Abstract
UL13 and Us3 are protein kinases encoded by herpes simplex virus 1. We report here that Us3 is a physiological substrate for UL13 in infected cells, based on the following observations. (i) The electrophoretic mobility, in denaturing gels, of Us3 isoforms from Vero cells infected with wild-type virus was slower than that of isoforms from cells infected with a UL13 deletion mutant virus (ΔUL13). After treatment with phosphatase, the electrophoretic mobility of the Us3 isoforms from cells infected with wild-type virus changed, with one isoform migrating as fast as one of the Us3 isoforms from ΔUL13-infected cells. (ii) A recombinant protein containing a domain of Us3 was phosphorylated by UL13 in vitro. (iii) The phenotype of ΔUL13 resembles that of a recombinant virus lacking the Us3 gene (ΔUs3) with respect to localization of the viral envelopment factors UL34 and UL31, whose localization has been shown to be regulated by Us3. UL34 and UL31 are localized in a smooth pattern throughout the nuclei of cells infected with wild-type virus, whereas their localization in ΔUL13- and ΔUs3-infected cells appeared as nuclear punctate patterns. These results indicate that UL13 phosphorylates Us3 in infected cells and regulates UL34 and UL31 localization, either by phosphorylating Us3 or by a Us3-independent mechanism.
Herpes simplex virus 1 (HSV-1) encodes at least three protein kinases, UL13, Us3, and UL39 (63). This report presents studies of the interaction between UL13 and Us3. The background for these studies is as follows.
First, UL13 is a serine/threonine protein kinase that is packaged in the tegument, a virion structural component located between the nucleocapsid and the envelope (9, 12, 13, 29, 52, 68). UL13 plays a role in viral replication in cell cultures, since UL13 deletion mutants exhibit impaired replication in some cell lines, including rabbit skin cells and baby hamster kidney (BHK) cells (10, 45, 56, 57, 70). Although the mechanism by which UL13 acts in HSV-1-infected cells remains unclear, infection of rabbit skin cells and BHK cells with UL13 deletion mutants reduces the expression levels of the α protein ICP0 and a subset of γ proteins, including UL26, UL26.5, UL38, UL41, and Us11 (56), suggesting that UL13 is involved in viral-gene expression in infected cells. UL13 would also be expected to function in early postinfection events, since tegument proteins are, in general, released into the cytoplasm of newly infected cells. In agreement with this possibility, phosphorylation of a tegument protein by UL13 has been implicated in promoting tegument disassembly in vitro (40).
Second, UL13 may function by phosphorylating specific viral and cellular proteins. Thus far, gI/gE, ICP0, ICP22, Us1.5, UL47, UL49, p60, elongation factor 1δ (EF-1δ), casein kinase IIβ subunit, and RNA polymerase II have been reported to be putative substrates for UL13 (4, 10, 20, 29, 32, 37, 44, 51, 57, 63). However, the biological significance of UL13-mediated phosphorylation in infected cells remains unclear. Since the UL13 amino acid sequence is conserved in all Herpesviridae subfamilies (9, 68), UL13 homologues may play a conserved role in herpesvirus replication by phosphorylating common host cellular targets and conserved herpesvirus gene products. The only substrate identified to date that is targeted by UL13 homologues from all Herpesviridae subfamilies is the cellular translation factor EF-1δ (26, 29, 30, 32). An interesting feature of the interaction between UL13 homologues and EF-1δ is that both cellular protein kinase cdc2 and UL13 homologues phosphorylate the same EF-1δ amino acid residue (29). These observations suggest that UL13 homologues may share a function that mimics the cellular cdc2 protein kinase (28). This hypothesis is supported by data showing that HSV-1 UL13 phosphorylates the cdc2 site of the casein kinase IIβ subunit in vitro (29). Moreover, reports that the Epstein-Barr virus (EBV) UL13 homologue BGLF4 and cdc2 phosphorylate the same sites of EBV regulatory proteins— EBNA-LP and EBNA-2, which are critical for the transcriptional activities of the proteins—both in vitro and in vivo are consistent with this hypothesis (27, 75, 76).
Third, Us3 is also a serine/threonine protein kinase and is packaged in the virion (18, 55, 62). In contrast to UL13, the Us3 amino acid sequence is conserved only in the subfamily Alphaherpesvirinae (9, 39, 63, 68), and the function of Us3 as a virion component has not been elucidated yet. Us3 is a positive regulator of viral replication, based on studies showing that recombinant Us3 mutant viruses have impaired growth properties in cell cultures and mouse models (49, 62, 65). Increasing data indicate that Us3 plays a role in viral replication by regulating apoptosis. It has been reported that Us3 protein kinase can prevent apoptosis induced by proapoptotic cellular proteins, osmotic shock, and replication-incompetent mutant virus (2, 6, 7, 23, 36, 41-43, 50). Benetti and Roizman have recently shown that Us3 activates protein kinase A (PKA), a cellular cyclic-AMP-dependent protein kinase with phosphorylation target sequences resembling those of Us3, and that both Us3 and PKA phosphorylate the same target protein residues (3). Us3 may express its antiapoptotic activity through phosphorylation of PKA substrates, by activating PKA, and/or by mimicking this cellular protein kinase.
Fourth, Us3 is involved in the nuclear egress of progeny nucleocapsids based on several observations. (i) In cells infected with mutant virus lacking functional Us3, virions were found to accumulate in the perinuclear space in large invaginations of the inner nuclear membrane (62). Similar structures were reported in cells infected with a recombinant pseudorabies virus, a member of Alphaherpesvirinae, lacking a Us3-homologous gene (34, 71). (ii) Us3 phosphorylates UL34 and UL31 (25, 58, 65), both of which are critical regulators for primary envelopment of nucleocapsids at the nuclear membrane (61, 64, 74). (iii) Us3 protein kinase activity is required for proper localization of UL34 and UL31 at the nuclear membrane (61, 62, 65). Us3 may function in the nuclear egress pathway by direct or indirect interactions with UL34 and UL31.
We report here studies showing that UL13 phosphorylates Us3 in infected cells and examine some possible effects of this phorphorylation.
MATERIALS AND METHODS
Cells and viruses.
Vero and Spodoptera frugiperda Sf9 cells were described previously (29, 69). A human neuroblastoma cell line (SK-N-SH cells) was kindly provided by B. Roizman (University of Chicago, Chicago, Ill.) and maintained in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal calf serum (FCS). The HSV-1 wild-type strain HSV-1(F) and UL13 deletion mutant virus R7356 were described previously (17, 29, 56, 69). The Us3 deletion mutant virus R7041 (55) was kindly provided by B. Roizman. The recombinant baculoviruses Bac-GST-UL13 and Bac-GST-UL13K176M were described previously (29).
Plasmids.
pBluescript II KS(+) (Stratagene) was digested with HindIII, treated with T4 DNA polymerase, and religated to produce pBluescript II KS(+) without the HindIII site. The resultant plasmid was designated pBSΔHdIII. BglII O in pBSΔHdIII was generated by cloning the 5.4-kbp BglII O fragment of pBC1015 (32) into the BamHI site of pBSΔHdIII. pMAL-Us3-P1 and pMAL-Us3-P2 were constructed by amplifying the domains encoding Us3 codons 405 to 481 and codons 254 to 411, respectively, by PCR from pBC1013 (33) and cloning the DNA fragments into pMAL-c (New England BioLabs) in frame with maltose binding protein (MBP). pBC1013 and pBC1015 were kindly provided by B. Roizman.
Generation of a recombinant virus.
To construct the recombinant virus R7356Rep with a repaired UL13 gene, the UL13 sequences deleted from R7356 were restored by cotransfecting rabbit skin cells with R7356 DNA and BglII O in pBSΔHdIII. Plaques were isolated, purified, and screened by PCR analysis for wild-type UL13 sequences. Restoration of the original sequence was confirmed by Southern blotting.
Purification of GST fusion proteins from baculovirus-infected cells.
The glutathione S-transferase (GST)-UL13 and GST-UL13K176M proteins were purified from Sf9 cells infected with Bac-GST-UL13 and Bac-GST-UL13K176M, respectively, as described previously (29).
Production and purification of MBP fusion proteins.
