Abstract
The initiation of DNA replication is tightly regulated in eukaryotic cells to ensure that the genome is precisely duplicated once and only once per cell cycle. This is accomplished by controlling the assembly of a prereplicative complex (pre-RC) which involves the sequential binding to replication origins of the origin recognition complex (ORC), Cdc6/Cdc18, Cdt1, and the minichromosome maintenance complex (Mcm2-Mcm7, or Mcm2-7). Several mechanisms of pre-RC regulation are known, including ATP utilization, cyclin-dependent kinase levels, protein turnover, and Cdt1 binding by geminin. Histone acetylation may also affect the initiation of DNA replication, but at present neither the enzymes nor the steps involved are known. Here, we show that Hbo1, a member of the MYST histone acetyltransferase family, is a previously unrecognized positive regulatory factor for pre-RC assembly. When Hbo1 expression was inhibited in human cells, Mcm2-7 failed to associate with chromatin even though ORC and Cdc6 loading was normal. When Xenopus egg extracts were immunodepleted of Xenopus Hbo1 (XHbo1), chromatin binding of Mcm2-7 was lost, and DNA replication was abolished. The binding of Mcm2-7 to chromatin in XHbo1-depleted extracts could be restored by the addition of recombinant Cdt1.
Replication licensing ensures the precise duplication of DNA once per cell division cycle and thus plays a crucial role in maintaining genome integrity in proliferating cells (5, 7, 8, 13, 21, 27). A key feature of the system is the formation of a prereplicative complex (pre-RC) on replication origins, beginning as cells exit M phase and continuing during the G1 period. The formation of the pre-RC, a prerequisite for the initiation of DNA replication, depends on the binding of the origin recognition complex (ORC) to origins (6); ORC, in turn, recruits Cdc6/Cdc18 and Cdt1. Eventually, the minichromosome maintenance complex (Mcm2-Mcm7, or Mcm2-7) is recruited to origins, completing the hallmark step in the formation of the pre-RC (11, 14, 28, 36). At the onset of S phase, cyclin-dependent kinase and Cdc7 kinase activate the pre-RC, converting it into an initiation complex. Once the pre-RC is activated, its reassembly is prohibited throughout S phase until the end of M phase. A major mechanism for preventing the formation of the pre-RC involves the action of cyclin-dependent kinase, which negatively regulates components of the licensing system (35). In metazoans, an additional licensing inhibitor, geminin, also acts to prevent pre-RC assembly (29). Geminin specifically binds to Cdt1 and inhibits its function (43, 47). The removal of geminin in Drosophila melanogaster tissue culture cells and human cancer cells (31, 33, 50) by small interfering RNAs (siRNAs) leads to overreplication of the genome. In Xenopus egg extracts, geminin is the major trans-acting inhibitor of replication licensing (24, 49).
In addition to factors directly involved in the formation of the pre-RC, recent studies suggest a potential role of chromatin structure in the control of initiation of DNA replication. Chromatin structure has been linked to the initiation of DNA replication in several studies (9, 25, 40), and histone modification has been implicated in regulating replication timing in budding yeast (4, 46). Recent studies further suggest that histone acetylation is involved in origin activation at the chorion gene loci in Drosophila follicle cells (1) and in Xenopus early development (12). In addition, the histone deacetylase Sir2 has been shown to negatively regulate pre-RC assembly in budding yeast (38). These results suggest that histone acetylation is potentially involved in the control of pre-RC assembly; however, the molecular mechanism of this role is unknown.
The human protein Hbo1 (hHbo1), a MYST family histone acetyltransferase (HAT) (45), was originally identified by Iizuka and Stillman (20) through its binding to the human Orc1 protein; subsequently, Hbo1 was found to bind mouse Mcm2 as well (10). More recently, Hbo1 was found to associate with the latent replication origin of Kaposi's sarcoma-associated herpes virus, and siRNA inhibition of Hbo1 expression partially impaired Kaposi's sarcoma-associated herpes virus replication (41). These physical and functional interactions suggest that the acetylase activity of Hbo1 might participate in pre-RC formation and replication licensing.
MATERIALS AND METHODS
Identification of Xenopus Hbo1 (XHbo1).
Two different cDNAs encoding putative Xenopus homologues of Hbo1 were identified in the public databases. They have the unique Mammalian Gene Collection identification numbers MGC78789 and MGC68869 assigned by the Mammalian Gene Collection project (44), with GenBank accession numbers of BC077173 and BC072987, respectively. The cDNAs were purchased from the I.M.A.G.E. Consortium, and their sequences were confirmed with an automatic DNA sequencer (ABI PRISM 310 Genetic Analyzer).
