsky, and K. L. Tyler. 2002. Reovirus-induced neuronal apoptosis is mediated by caspase 3 and is associated with the activation of death receptors. J. Neurovirol. 8:365-380. [DOI] [PubMed] [Google Scholar]
- 58.Roberg, K. 2001. Relocalization of cathepsin D and cytochrome c early in apoptosis revealed by immunoelectron microscopy. Lab. Investig. 81:149-158. [DOI] [PubMed] [Google Scholar]
- 59.Rodgers, S. E., E. S. Barton, S. M. Oberhaus, B. Pike, C. A. Gibson, K. L. Tyler, and T. S. Dermody. 1997. Reovirus-induced apoptosis of MDCK cells is not linked to viral yield and is blocked by Bcl-2. J. Virol. 71:2540-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaw, J. E., and D. C. Cox. 1973. Early inhibition of cellular DNA synthesis by high multiplicities of infectious and UV-irradiated reovirus. J. Virol. 12:704-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shepard, D. A., J. G. Ehnstrom, and L. A. Schiff. 1995. Association of reovirus outer capsid proteins σ3 and μ1 causes a conformational change that renders σ3 protease sensitive. J. Virol. 69:8180-8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shepard, D. A., J. G. Ehnstrom, P. J. Skinner, and L. A. Schiff. 1996. Mutations in the zinc-binding motif of the reovirus capsid protein σ3 eliminate its ability to associate with capsid protein μ1. J. Virol. 70:2065-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silverstein, S. C., C. Astell, D. H. Levin, M. Schonberg, and G. Acs. 1972. The mechanism of reovirus uncoating and gene activation in vivo. Virology 47:797-806. [DOI] [PubMed] [Google Scholar]
- 64.Smith, R. E., H. J. Zweerink, and W. K. Joklik. 1969. Polypeptide components of virions, top component and cores of reovirus type 3. Virology 39:791-810. [DOI] [PubMed] [Google Scholar]
- 65.Stoka, V., B. Turk, S. L. Schendel, T. H. Kim, T. Cirman, S. J. Snipas, L. M. Ellerby, D. Bredesen, H. Freeze, M. Abrahamson, D. Bromme, S. Krajewski, J. C. Reed, X. M. Yin, V. Turk, and G. S. Salvesen. 2001. Lysosomal protease pathways to apoptosis. Cleavage of bid, not pro-caspases, is the most likely route. J. Biol. Chem. 276:3149-3157. [DOI] [PubMed] [Google Scholar]
- 66.Sturzenbecker, L. J., M. L. Nibert, D. B. Furlong, and B. N. Fields. 1987. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J. Virol. 61:2351-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tillotson, L., and A. J. Shatkin. 1992. Reovirus polypeptide σ3 and N-terminal myristoylation of polypeptide μ1 are required for site-specific cleavage to μ1C in transfected cells. J. Virol. 66:2180-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tipper, D. J., and M. J. Schmitt. 1991. Yeast dsRNA viruses: replication and killer phenotypes. Mol. Microbiol. 5:2331-2338. [DOI] [PubMed] [Google Scholar]
- 69.Tosteson, M. T., M. L. Nibert, and B. N. Fields. 1993. Ion channels induced in lipid bilayers by subvirion particles of the nonenveloped mammalian reoviruses. Proc. Natl. Acad. Sci. USA 90:10549-10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tyler, K. L. 2001. Mammalian reoviruses, p. 1729-1745. In D. M. Knipe and P. M. Howley (ed.), Fields Virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 71.Tyler, K. L., M. K. Squier, S. E. Rodgers, S. E. Schneider, S. M. Oberhaus, T. A. Grdina, J. J. Cohen, and T. S. Dermody. 1995. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein σ1. J. Virol. 69:6972-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tyler, K. L., M. K. T. Squier, A. L. Brown, B. Pike, D. Willis, S. M. Oberhaus, T. S. Dermody, and J. J. Cohen. 1996. Linkage between reovirus-induced apoptosis and inhibition of cellular DNA synthesis: role of the S1 and M2 genes. J. Virol. 70:7984-7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Virgin, H. W., IV, M. A. Mann, B. N. Fields, and K. L. Tyler. 1991. Monoclonal antibodies to reovirus reveal structure/function relationships between capsid proteins and genetics of susceptibility to antibody action. J. Virol. 65:6772-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weiner, H. L., K. A. Ault, and B. N. Fields. 1980. Interaction of reovirus with cell surface receptors. I. Murine and human lymphocytes have a receptor for the hemagglutinin of reovirus type 3. J. Immunol. 124:2143-2148. [PubMed] [Google Scholar]
- 75.Weinrauch, Y., and A. Zychlinsky. 1999. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 53:155-187. [DOI] [PubMed] [Google Scholar]
- 76.Wetzel, J. D., J. D. Chappell, A. B. Fogo, and T. S. Dermody. 1997. Efficiency of viral entry determines the capacity of murine erythroleukemia cells to support persistent infections by mammalian reoviruses. J. Virol. 71:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wetzel, J. D., G. J. Wilson, G. S. Baer, L. R. Dunnigan, J. P. Wright, D. S. H. Tang, and T. S. Dermody. 1997. Reovirus variants selected during persistent infections of L cells contain mutations in the viral S1 and S4 genes and are altered in viral disassembly. J. Virol. 71:1362-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wickner, R. B. 1996. Double-stranded RNA viruses of Saccharomyces cerevisiae. Microbiol. Rev. 60:250-265. [DOI] [PMC free article] [PubMed] [Google Scholar]