MBP fusion proteins (MBP-Us3-P1, MBP-Us3-P2, and MBP-LacZ) were expressed in Escherichia coli that had been transformed with pMAL-Us3-P1, pMAL-Us3-P2, and pMAL-c, respectively, and purified as described previously (29).
Antibodies.
Rabbit polyclonal antibodies to Us3, UL34, and UL31 were described previously (14, 21, 25, 66, 78). Chicken polyclonal antibody to UL34 (61) was kindly provided by R. Roller (University of Iowa). Mouse monoclonal antibody to nucleoporin p62 was purchased from Transduction Laboratories.
In vitro kinase assays.
MBP fusion proteins were captured on amylose beads (New England BioLabs) and used as substrates in in vitro kinase assays with 2 μg of purified GST-UL13 and GST-UL13K176M, as described previously (29). The relative amounts of radioactivity in substrates phosphorylated by GST-UL13 were quantified with the aid of Dolphin Doc and the software Dolphin-1D (Wealtec).
Immune complex kinase assays.
Vero cells were infected with either HSV-1(F), R7041, or R7356 at a multiplicity of infection (MOI) of 5 PFU per cell. Infected cells were harvested at 12 h postinfection and lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Nonidet P-40 [NP-40], 0.5% deoxycholate, 0.1% sodium dodecyl sulfate) containing a protease inhibitor cocktail (Sigma). Supernatant fluids obtained after centrifugation of the cell lysate were precleared by incubation with protein A-Sepharose beads (Amersham-Pharmacia) at 4°C for 30 min and then reacted with rabbit polyclonal antibody to Us3 at 4°C for 2 h. Additional protein A-Sepharose beads were added, and the reaction continued for another 1.5 h. Immunoprecipitates were collected by a brief centrifugation; washed twice with high-salt buffer (1 M NaCl, 10 mM Tris-HCl [pH 8.0], 0.2% NP-40), once with low-salt buffer (0.1 M NaCl, 10 mM Tris-HCl [pH 8.0], 0.2% NP-40), six times with RIPA buffer, and twice with Us3 kinase buffer (50 mM Tris-HCl [pH 9.0], 20 mM MgCl2, 0.1% NP-40, and 1 mM dithiothreitol) (25); and analyzed by in vitro kinase assays. For these assays, Us3 kinase buffer containing 10 μM ATP and 10 μCi [γ-32P]ATP was added to the protein A-Sepharose beads (15 μl) containing immunoprecipitated Us3 protein kinase, and the samples were reacted at 30°C for 30 min. After incubation, the samples were washed twice with TNE buffer (20 mM Tris-HCl [pH 8.0], 100 mM NaCl, and 1 mM EDTA) and analyzed by electrophoresis in denaturing gels with or without phosphatase treatment. After electrophoresis, the separated proteins were transferred from the gels to nitrocellulose membranes (Bio-Rad), and the membranes were exposed to X-ray film and then immunoblotted with anti-Us3 antibody.
Phosphatase treatment.
After the in vitro kinase assays, the MBP fusion proteins or immunoprecipitates were washed twice with TNE buffer and twice with lambda protein phosphatase (λ-PPase) reaction buffer supplemented with 2 mM MnCl2 (New England BioLabs). Then, λ-PPase reaction buffer containing 2,000 U λ-PPase (New England BioLabs) was added to the beads, and the samples were incubated at 37°C for 30 min. For MBP fusion proteins, after electrophoresis in denaturing gels, the gel was stained with Coomassie brilliant blue (CBB) and exposed to X-ray film. For immunoprecipitates, the samples were electrophoretically separated and transferred to nitrocellulose membranes, and the membranes were exposed to X-ray film and then immunoblotted with anti-Us3 antibody. In other studies, infected cells were lysed in NP-40 buffer (10 mM Tris-HCl [pH 7.8], 0.15 M NaCl, 1 mM EDTA, and 1% NP-40) containing a protease inhibitor cocktail. The supernatants obtained after centrifugation of the cell lysates were incubated with 20 U alkaline phosphatase (CIP; New England BioLabs) for 2 h at 37°C, after which they were electrophoretically separated and then immunoblotted with anti-Us3 antibody.
Southern blotting, immunoblotting, and immunofluorescence.
Southern blotting and immunoblotting were performed as described previously (24, 69). Indirect immunofluorescence assays were performed as described previously (31), except that anti-mouse or anti-rabbit immunoglobulin G (IgG) conjugated to Alexa Fluor 488, anti-rabbit IgG conjugated to Alexa Fluor 546, or anti-chicken IgG conjugated to fluorescein isothiocyanate (FITC) was used as a secondary antibody, in addition to anti-rabbit IgG conjugated to FITC, and samples were examined with a Zeiss LSM510 or LSM5 laser scanning microscope.
Induction of apoptosis and measurement of caspase 3/7 activity.
SK-N-SH cells were mock infected or infected with HSV-1(F), R7041, or R7356 at an MOI of 5. After a 1-h virus adsorption, the virus inoculum was replaced with DMEM containing 10% FCS. At 12 h postinfection, the cell culture medium was removed and the cells were exposed to 1 M sorbitol in DMEM containing 1% FCS for 1 h to produce osmotic shock and induce apoptosis. After sorbitol treatment, the cells were washed with DMEM and incubated in DMEM containing 1% FCS for an additional 5 h. The cells were then harvested and assayed for caspase 3/7 activity using a Caspase-Glo 3/7 assay kit with a tetrapeptide (Z-DEVD)-conjugated aminoluciferin substrate, according to the manufacturer's instructions (Promega).
RESULTS
UL13 mediates phosphorylation of Us3 in infected cells.
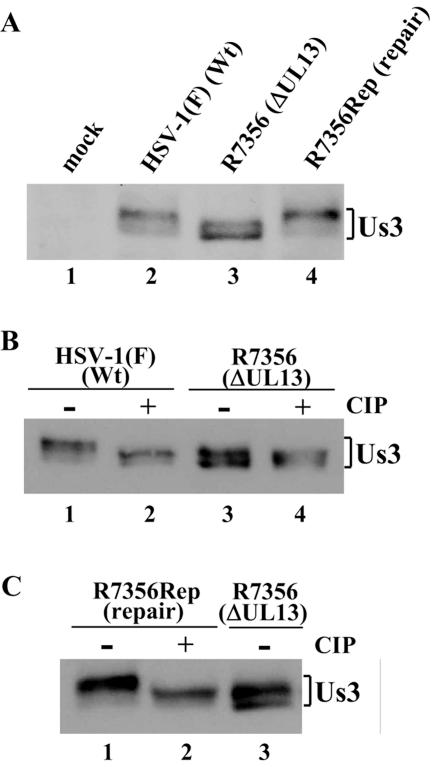
For these experiments, Vero cells were mock infected or infected with wild-type virus [HSV-1(F)], UL13 deletion mutant virus (R7356), or UL13-repaired R7356 virus (R7356Rep) at an MOI of 5; harvested at 12 h postinfection; solubilized; and analyzed by immunoblotting with polyclonal antibody to Us3. As reported previously (41), Us3 protein from HSV-1(F)-infected Vero cells was detected in a denaturing gel as doublet bands, with the more slowly migrating isoform predominating (Fig. 1A, lane 2). In R7356-infected Vero cells, the Us3 protein was also detected as doublet bands, but the amounts of Us3 in the two isoforms were more equal than in HSV-1(F)-infected cells, and both isoforms from R7356-infected cells appeared to migrate faster than those from HSV-1(F)-infected cells (Fig. 1A, lanes 2 and 3).
FIG. 1.
(A) Immunoblot of electrophoretically separated lysates from Vero cells mock infected (lane 1) or infected with HSV-1(F) (lane 2), R7356 (lane 3), or R7356Rep (lane 4). Infected cells were harvested at 12 h postinfection and analyzed by immunoblotting with polyclonal antibody to Us3. Wt, wild type. (B) Immunoblots of electrophoretically separated lysates from Vero cells infected with HSV-1(F) (lanes 1 and 2) and R7356 (lanes 3 and 4). The infected cells were harvested at 12 h postinfection, solubilized, mock treated (lanes 1 and 3) or treated with CIP (lanes 2 and 4), and immunoblotted with antibody to Us3. (C) Immunoblots of electrophoretically separated lysates from Vero cells infected with R7356Rep (lanes 1 and 2) and R7356 (lane 3). The infected cells were harvested at 12 h postinfection, solubilized, mock treated (lanes 1 and 3) or treated with CIP (lane 2), and immunoblotted with antibody to Us3.