Recombinant proteins.
Plasmids for the expression of human His-tagged geminin (39) and glutathione S-transferase (GST)-Orc2 (16), were generous gifts from A Dutta and B. Stillman, respectively. Human His6-Hbo1 (amino acids 1 to 611) and His6-geminin proteins were expressed in bacteria and purified using Ni-NTA agarose (QIAGEN). Human GST-Orc2 protein was expressed and purified with glutathione-agarose (Sigma). His6-Mcm2 and His6-Cdc6 proteins were the generous gift of M. Alexandrow (3). For recombinant Xenopus Hbo1, the cDNA (XHbo1a) containing the full-length open reading frame was subcloned into pGEX6P-1 (GST-XHbo1) and was expressed in bacteria (strain BL21). The cleared lysates were incubated with glutathione-Sepharose 4B (Amersham Biosciences) at 4°C for 3 h. After being washed with phosphate-buffered saline (PBS), the GST moiety was cleaved with Precision Protease (Amersham Biosciences).
Antibodies and peptides.
Rabbit polyclonal antibodies to hOrc2 and hMcm2 were a generous gift from B. Stillman. A rabbit antibody selective for the phosphorylated form of histone H1 was a gift from C. D. Allis (26). An affinity-purified rabbit polyclonal antibody to human Cdt1 was a gift from Hideo Nishitani. Commercial antibodies were purchased for antiMEK2 (N-20), anti-cyclin B1, anti-Cdc6, anti-cyclin E (Santa Cruz Biotechnology), anti-tetra-acetyl-histone H4 (Upstate Biotechnology), and antiactin (Sigma). Antibodies against human Hbo1 were raised in rabbits using peptide L74-1 (residues 158 to 172 of human Hbo1) synthesized by Sawady Technology (Tokyo, Japan). An affinity-purified fraction was used for immunofluorescence microscopy (IMF). Polyclonal antibodies against Xenopus Hbo1 were generated with recombinant Hbo1 protein expressed in bacteria, as well as two synthetic peptides. Peptide 1 (SLKDSGSDLSHRPKR) corresponds to residues 158 to 172 of human Hbo1; peptide 2 (CKVRAQSRDKQEDER) corresponds to residues 220 to 234 of human Hbo1. Affinity-purified antibody against peptide 1 was used for immunoprecipitation and immunodepletion. All antisera were affinity purified using full-length recombinant Xenopus Hbo1 and used for immunoblotting.
Acetylation, immunoprecipitation, and H1 kinase assay.
Acetylation assays were performed in 20-μl reaction mixtures (50 mM HEPES [pH 8.0], 10% glycerol, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 10 mM sodium butyrate, 1 μl of [3H]acetyl-coenzyme A [CoA] [2.1 Ci/mmol; Amersham]), 1 to 3 μg of highly purified substrate proteins or 2 μg of GST fusion proteins, and 2 μg of His-Hbo1 and were incubated at 30°C for 1 h. The reaction mixture was subjected to electrophoresis with a 10% polyacrylamide gel electrophoresis (PAGE) gel and autoradiography. Gels containing [3H]acetate-labeled proteins were fixed with 10% glacial acetic acid and 40% methanol for 1 h and were enhanced by impregnation with a commercial fluorography enhancing solution (Amplify; Perkin-Elmer) for 30 min. Gels were then dried, and autoradiography was performed at −70°C for 7 to 21 days. Immunoprecipitation and histone acetyltransferase assays were as described previously (20), except that chicken core histones were used as substrates. H1 kinase assays were carried out as described previously (2).
Cell lines, RNAi, and Ads.
A549 and HeLa cell lines were purchased from the American Type Culture Collection. For synchronization at the G1/S boundary, cells were incubated in medium containing 2 mM thymidine (Sigma) for 12 h and then in thymidine-free medium for 6 h, followed by an additional 12-h incubation in medium containing 2 mM thymidine. To synchronize HeLa cells at mitosis, cultures were treated with 50-ng/ml nocodazole (Sigma) for 16 h, and rounded mitotic cells were then detached from the dishes by manual shaking. After several washes with PBS, prewarmed regular medium was added to release the cells from the arrest. For analyses of cell cycle progression, ethanol-fixed cells were stained with propidium iodide and analyzed by flow cytometry (FACS Core Facility, University of Virginia). RNA interference (RNAi) was accomplished as described previously (15) by transfection with duplex RNA (Dharmacon) corresponding to nucleotides 92 to 112 of the Hbo1 coding region. Replication-deficient recombinant viruses were created as described previously (17). Adenovirus (Ad) stocks were maintained as described previously (34) and purified by cesium chloride density gradient centrifugation. Viruses were used at a multiplicity of infection of 900. Details of the construction of the green fluorescent protein (GFP) and Hbo1 antisense adenoviruses are available upon request.