To verify that the phenotype observed in these studies was due to the UL13 deletion, this deletion in the R7356 mutant virus was repaired to yield the UL13 repaired virus R7356Rep, as described in Materials and Methods. The genotype of R7356Rep was confirmed by Southern blotting when restricted with BglII and probed with the BglII O DNA fragment (data not shown). The electrophoretic pattern of Us3 isoforms from R7356Rep-infected cells could not be differentiated from that of Us3 isoforms from cells infected with wild-type virus (Fig. 1A, lanes 2 and 4). These results indicate that UL13 mediates posttranslational processing of Us3 in HSV-infected cells.
To examine whether the UL13-mediated posttranslational processing of Us3 is due to phosphorylation, the infected-cell lysates were phosphatase treated with CIP, solubilized, and analyzed by immunoblotting with polyclonal antibody to Us3. After CIP treatment, the electrophoretic mobilities of both Us3 isoforms from Vero cells infected with wild-type HSV-1(F) or UL13 repaired R7356Rep virus changed, with one of the Us3 isoforms migrating as fast as one from R7356-infected cells (Fig. 1B, lanes 1 to 3, and Fig. 1C, lanes 1 to 3). CIP treatment of lysate from cells infected with R7356 had little effect on the migration of the Us3 isoforms (Fig. 1B, lanes 3 and 4). These results indicate that UL13 mediates phosphorylation of Us3 in infected cells. Consistent with our observations, Poon and Roizman (54) recently reported that Us3 proteins produced by cells infected with the UL13 deletion mutant virus R7356 migrated in a denaturing gel faster than those produced by cells infected with wild-type virus. However, the study did not address whether the wild-type phenotype was restored in cells infected with a recombinant virus in which the UL13 sequence was repaired and whether the posttranslational modification of Us3 mediated by UL13 was due to phosphorylation (54).
UL13 phosphorylates Us3 in vitro.
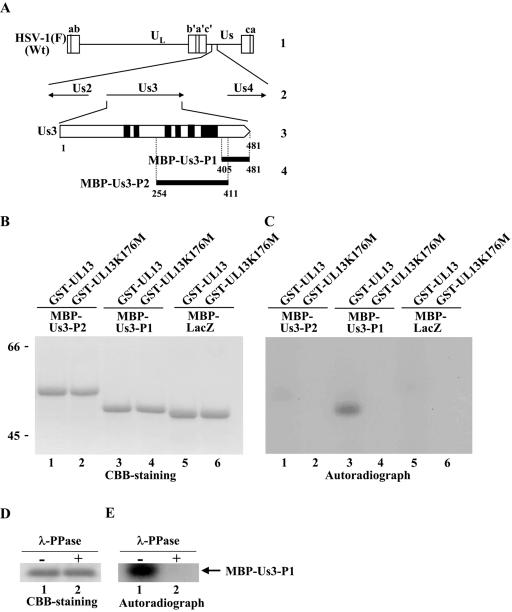
To investigate whether UL13 directly phosphorylates Us3, we generated and purified chimeric proteins consisting of MBP fused to peptides encoded by Us3 codons 254 to 411 (MBP-Us3-P2) and codons 405 to 481 (MBP-Us3-P1) (Fig. 2A). We also used MBP-LacZ protein (25) as a control. MBP-Us3-P1, MBP-Us3-P2, and MBP-LacZ contain 45, 56, and 47 serines/threonines, respectively. The MBP fusion proteins were captured on amylose beads and used as substrates in in vitro kinase assays with purified wild-type GST-UL13 or the kinase-negative mutant GST-UL13K176M. As shown in Fig. 2C, MBP-Us3-P1 was labeled with [γ-32P]ATP in kinase assays using GST-UL13 (Fig. 2C, lane 3), while the MBP-Us3-P2 and MBP-LacZ proteins were not (Fig. 2C, lanes 1 and 5). When the gel was overexposed, the MBP-LacZ was labeled very faintly in the presence of GST-UL13 (data not shown). However, the relative amount of radioactivity in MBP-Us3-P1 was >100 higher than that in MBP-LacZ (data not shown). When the kinase-negative mutant GST-UL13K176M was used, none of the MBP fusion proteins were labeled (Fig. 2C, lanes 2, 4, and 6). To confirm that MBP-Us3-P1 labeling by GST-UL13 was due to phosphorylation, the labeled MBP-Us3-P1 was treated with λ-PPase. As shown in Fig. 2E, MBP-Us3-P1 labeling by GST-UL13 was eliminated by phosphatase treatment, indicating that MBP-Us3-P1 was labeled by phosphorylation. The presence of each MBP fusion protein and the radiolabeled MBP-Us3-P1 band was verified by CBB staining (Fig. 2B and D).
FIG. 2.
(A) Schematic diagram of the genome structures of wild-type (Wt) virus HSV-1(F) and the location of the Us3 gene. Line 1, linear representation of the HSV-1(F) genome. The unique sequences are represented as unique long (UL) and short (Us) domains, and the terminal repeats flanking them are shown as open rectangles with the designation above each repeat. Line 2, structure of the genome domain containing the Us2, Us3, and Us4 open reading frames. Line 3, structure of the Us3 open reading frame. The shaded areas represent subdomains I to VI, which are conserved in eukaryotic protein kinases (68). Line 4, the domains of the Us3 gene used in these studies to generate MBP-Us3 fusion proteins. (B) CBB-stained images of phosphorylated Us3. Purified MBP-Us3-P2 (lanes 1 and 2), MBP-Us3-P1 (lanes 3 and 4), and MBP-LacZ (lanes 5 and 6) incubated in kinase buffer containing [γ-32P]ATP and purified GST-UL13 (lanes 1, 3, and 5) or GST-UL13K176M (lanes 2, 4, and 6), separated on a denaturing gel, and stained with CBB. Molecular masses (kDa) are shown on the left. (C) Autoradiograph of the gel in panel B. (D) Purified MBP-Us3-P1 incubated in kinase buffer containing [γ-32P]ATP and purified GST-UL13 and then either mock treated (lane 1) or treated with λ-PPase (lane 2), separated on a denaturing gel, and stained with CBB. (E) Autoradiograph of the gel in panel D.
These results indicate that UL13 specifically and directly phosphorylates the Us3 peptide encoded by codons 405 to 481 in vitro.
UL13-mediated phosphorylation of Us3 does not affect Us3 protein kinase activity in infected cells.
Phosphorylation of a protein often leads to a change in function(s) of the target protein. The result, described above, showing that Us3 is phosphorylated by UL13 suggested three possible effects of this modification: (i) UL13 may affect the intrinsic protein kinase activity of Us3, (ii) UL13 may affect the ability of Us3 to regulate apoptosis, and (iii) UL13 may affect the ability of Us3 to determine the localization of UL31 and UL34.
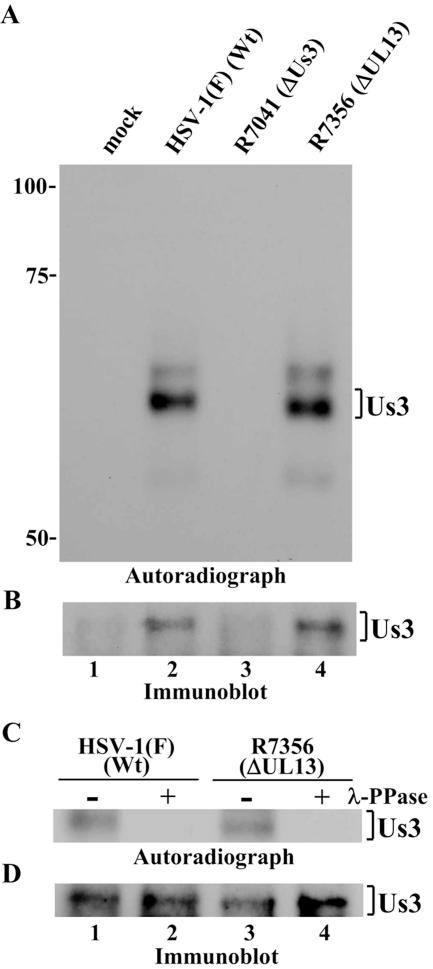
To test the first possibility, two series of experiments were done. In the first, Vero cells infected with HSV-1(F), R7041 (ΔUs3), or R7356 (ΔUL13) were harvested at 12 h postinfection, solubilized, and immunoprecipitated with antibody to Us3. The immunoprecipitates were then used in kinase assays. To reduce the possibility that the anti-Us3 antibody might bring down contaminating kinase(s), the immunoprecipitates containing Us3 protein kinase were washed with high-salt buffer containing 1 M NaCl prior to in vitro kinase assays. As shown in Fig. 3A, Us3 protein in immunoprecipitates from HSV-1(F)- and R7356 (ΔUL13)-infected cells were labeled with [γ-32P]ATP at similar levels, but no labeled protein bands at the apparent Mr corresponding to Us3 were detected in immunoprecipitates from R7041 (ΔUs3)-infected cells. The labeling of Us3 proteins was due to phosphorylation, as determined by studies showing that the labeling was eliminated by phosphatase treatment (Fig. 3C). The expression of each Us3 protein and identification of the Us3 radiolabeled band were verified by immunoblotting (Fig. 3B and D). These results indicate that Us3 proteins in lysates from cells infected with HSV-1(F) and R7356 (ΔUL13) have similar autophosphorylation activities.
FIG. 3.
Autoradiographic images of Us3 immunoprecipitates subjected to in vitro kinase assay. (A) Vero cells were mock infected (lane 1) or infected with HSV-1(F) (lane 2), R7041 (lane 3), or R7356 (lane 4); harvested at 12 h postinfection; and immunoprecipitated with antibody to Us3. The immunoprecipitates were incubated in kinase buffer containing [γ-32P]ATP, separated on a denaturing gel, transferred to a nitrocellulose membrane, and analyzed by autoradiography. (B) Immunoblot of the nitrocellulose membrane in panel A using anti-Us3 antibody. (C) Immunoprecipitates prepared as in panel A were either mock treated (lanes 1 and 3) or treated with λ-PPase (lanes 2 and 4), separated on a denaturing gel, transferred to a nitrocellulose membrane, and analzyed by autoradiography. Wt, wild type. (D) Immunoblot of the nitrocellulose membrane in panel C using anti-Us3 antibody.
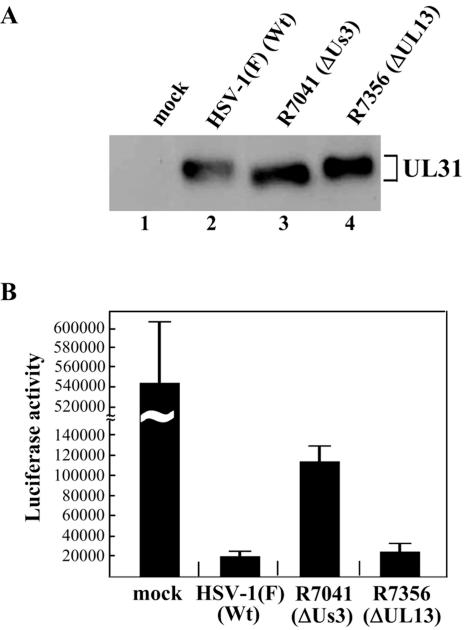
We recently reported that Us3 directly phosphorylates UL31 in vitro and mediates posttranslational processing of UL31, which involves phosphorylation, in infected cells (25). To examine whether UL13 affects the posttranslational modification of UL31 in infected cells, in a second series of experiments, Vero cells mock infected or infected with HSV-1(F), R7041 (ΔUs3), or R7356 (ΔUL13) were harvested at 12 h postinfection, solubilized, electrophoretically separated in a denaturing gel, and subjected to immunoblotting with antibody to UL31. These data showed that UL31 protein produced in cells infected with R7041 (ΔUs3) migrated faster than that produced in cells infected with HSV-1(F) (Fig. 4A, lanes 2 and 3). In contrast, UL31 from cells infected with R7356 (ΔUL13) migrated as slowly as UL31 from cells infected with HSV-(F) (Fig. 4A, lanes 2 and 4). These results suggest that Us3 proteins expressed in cells infected with wild-type virus and UL13 deletion mutant virus induce similar posttranslational modifications of UL31.
FIG. 4.
(A) Immunoblot of electrophoretically separated lysates from Vero cells mock infected (lane 1) or infected with HSV-1(F) (lane 2), R7041 (lane 3), or R7356 (lane 4) at an MOI of 5. Infected cells were harvested at 12 h postinfection and immunoblotted with anti-UL31 antibody. (B) Caspase 3/7 activity of infected SK-N-SH cells after induction of apoptosis by osmotic shock. SK-N-SH cells were mock infected or infected with HSV-1(F), R7041, or R7356. At 12 h postinfection, the cells were exposed to sorbitol for 1 h, incubated for an additional 5 h, harvested, and assayed for caspase 3/7 activity using a Z-DEVD-aminoluciferin substrate. The values are the means and standard deviations for three independent experiments. Wt, wild type.
Taken together, these experiments suggest that UL13-mediated phosphorylation of Us3 is not required for optimal Us3 protein kinase activity in infected cells. However, we cannot completely exclude the possibility that UL13 affects the Us3 protein kinase activity in vivo if cofactors are necessary for optimal Us3 protein kinase activity and/or that UL13 modulates the Us3 protein kinase activity against other Us3 substrates, except UL31, in vivo.
Level of caspase 3/7 activity in virus-infected SK-N-SH cells in which apoptosis was induced.
To investigate whether UL13-mediated phosphorylation of Us3 affects Us3 regulation of apoptosis in infected cells, SK-N-SH cells were infected with HSV-1(F), R7041 (ΔUs3), or R7356 (ΔUL13), and at 12 h postinfection, apoptosis was induced by osmotic shock. The cells were then harvested and assayed for caspase 3/7 activity. As shown in Fig. 4B, caspase 3/7 activity induced by osmotic shock was significantly reduced (26.3-fold) in HSV-1(F)-infected cells (Fig. 4B). In R7041 (ΔUs3)-infected cells, there was less reduction of caspase 3/7 activity (4.7-fold), probably due to the lack of Us3 antiapoptotic activity. Similar results were reported previously (7). In R7356 (ΔUL13)-infected cells, caspase 3/7 activity was similar to that in HSV-1(F)-infected cells. The activity of Us3 to regulate apoptosis was not detected in SK-N-SH cells without induction of apoptosis, based on the observation that the level of the caspase 3/7 activity in SK-N-SH cells infected with R7041 (ΔUs3), without osmotic shock, was comparable to that in cells infected with wild-type virus (data not shown). These results suggest that the presence of UL13 does not affect caspase 3/7 activity in infected SK-N-SH cells.
UL13 is required for proper localization of UL34 and UL31 in infected cells.
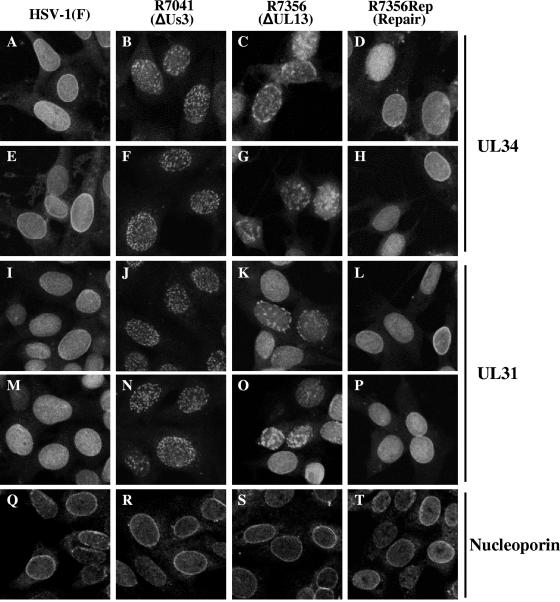
To investigate whether UL13-mediated phosphorylation of Us3 affects the role of Us3 in UL34 and UL31 localization, Vero cells were mock infected or infected with HSV-1(F), R7041 (ΔUs3), R7356 (ΔUL13), or R7356Rep (repair) at an MOI of 5; fixed at 12 or 15 h postinfection; and processed for indirect immunofluorescence assay with antibodies to UL34, UL31, and nucleoporin p62.