Indirect IMF.
Cells were treated with hypotonic buffer (10 mM HEPES-KOH [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.5 mM dithiothreitol, 0.1% Triton X-100) (37). Cells were fixed with 4% paraformaldehyde and permeablized with 0.2% Triton X-100. After being blocked with PBS containing 2% goat serum, the fixed cells were stained with the appropriate primary antibody for 1 h. After several PBS washes, cells were incubated for 1 h with Cy3-labeled goat anti-rabbit immunoglobulin G (IgG) (1:1,000 dilution) and/or fluorescein isothiocyanate-labeled donkey anti-mouse IgG (1:1,000 dilution) (both from Jackson ImmunoResearch Laboratories). For the Xenopus experiments, interphase egg extracts were supplemented with sperm chromatin (4,000 sperm heads per μl) and 1 μM Cy3-dCTP. At appropriate times after incubation, extracts were diluted 10 fold with extraction buffer (EB; 50 mM HEPES-KOH [pH 7.5], 100 mM KCl, 2.5 mM MgCl2) containing 0.25% NP-40, and then formaldehyde was added to a final concentration of 3.7%. Samples were recovered by centrifugation though EB containing 30% sucrose onto a cover glass. The cover glass was washed with Tris-buffered saline plus 0.05% Tween 20 and incubated overnight at 4°C with primary rabbit antibody in Tris-buffered saline plus 0.05% Tween 20 containing 5% skim milk and then with a second antibody of Alexa Fluor 488-labeled anti-rabbit goat IgG (Invitrogen).
Preparation of egg extracts and immunodepletion.
Interphase Xenopus egg extracts and sperm chromatin were prepared as previously described (22). The extracts were supplemented with 40-μg/ml cycloheximide, 30 mM phosphocreatine, and 15-μg/ml creatine phosphokinase. Immunodepletion of Hbo1 from the extracts was performed as follows. Affinity-purified anti-Hbo1 peptide antibody or control IgG was bound to protein A-Sepharose Fast Flow (Amersham Bioscience) at 5 μg of antibody per μl of beads and washed with EB. Interphase egg extract was mixed with a 0.1 volume of the beads for 30 min at 4°C. After removal of the beads, the extracts were similarly treated twice with freshly prepared antibody beads, and the obtained extracts were used for further experiments.
Chromatin preparation from frog eggs.
Egg extracts were supplemented with sperm chromatin at a concentration of 4,000 sperm heads per μl and 40-μg/ml aphidicolin. At appropriate times after incubation at 23°C, extracts were diluted 10 fold with EB containing 0.25% NP-40, and the same buffer containing 10% (wt/wt) and 30% (wt/wt) sucrose were underlayered. The extracts were then centrifuged at 5,500 × g in a swinging-bucket rotor at 4°C for 5 min, and only the top layer was removed. Next, EB containing 0.25% NP-40 was layered on top of the samples, and the samples were centrifuged at 8,500 × g in a swinging-bucket rotor for 5 min. The top and second layers were then removed, leaving the bottom layer containing 30% sucrose, and the bottom layer diluted with EB was further centrifuged at 8,500 × g to pellet the chromatin fractions.
RESULTS
Hbo1 is required for the chromatin association of Mcm2-7.