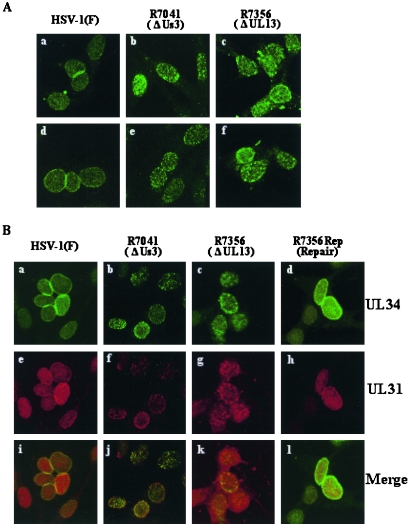
Previous studies reported that in HSV-1(F)-infected Vero and HEp-2 cells at 8 and 12 h postinfection, the UL34 and UL31 proteins colocalize at the nuclear envelope in a uniform distribution (61, 65). However, as shown in Fig. 5, the UL34 and UL31 distributions observed in the studies reported here differed from those results (61, 65), with both UL34 and UL31 showing nucleoplasmic localization in addition to nuclear-membrane localization (Fig. 5A, E, I, and M). The antibodies to UL34 and UL31 used in these studies were not able to detect any specific fluorescence in mock-infected cells (data not shown). In R7041 (ΔUs3)-infected cells, in agreement with previous reports (61, 65), UL34 and UL31 proteins were detected as punctate structures at the nuclear membrane (Fig. 5B, F, J, and N). However, although the previous studies found only UL34 and UL31 localized at the nuclear membrane in R7041 (ΔUs3)-infected cells, in the studies reported here, UL34 and UL31 were also detected as punctate structures in the nucleoplasm of R7041 (ΔUs3)-infected cells (Fig. 5B, F, J, and N). The nucleoplasmic staining of UL34 and UL31 in HSV-1(F)- and R7041 (ΔUs3)-infected cells did not appear to be specific to rabbit polyclonal antibodies generated in our laboratory. Thus, in HSV-1(F)- or R7041 (ΔUs3)-infected cells, the patterns of UL34 fluorescence detected by chicken polyclonal antibody to UL34, which was used in previously published studies (61, 62, 65), were almost identical to those of UL34 fluorescence detected by rabbit polyclonal antibody to UL34 generated in our laboratory (Fig. 6A, a, b, d, and e). As reported earlier (61), UL34 detected by chicken polyclonal antibody was clearly colocalized with UL31 detected by rabbit polyclonal antibody to UL31 in discrete punctate structures of R7041 (ΔUs3)-infected cells (Fig. 6B, b, f, and j). However, in the studies reported here, the punctate regions containing both UL34 and UL31 in R7041 (ΔUs3)-infected cells were detected not only at the nuclear membrane, but also in the nucleoplasm (Fig. 6B, b, f, and j).
FIG. 5.
Digital confocal microscope images showing localization of UL34, UL31, and nucleoporin p62 proteins in Vero cells infected with HSV-1(F) (A, E, I, M, and Q), R7041 (B, F, J, N, and R), R7356 (C, G, K, O, and S), and R7356Rep (D, H, L, P, and T). At 12 h postinfection, infected cells were fixed, permeabilized, and immunostained with rabbit polyclonal antibody to UL34 (A to H) detected with FITC-conjugated anti-rabbit IgG antibody, rabbit polyclonal antibody to UL31 (I to P) detected with Alexa Fluor 488-conjugated anti-rabbit IgG antibody, or mouse monoclonal antibody to nucleoporin p62 (Q to T) detected with Alexa Fluor 488-conjugated anti-mouse IgG antibody.
FIG. 6.
(A) Digital confocal microscope images showing localization of UL34 in Vero cells infected with HSV-1(F) (a and d), R7041 (b and e), and R7356 (c and f). At 15 h postinfection, infected cells were fixed, permeabilized, and immunostained with rabbit polyclonal antibody to UL34 (a to c) detected with FITC-conjugated anti-rabbit IgG antibody or chicken polyclonal antibody to UL34 (d to f) detected with FITC-conjugated anti-chicken IgG antibody. (B) Digital confocal microscope images showing localization of UL34 and UL31 in Vero cells infected with HSV-1(F) (a, e, and i), R7041 (b, f, and j), R7356 (c, g, and k), and R7356Rep (d, h, and l). At 15 h postinfection, infected cells were fixed, permeabilized, and double labeled with a combination of chicken polyclonal antibody to UL34 (a to d) and rabbit polyclonal antibody to UL31 (e to h) and then detected with FITC-conjugated anti-chicken IgG antibody (green fluorescence) and Alexa-546-conjugated anti-rabbit IgG antibody (red fluorescence). Single-color images were captured separately and are shown in the upper (UL34) (a to d) and middle (UL31) (e to h) panels; the lower panels (i to l) represent simultaneous acquisitions of both colors. The yellow colors visualized in the merged images represent colocalization of UL34 and UL31.
In R7356 (ΔUL13)-infected cells, the UL34 protein was detected as punctate structures in the nucleus by rabbit polyclonal antibody to UL34 (Fig. 5C and G and 6A, c), as well as chicken polyclonal antibody to UL34 (Fig. 6A, f, and 6B, c). Similarly, R7356 (ΔUL13)-infected cells showed UL31 localization as nuclear punctate staining (Fig. 5K and O and 6B, g). Furthermore, UL34 and UL31 colocalized in the nuclear punctate structures of R7356 (ΔUL13)-infected cells (Fig. 6B, c, g, and k). These localization features of UL34 and UL31 in R7356 (ΔUL13)-infected cells seemed to be similar to those of the viral proteins in R7041 (ΔUs3)-infected cells (61). It should be noted, however, that the sizes of UL34 and UL31 stained speckles in R7356 (ΔUL13)-infected cells appeared to be larger than those in R7041 (ΔUs3)-infected cells, and the number of speckles in R7356 (ΔUL13)-infected cells was less than in R7041 (ΔUs3)-infected cells (Fig. 5B, C, F, G, J, K, N, and O and 6A, b, c, e, and f, and B, b, c, f, g, j, and k). Furthermore, it appeared that the effect of UL13 deletion on localization of UL31 was less than that on UL34 at 12 h postinfection. Thus, in most (approximately 80%) of the R7356 (ΔUL13)-infected cells, the UL34 protein appeared as punctate structures in the nucleus, but in the remainder (approximately 20%), UL34 staining was similar to that in HSV-1(F)-infected cells (Fig. 5C and G). In contrast, most (approximately 80%) R7356 (ΔUL13)-infected cells showed UL31 localization similar to that in HSV-1(F)-infected cells, and the remainder (approximately 20%) showed UL31 localization as nuclear punctate staining (Fig. 5K and O). At later times of infection (15 h postinfection), however, the UL31 protein appeared as punctate structures in the nuclei of most R7356 (ΔUL13)-infected cells, as observed with the UL34 protein (Fig. 6B, c to g).
As expected, in R7356Rep-infected cells with the UL13 deletion repaired, UL34 and UL31 localization was similar to that in HSV-1(F)-infected cells, confirming that the change in localization of UL34 and UL31 proteins in R7356 (ΔUL13)-infected cells was a result of the deletion of the UL13 open reading frame (Fig. 5D, H, L, and P and 6B, d, h, and l). Nucleoporin p62, a marker for the nuclear envelope, was evenly distributed in HSV-1(F)-, R7041 (ΔUs3)-, R7356 (ΔUL13)-, and R7356Rep-infected cells (Fig. 5Q, R, S, and T). These results indicate that UL13 plays a role in the proper localization of UL34 and UL31 in HSV-1-infected cells.
DISCUSSION
Cellular protein kinases are often regulated by phosphorylation cascades organized by other protein kinases (15, 77). The question we have investigated in the studies reported here is whether one HSV-encoded protein kinase can target another HSV-encoded protein kinase and what effect this might have on infected cells. The conclusions of these studies are as follows.