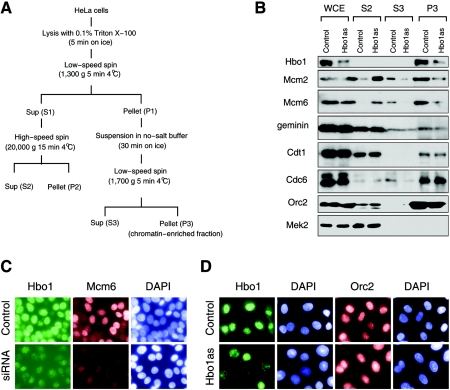
Since Hbo1 has protein-protein interactions with both ORC and Mcm2-7, we reasoned that it might play a role in the chromatin loading of one or more pre-RC components. To explore this prediction, we inhibited Hbo1 expression in HeLa cells by transcribing Hbo1 antisense RNA from a transfected recombinant adenovirus. An equivalent adenovirus expressing mRNA for GFP served as a control. Following transfection with these adenovirus vectors, cells were separated into cytoplasmic, nucleus-soluble, and chromatin-enriched fractions and the distribution of pre-RC components were assayed by immunoblot analysis (Fig. 1A and B). Good separation of subcellular compartments was achieved in these experiments, as indicated by the fact that the cytoplasmic kinase MEK2 was detected exclusively in cytoplasmic fraction (Fig. 1B, lanes S2). The expression of antisense Hbo1, but not control GFP, markedly decreased the expression of Hbo1 in the whole-cell extracts without affecting the protein levels of the pre-RC components Orc2, Cdc6, Cdt1, Mcm2, or Mcm6 (Fig. 1B, lanes WCE). However, the depletion of Hbo1 did cause a significant redistribution of Mcm2 and Mcm6. In control cells, Mcm2 and Mcm6 were mainly recovered in the chromatin fractions, as expected (Fig. 1B, lane P3 Control). However, in Hbo1-depleted cells the amounts of Mcm2 and Mcm6 were reduced in this fraction and recovered instead in the cytoplasmic fraction (Fig. 1B, lane S2 Hbo1as). In contrast, Orc2 and Cdc6 were found in the chromatin-enriched fractions in both the control and the Hbo1-depleted cells. These results suggested that Hbo1 function is required for Mcm2-7 loading and normal replication licensing.
FIG. 1.
Hbo1 is required for the chromatin association of Mcm2 and Mcm6. (A) Fractionation scheme. HeLa cell lysates were separated into soluble cytoplasmic (S2), soluble nucleoplasmic (S3), and chromatin-enriched (P3) fractions as described previously (32). (B) HeLa cells infected with Ad-GFP (Control) or Ad-antisense Hbo1 (Hbo1as) were fractionated as outlined in panel A. Whole-cell extracts (WCE) were prepared by lysis in sodium dodecyl sulfate. WCE, S2, S3, and P3 fractions from equivalent numbers of cells were analyzed by Western blotting using antibodies against the proteins, indicated to the left of each set. (C) Mock-transfected HeLa cells (Control) or HeLa cells transfected with a 21-bp double-stranded RNA oligonucleotide (siRNA) were treated with Triton X-100, fixed with paraformaldehyde, and costained with rabbit polyclonal anti-Hbo1 (Hbo1), mouse monoclonal anti-Mcm6 (Mcm6), and 4′,6′-diamidino-2-phenylindole (DAPI). (D) HeLa cells infected with adenoviruses expressing GFP (Control) or antisense Hbo1 (Hbo1as) were treated with Triton X-100 and then fixed with paraformaldehyde. Cells were stained with rabbit polyclonal anti-Hbo1 and DAPI or with rabbit polyclonal anti-Orc2 and DAPI.
To test this interpretation using immunofluorescence microscopy (IMF), we inhibited Hbo1 expression by either RNAi or adenovirus antisense expression and examined the chromatin binding of Hbo1, Orc2, and Mcm6 in cells extracted with Triton X-100 (Materials and Methods). The downregulation of Hbo1 expression by Hbo1 RNAi, but not control RNAi, resulted in a loss of both Hbo1 and Mcm6 from the chromatin in the same nuclei (Fig. 1C). This effect on Mcm6 was specific, since the downregulation of Hbo1 by antisense expression did not inhibit the binding of Orc2 (Fig. 1D). Thus, the results of both the IMF and the biochemical fractionation experiments show that depletion of Hbo1 inhibits the loading of the Mcm2-7 complex onto chromatin, without affecting the expression of proteins involved in the licensing reaction.
Hbo1 is required for pre-RC assembly during G1 phase.
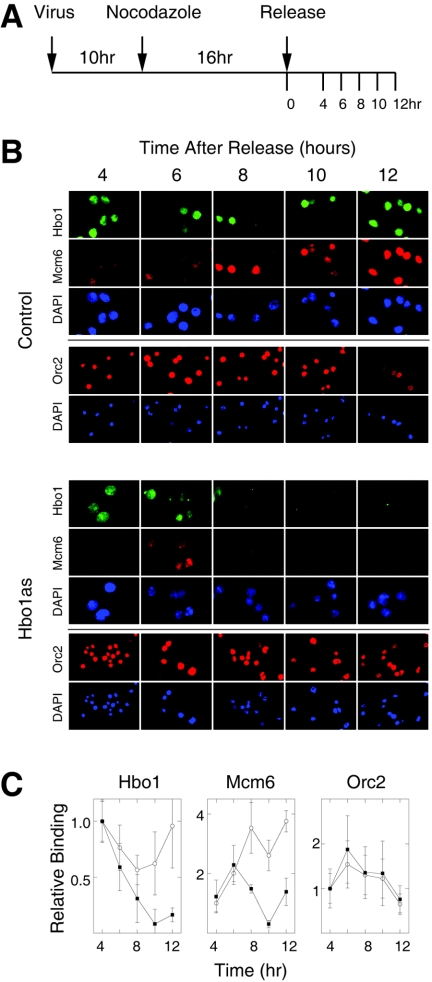
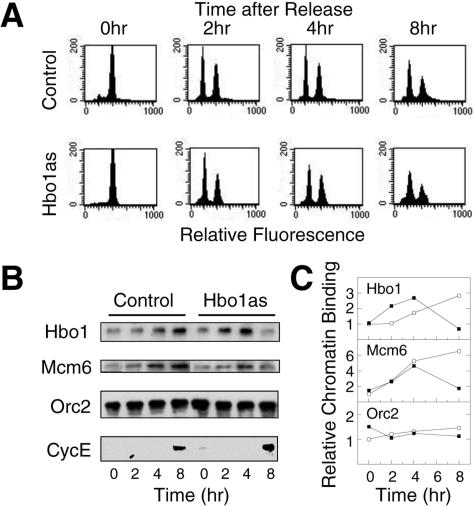
Since normal pre-RC formation takes place during G1 phase, we next examined the chromatin loading of Mcm2-7 and ORC in synchronously dividing cultures. Our strategy was to inhibit Hbo1 expression with antisense adenovirus in cultures that were synchronized in mitosis by inhibiting microtubule function with the drug nocodazole. Since microtubule function is required for the directed targeting of adenovirus particles to the nucleus (42), adenovirus infection had to be done prior to nocodazole treatment. Different times of infection were tested and optimized at 10 h (Fig. 2A). Shorter times did not achieve adequate Hbo1 inhibition, while longer times affected the release of antisense-treated cells from mitosis, presumably due to checkpoint activation as a result of damage in the previous cell cycle. Thus, cells were first infected with either control GFP adenovirus or Hbo1 antisense adenovirus and allowed to grow for 10 h to begin expression of the transcripts. Infected cells were then synchronized in M phase by treatment with nocodazole for 16 h and finally released into G1 phase by washing out the nocodazole. Following release from nocodazole, we assayed the expression of Hbo1 and the chromatin association of Mcm6 and Orc2 by IMF. Using this regimen, Hbo1 inhibition was timed to occur during the course of G1 phase. As shown in Fig. 2B and C, the levels of Hbo1 protein in control and antisense cells were initially similar at 4 h postrelease. However, in cells treated with antisense adenovirus, Hbo1 protein began to be preferentially depleted starting at 6 to 8 h and was significantly diminished by 10 and 12 h postrelease, compared to controls. In the Hbo1 antisense cells, the chromatin association of Mcm6 increased for 4 to 6 h postrelease, but by 8 h it lagged behind that of control cells; by 10 to 12 h, its association was markedly decreased. In contrast, the chromatin association of Orc2 was essentially unaffected by Hbo1 antisense expression in these experiments. To complement these IMF experiments, we also carried out biochemical fractionation experiments on synchronously dividing populations (Fig. 3). At time intervals after nocodazole release, cells were sampled and fractionated into cytoplasmic and chromatin-bound fractions (Fig. 1A). The amounts of Hbo1, Mcm6, and Orc2 in the S3 chromatin fractions then were assayed by immunoblotting (Fig. 3B and C). For cells treated with antisense adenovirus, but not control adenovirus, the amount of chromatin-bound Hbo1 was substantially lost by 8 h. In control cells, the amount of Mcm6 was found to increase during the course of the experiment. Consistent with the IMF results, the chromatin association of Mcm6 in Hbo1as-treated cells began to increase normally but then decreased significantly between 4 to 8 h, coincident with the loss of Hbo1. As before, the chromatin association of Orc2 was unaffected by Hbo1 inhibition.