First, UL13 phosphorylates Us3 in vitro and in infected cells. Identification of the physiological substrate of a viral protein kinase requires demonstration that the substrate is specifically and directly phosphorylated by the kinase in vitro and that phosphorylation of the substrate in cells infected with a mutant virus lacking the protein kinase activity is altered. Although about 10 potential substrates of UL13 have been reported, only 3 (including gI/gE, ICP0, and EF-1δ) appear to fulfill the requirements to be natural UL13 substrates (4, 10, 20, 29, 32, 37, 44, 51, 57, 63). In the studies presented here, we have shown that a purified Us3 preparation was phosphorylated in vitro in the presence of purified recombinant UL13. The phosphorylation of Us3 was shown to be a direct effect of UL13 protein kinase activity and not of a contaminating kinase(s), because a kinase-negative mutant (GST-UL13K176M) was unable to phosphorylate Us3 in vitro. Furthermore, we found that Us3 phosphorylation was altered in cells infected with the UL13 deletion mutant virus. Thus, Us3 also fulfills the requirements to be a natural substrate of UL13 in infected cells.
Second, UL13 plays a role in the proper localization of UL34 and UL31 in infected cells. Previous studies have demonstrated that Us3 regulates the normal localization of the HSV-1 envelopment factors UL34 and UL31, showing that these viral proteins are localized abnormally in punctate structures at the nuclear membrane in cells infected with recombinant viruses lacking a functional Us3 protein (61, 62, 65). In the studies reported here, we have shown that the phenotype of the UL13 deletion mutant virus with respect to UL34 and UL31 localization is similar to that of the Us3 deletion mutant. Together with the observation that UL13 phosphorylates Us3 in infected cells, a reasonable hypothesis is that UL13-mediated phosphorylation of Us3 may regulate the ability of Us3 to determine the proper localization of the viral proteins UL34 and UL31. Although we have shown here that UL13-mediated phosphorylation of Us3 is not required for optimal Us3 protein kinase activity, such phosphorylation might alter some other Us3 activity, such as substrate specificity or subcellular localization. Further studies will be needed to clarify whether UL13-mediated phosphorylation of Us3 is required for regulation of UL34 and UL31 localization. Such studies will need to include identification of the Us3 site(s) for UL13-mediated phosphorylation, construction of a recombinant virus with a mutated phosphorylaton site(s) in Us3, and investigation of the phenotype of this mutant virus with respect to UL34 and UL31 localization.
Alternatively, UL13 may regulate UL34 and UL31 localization independently of Us3. We previously reported that HSV-1 UL13 and its counterparts in other herpesviruses, including human cytomegalovirus UL97 and EBV BGLF4, and cellular protein kinase cdc2 phosphorylate the same amino acid residues on target proteins (27-29). In addition, Advani et al. have shown that HSV-1 infection activates cdc2 and that UL13 is required for this activation (1). It is well known that cdc2 modifies nuclear membranes by direct phosphorylation of nuclear envelope proteins (5, 46). In particular, phosphorylation of nuclear lamina and the lamin B receptor by cdc2 results in disassembly of nuclear lamina during mitosis (11, 16, 22, 38, 47, 48, 53, 72). Interestingly, both the UL34 and UL31 proteins have been reported to interact in vitro with lamin A/C, a major component of nuclear lamina, and to be required for HSV-mediated modification of lamin A/C and chromatin (60, 67). Therefore, UL13 may act like a cdc2 kinase or may activate cdc2 kinase to phosphorylate nuclear envelope proteins for proper targeting of UL34 and UL31 proteins at the nuclear membrane.
The possibility that UL13 directly phosphorylates UL34 and/or UL31 to regulate their localization seems less likely, based on the following observations. First, we have shown here that, in cells infected with UL13 deletion mutant virus, posttranslational processing of UL31, which is associated with phosphorylation (25), could not be differentiated from that in cells infected with wild-type virus. Second, Ryckman and Roller (65) reported that UL34 phosphorylation was completely abolished in infected cells when the Us3 kinase target sites in UL34 (threonine 195 and serine 198) (25, 58, 65) were mutated, indicating that UL34 is phosphorylated only at threonine 195 and serine 198. There are no reports that UL13 and Us3 target the same substrate phosphorylation site(s).
In the present study, we have demonstrated that UL34 and UL31 exhibited nucleoplasmic localization, in addition to nuclear membrane localization. Although several laboratories have investigated the localization of UL34 and UL31, it remains enigmatic. Thus, some laboratories clearly demonstrated that UL34 and UL31 were detected only at the nuclear membrane in HSV-1-infected cells and in cells cotransfected with a UL34- and UL31-expressing plasmid (61, 62, 65). In contrast, the others, including this laboratory, reported nucleoplasmic localization of UL34 and/or UL31, in addition to nuclear membrane localization, in cells infected with HSV-1 or HSV-2 and in cells transiently coexpressing UL31 and UL34 (8, 66, 67, 73, 74). Furthermore, the nucleoplasmic distribution of pseudorabies virus UL31 and UL34 homologues in infected cells has also been reported (19). At present, we do not know how to explain these discrepancies. Although it has been reported that UL34 is a membrane-anchored protein (58, 59, 66), UL34 could also function as a nucleoplasmic protein. There is also a possibility that the methods for fixation of cells, staining conditions, and equipment used in immunofluorescence assays affect the detection of the viral proteins. Further studies will be needed to clarify this subject.
In conclusion, we have provided data showing that Us3 is a physiological substrate of UL13 and that UL13 regulates the localization of the HSV envelopment factors UL34 and UL31. Although direct linkage between UL13-mediated phosphorylation of Us3 and the regulatory effects of Us3 on UL34 and UL31 localization remains to be elucidated, our observations raise the interesting possibility that UL13 is also involved in the nuclear egress pathway of HSV-1. In agreement with this possibility, it has been reported that the ability of mutant human cytomegalovirus UL97 to bud through the nuclear membrane is severely impaired (35).
Acknowledgments
We thank B. Roizman for R7356, R7041, pBC1013, pBC1015, and SK-N-SH cells and R. Roller for chicken polyclonal antibody to UL34. We thank H. Noma for excellent technical assistance.
This study was supported in part by Grants for Scientific Research and Grants for Scientific Research in Priority Areas from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Advani, S. J., R. Brandimarti, R. R. Weichselbaum, and B. Roizman. 2000. The disappearance of cyclins A and B and the increase in activity of the G2/M-phase cellular kinase cdc2 in herpes simplex virus 1-infected cells require expression of the alpha22/U(S)1.5 and U(L)13 viral genes. J. Virol. 74:8-15. [PMC free article] [PubMed] [Google Scholar]
- 2.Asano, S., T. Honda, F. Goshima, D. Watanabe, Y. Miyake, Y. Sugiura, and Y. Nishiyama. 1999. US3 protein kinase of herpes simplex virus type 2 plays a role in protecting corneal epithelial cells from apoptosis in infected mice. J. Gen. Virol. 80:51-56. [DOI] [PubMed] [Google Scholar]
- 3.Benetti, L., and B. Roizman. 2004. Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc. Natl. Acad. Sci. USA 101:9411-9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruni, R., B. Fineschi, W. O. Ogle, and B. Roizman. 1999. A novel cellular protein, p60, interacting with both herpes simplex virus 1 regulatory proteins ICP22 and ICP0 is modified in a cell-type-specific manner and is recruited to the nucleus after infection. J. Virol. 73:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buendia, B., J. C. Courvalin, and P. Collas. 2001. Dynamics of the nuclear envelope at mitosis and during apoptosis. Cell Mol. Life Sci. 58:1781-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartier, A., E. Broberg, T. Komai, M. Henriksson, and M. G. Masucci. 2003. The herpes simplex virus-1 Us3 protein kinase blocks CD8T cell lysis by preventing the cleavage of Bid by granzyme B. Cell Death Differ. 10:1320-1328. [DOI] [PubMed] [Google Scholar]
- 7.Cartier, A., T. Komai, and M. G. Masucci. 2003. The Us3 protein kinase of herpes simplex virus 1 blocks apoptosis and induces phosporylation of the Bcl-2 family member Bad. Exp. Cell Res. 291:242-250. [DOI] [PubMed] [Google Scholar]
- 8.Chang, Y. E., and B. Roizman. 1993. The product of the UL31 gene of herpes simplex virus 1 is a nuclear phosphoprotein which partitions with the nuclear matrix. J. Virol. 67:6348-6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chee, M. S., G. L. Lawrence, and B. G. Barrell. 1989. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J. Gen. Virol. 70:1151-1160. [DOI] [PubMed] [Google Scholar]
- 10.Coulter, L. J., H. W. Moss, J. Lang, and D. J. McGeoch. 1993. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J. Gen. Virol. 74:387-395. [DOI] [PubMed] [Google Scholar]
- 11.Courvalin, J. C., N. Segil, G. Blobel, and H. J. Worman. 1992. The lamin B receptor of the inner nuclear membrane undergoes mitosis-specific phosphorylation and is a substrate for p34cdc2-type protein kinase. J. Biol. Chem. 267:19035-19038. [PubMed] [Google Scholar]
- 12.Cunningham, C., A. J. Davison, A. Dolan, M. C. Frame, D. J. McGeoch, D. M. Meredith, H. W. Moss, and A. C. Orr. 1992. The UL13 virion protein of herpes simplex virus type 1 is phosphorylated by a novel virus-induced protein kinase. J. Gen. Virol. 73:303-311. [DOI] [PubMed] [Google Scholar]
- 13.Daikoku, T., S. Shibata, F. Goshima, S. Oshima, T. Tsurumi, H. Yamada, Y. Yamashita, and Y. Nishiyama. 1997. Purification and characterization of the protein kinase encoded by the UL13 gene of herpes simplex virus type 2. Virology 235:82-93. [DOI] [PubMed] [Google Scholar]
- 14.Daikoku, T., Y. Yamashita, T. Tsurumi, K. Maeno, and Y. Nishiyama. 1993. Purification and biochemical characterization of the protein kinase encoded by the US3 gene of herpes simplex virus type 2. Virology 197:685-694. [DOI] [PubMed] [Google Scholar]
- 15.Dancey, J. E. 2004. Molecular targeting: PI3 kinase pathway. Ann. Oncol. 15(Suppl. 4):iv233-iv239. [DOI] [PubMed] [Google Scholar]
- 16.Dessev, G., C. Iovcheva-Dessev, J. R. Bischoff, D. Beach, and R. Goldman. 1991. A complex containing p34cdc2 and cyclin B phosphorylates the nuclear lamin and disassembles nuclei of clam oocytes in vitro. J. Cell Biol. 112:523-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 18.Frame, M. C., F. C. Purves, D. J. McGeoch, H. S. Marsden, and D. P. Leader. 1987. Identification of the herpes simplex virus protein kinase as the product of viral gene US3. J. Gen. Virol. 68:2699-2704. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs, W., B. G. Klupp, H. Granzow, N. Osterrieder, and T. C. Mettenleiter. 2002. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. J. Virol. 76:364-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiss, B. J., J. E. Tavis, L. M. Metzger, D. A. Leib, and L. A. Morrison. 2001. Temporal regulation of herpes simplex virus type 2 VP22 expression and phosphorylation. J. Virol. 75:10721-10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goshima, F., T. Daikoku, H. Yamada, S. Oshima, T. Tsurumi, and Y. Nishiyama. 1998. Subcellular localization of the US3 protein kinase of herpes simplex virus type 2. Arch. Virol. 143:613-622. [DOI] [PubMed] [Google Scholar]
- 22.Heald, R., and F. McKeon. 1990. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell 61:579-589. [DOI] [PubMed] [Google Scholar]
- 23.Jerome, K. R., R. Fox, Z. Chen, A. E. Sears, H. Lee, and L. Corey. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J. Virol. 73:8950-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanamori, M., S. Watanabe, R. Honma, M. Kuroda, S. Imai, K. Takada, N. Yamamoto, Y. Nishiyama, and Y. Kawaguchi. 2004. Epstein-Barr virus nuclear antigen leader protein induces expression of thymus- and activation-regulated chemokine in B cells. J. Virol. 78:3984-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato, A., M. Yamamoto, T. Ohno, H. Kodaira, Y. Nishiyama, and Y. Kawaguchi. 2005. Identification of proteins phosphorylated directly by the Us3 protein kinase encoded by herpes simplex virus 1. J. Virol. 79:9325-9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato, K., Y. Kawaguchi, M. Tanaka, M. Igarashi, A. Yokoyama, G. Matsuda, M. Kanamori, K. Nakajima, Y. Nishimura, M. Shimojima, H. T. Phung, E. Takahashi, and K. Hirai. 2001. Epstein-Barr virus-encoded protein kinase BGLF4 mediates hyperphosphorylation of cellular elongation factor 1δ (EF-1δ): EF-1δ is universally modified by conserved protein kinases of herpesviruses in mammalian cells. J. Gen. Virol. 82:1457-1463. [DOI] [PubMed] [Google Scholar]
- 27.Kato, K., A. Yokoyama, Y. Tohya, H. Akashi, Y. Nishiyama, and Y. Kawaguchi. 2003. Identification of protein kinases responsible for phosphorylation of Epstein-Barr virus nuclear antigen leader protein at serine-35, which regulates its coactivator function. J. Gen. Virol. 84:3381-3392. [DOI] [PubMed] [Google Scholar]
- 28.Kawaguchi, Y., and K. Kato. 2003. Protein kinases conserved in herpesviruses potentially share a function mimicking the cellular protein kinase cdc2. Rev. Med. Virol. 13:331-340. [DOI] [PubMed] [Google Scholar]
- 29.Kawaguchi, Y., K. Kato, M. Tanaka, M. Kanamori, Y. Nishiyama, and Y. Yamanashi. 2003. Conserved protein kinases encoded by herpesviruses and cellular protein kinase cdc2 target the same phosphorylation site in eukaryotic elongation factor 1δ. J. Virol. 77:2359-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawaguchi, Y., T. Matsumura, B. Roizman, and K. Hirai. 1999. Cellular elongation factor 1δ is modified in cells infected with representative alpha-, beta-, or gammaherpesviruses. J. Virol. 73:4456-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawaguchi, Y., K. Nakajima, M. Igarashi, T. Morita, M. Tanaka, M. Suzuki, A. Yokoyama, G. Matsuda, K. Kato, M. Kanamori, and K. Hirai. 2000. Interaction of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP) with HS1-associated protein X-1: implication of cytoplasmic function of EBNA-LP. J. Virol. 74:10104-10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1998. Eukaryotic elongation factor 1δ is hyperphosphorylated by the protein kinase encoded by the U(L)13 gene of herpes simplex virus 1. J. Virol. 72:1731-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1997. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 71:7328-7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2001. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus. J. Gen. Virol. 82:2363-2371. [DOI] [PubMed] [Google Scholar]
- 35.Krosky, P. M., M. C. Baek, and D. M. Coen. 2003. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77:905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA 94:7891-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long, M. C., V. Leong, P. A. Schaffer, C. A. Spencer, and S. A. Rice. 1999. ICP22 and the UL13 protein kinase are both required for herpes simplex virus-induced modification of the large subunit of RNA polymerase II. J. Virol. 73:5593-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luscher, B., L. Brizuela, D. Beach, and R. N. Eisenman. 1991. A role for the p34cdc2 kinase and phosphatases in the regulation of phosphorylation and disassembly of lamin B2 during the cell cycle. EMBO J. 10:865-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGeoch, D. J., and A. J. Davison. 1986. Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 14:1765-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison, E. E., Y. F. Wang, and D. M. Meredith. 1998. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J. Virol. 72:7108-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munger, J., A. V. Chee, and B. Roizman. 2001. The U(S)3 protein kinase blocks apoptosis induced by the d120 mutant of herpes simplex virus 1 at a premitochondrial stage. J. Virol. 75:5491-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munger, J., and B. Roizman. 2001. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc. Natl. Acad. Sci. USA 98:10410-10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murata, T., F. Goshima, Y. Yamauchi, T. Koshizuka, H. Takakuwa, and Y. Nishiyama. 2002. Herpes simplex virus type 2 US3 blocks apoptosis induced by sorbitol treatment. Microbes Infect. 4:707-712. [DOI] [PubMed] [Google Scholar]
- 44.Ng, T. I., W. O. Ogle, and B. Roizman. 1998. UL13 protein kinase of herpes simplex virus 1 complexes with glycoprotein E and mediates the phosphorylation of the viral Fc receptor: glycoproteins E and I. Virology 241:37-48. [DOI] [PubMed] [Google Scholar]
- 45.Ng, T. I., C. Talarico, T. C. Burnette, K. Biron, and B. Roizman. 1996. Partial substitution of the functions of the herpes simplex virus 1 U(L)13 gene by the human cytomegalovirus U(L)97 gene. Virology 225:347-358. [DOI] [PubMed] [Google Scholar]
- 46.Nigg, E. A. 1993. Cellular substrates of p34cdc2 and its companion cyclin-dependent kinases. Trends Cell Biol. 3:296-301. [DOI] [PubMed] [Google Scholar]
- 47.Nikolakaki, E., J. Meier, G. Simos, S. D. Georgatos, and T. Giannakouros. 1997. Mitotic phosphorylation of the lamin B receptor by a serine/arginine kinase and p34(cdc2). J. Biol. Chem. 272:6208-6213. [DOI] [PubMed] [Google Scholar]
- 48.Nikolakaki, E., G. Simos, S. D. Georgatos, and T. Giannakouros. 1996. A nuclear envelope-associated kinase phosphorylates arginine-serine motifs and modulates interactions between the lamin B receptor and other nuclear proteins. J. Biol. Chem. 271:8365-8372. [DOI] [PubMed] [Google Scholar]
- 49.Nishiyama, Y., Y. Yamada, R. Kurachi, and T. Daikoku. 1992. Construction of a US3 lacZ insertion mutant of herpes simplex virus type 2 and characterization of its phenotype in vitro and in vivo. Virology 190:256-268. [DOI] [PubMed] [Google Scholar]
- 50.Ogg, P. D., P. J. McDonell, B. J. Ryckman, C. M. Knudson, and R. J. Roller. 2004. The HSV-1 Us3 protein kinase is sufficient to block apoptosis induced by overexpression of a variety of Bcl-2 family members. Virology 319:212-224. [DOI] [PubMed] [Google Scholar]
- 51.Ogle, W. O., T. I. Ng, K. L. Carter, and B. Roizman. 1997. The UL13 protein kinase and the infected cell type are determinants of posttranslational modification of ICP0. Virology 235:406-413. [DOI] [PubMed] [Google Scholar]
- 52.Overton, H. A., D. J. McMillan, L. S. Klavinskis, L. Hope, A. J. Ritchie, and P. Wong-Kai-In. 1992. Herpes simplex virus type 1 gene UL13 encodes a phosphoprotein that is a component of the virion. Virology 190:184-192. [DOI] [PubMed] [Google Scholar]
- 53.Peter, M., J. Nakagawa, M. Doree, J. C. Labbe, and E. A. Nigg. 1990. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell 61:591-602. [DOI] [PubMed] [Google Scholar]
- 54.Poon, A. P., and B. Roizman. 2005. Herpes simplex virus 1 ICP22 regulates the accumulation of a shorter mRNA and of a truncated US3 protein kinase that exhibits altered functions. J. Virol. 79:8470-8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Purves, F. C., R. M. Longnecker, D. P. Leader, and B. Roizman. 1987. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J. Virol. 61:2896-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Purves, F. C., W. O. Ogle, and B. Roizman. 1993. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc. Natl. Acad. Sci. USA 90:6701-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Purves, F. C., and B. Roizman. 1992. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein alpha 22. Proc. Natl. Acad. Sci. USA 89:7310-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Purves, F. C., D. Spector, and B. Roizman. 1991. The herpes simplex virus 1 protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J. Virol. 65:5757-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Purves, F. C., D. Spector, and B. Roizman. 1992. UL34, the target of the herpes simplex virus U(S)3 protein kinase, is a membrane protein which in its unphosphorylated state associates with novel phosphoproteins. J. Virol. 66:4295-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reynolds, A. E., L. Liang, and J. D. Baines. 2004. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes U(L)31 and U(L)34. J. Virol. 78:5564-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynolds, A. E., B. J. Ryckman, J. D. Baines, Y. Zhou, L. Liang, and R. J. Roller. 2001. U(L)31 and U(L)34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 75:8803-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott-Williams &Wilkins, Philadelphia, Pa.
- 64.Roller, R. J., Y. Zhou, R. Schnetzer, J. Ferguson, and D. DeSalvo. 2000. Herpes simplex virus type 1 U(L)34 gene product is required for viral envelopment. J. Virol. 74:117-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryckman, B. J., and R. J. Roller. 2004. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J. Virol. 78:399-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shiba, C., T. Daikoku, F. Goshima, H. Takakuwa, Y. Yamauchi, O. Koiwai, and Y. Nishiyama. 2000. The UL34 gene product of herpes simplex virus type 2 is a tail-anchored type II membrane protein that is significant for virus envelopment. J. Gen. Virol. 81:2397-2405. [DOI] [PubMed] [Google Scholar]
- 67.Simpson-Holley, M., J. Baines, R. Roller, and D. M. Knipe. 2004. Herpes simplex virus 1 U(L)31 and U(L)34 gene products promote the late maturation of viral replication compartments to the nuclear periphery. J. Virol. 78:5591-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith, R. F., and T. F. Smith. 1989. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J. Virol. 63:450-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tanaka, M., H. Kagawa, Y. Yamanashi, T. Sata, and Y. Kawaguchi. 2003. Construction of an excisable bacterial artificial chromosome containing a full-length infectious clone of herpes simplex virus type 1: viruses reconstituted from the clone exhibit wild-type properties in vitro and in vivo. J. Virol. 77:1382-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanaka, M., Y. Nishiyama, T. Sata, and Y. Kawaguchi. 2005. The role of protein kinase activity expressed by the UL13 gene of herpes simplex virus 1: the activity is not essential for optimal expression of UL41 and ICP0. Virology 341:301-312. [DOI] [PubMed] [Google Scholar]
- 71.Wagenaar, F., J. M. Pol, B. Peeters, A. L. Gielkens, N. de Wind, and T. G. Kimman. 1995. The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J Gen. Virol. 76:1851-1859. [DOI] [PubMed] [Google Scholar]
- 72.Ward, G. E., and M. W. Kirschner. 1990. Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell 61:561-577. [DOI] [PubMed] [Google Scholar]
- 73.Yamada, H., Y. M. Jiang, S. Oshima, T. Daikoku, Y. Yamashita, T. Tsurumi, and Y. Nishiyama. 1998. Characterization of the UL55 gene product of herpes simplex virus type 2. J. Gen. Virol. 79:1989-1995. [DOI] [PubMed] [Google Scholar]
- 74.Yamauchi, Y., C. Shiba, F. Goshima, A. Nawa, T. Murata, and Y. Nishiyama. 2001. Herpes simplex virus type 2 UL34 protein requires UL31 protein for its relocation to the internal nuclear membrane in transfected cells. J. Gen. Virol. 82:1423-1428. [DOI] [PubMed] [Google Scholar]
- 75.Yokoyama, A., M. Tanaka, G. Matsuda, K. Kato, M. Kanamori, H. Kawasaki, H. Hirano, I. Kitabayashi, M. Ohki, K. Hirai, and Y. Kawaguchi. 2001. Identification of major phosphorylation sites of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP): ability of EBNA-LP to induce latent membrane protein 1 cooperatively with EBNA-2 is regulated by phosphorylation. J. Virol. 75:5119-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yue, W., E. Gershburg, and J. S. Pagano. 2005. Hyperphosphorylation of EBNA2 by Epstein-Barr virus protein kinase suppresses transactivation of the LMP1 promoter. J. Virol. 79:5880-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zarubin, T., and J. Han. 2005. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15:11-18. [DOI] [PubMed] [Google Scholar]
- 78.Zhu, H. Y., H. Yamada, Y. M. Jiang, M. Yamada, and Y. Nishiyama. 1999. Intracellular localization of the UL31 protein of herpes simplex virus type 2. Arch. Virol. 144:1923-1935. [DOI] [PubMed] [Google Scholar]