FIG. 2.
Hbo1 is required for Mcm6 binding to chromatin during G1 phase. (A) Experimental protocol. Cells were infected with adenovirus for 10 h, arrested with nocodazole for 16 h, and then released. (B) At the time points indicated, HeLa cells infected with adenoviruses expressing GFP (Control) or antisense Hbo1 (Hbo1as) were sampled, treated with Triton X-100 to extract soluble proteins, and then fixed with paraformaldehyde for IMF. In the top three rows of each set, cells were costained with rabbit polyclonal anti-Hbo1 (Hbo1), mouse monoclonal anti-Mcm6 (Mcm6), and DAPI (magnification, ×100). In the bottom two rows, an independent sample of cells from the same cultures were stained with rabbit polyclonal anti-Orc2 (Orc2) and DAPI (magnification, ×40). (C) Relative chromatin association of Hbo1, Mcm6, and Orc2. The integrated fluorescence intensity of individual nuclei from the experiments shown in panel B was measured, and the averages are shown plotted versus time after release from the nocodazole block. The values shown in each set are normalized by the intensity of the control cells at the 4-h time point. Error bars indicate 1 standard deviation. The data for control cells treated with GFP adenovirus are plotted as open circles, and the data for cells treated with Hbo1 antisense adenovirus are plotted as solid squares.
FIG. 3.
Hbo1 is required for Mcm6 binding to chromatin during G1. (A) Synchronous cultures infected with either GFP adenovirus (Control) or Hbo1 antisense adenovirus (Hbo1as) were established as outlined in Fig. 2A. At the times indicated, the cultures were sampled, and the cells were stained for DNA content with propidium iodide. Histograms of the DNA fluorescence distributions were determined by flow cytometry and are plotted for each condition. (B) S3 chromatin-enriched fractions were prepared from cells infected with either Ad-GFP (Control) or Ad-antisense Hbo1 (Hbo1as), as outlined in the legend to Fig. 1A. The chromatin factions were assayed by Western blotting with antibodies against Hbo1, Mcm6, and Orc2. The S2 cytoplasmic fraction was assayed for cyclin E (CycE), a cell cycle marker, using a rabbit polyclonal antibody. (C) The chromatin binding of Hbo1, Mcm6, and Orc2 were quantified for the experiment shown in panel B. The open circles indicate the chromatin binding for GFP adenovirus treatment (Control), and the solid squares indicate the binding for Hbo1 antisense adenovirus treatment (Hbo1as). The results for each set are expressed as the fraction of the control signal at 0 h.
In both the IMF and biochemical fractionation experiments, cell cycle synchrony and progression were monitored by both flow cytometry and an analysis of cell cycle markers. These assays showed that cells from both control and Hbo1as cultures entered G1 phase from the nocodazole block with similar kinetics and proportions (Fig. 3A). Moreover, comparable levels of cyclin E, a G1/S stage marker, were expressed in the control and antisense cells at 8 h (Fig. 3B), indicating equivalent enrichment of G1/S at that time. Finally, significant H1 phosphorylation, an M-phase marker, was first detected in a few cells only after 12 h in both control and antisense cells, confirming similar G1- and S-phase synchrony in the populations (data not shown). Together, these results show that the loss of Mcm6 binding to chromatin specifically parallels the inhibition of Hbo1 expression. Thus, both the biochemical and the IMF results argue that Hbo1 is required for the chromatin association of the Mcm2-7 complex at G1 phase, a crucial step for licensing DNA replication.
Cell cycle regulation of Hbo1 HAT activity.
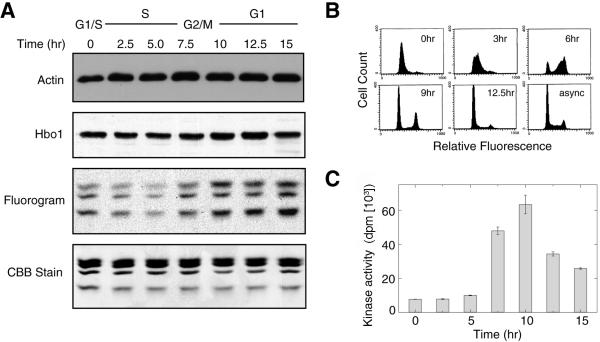
Since the previous experiments showed that Hbo1 is required for pre-RC assembly during G1 phase, we asked whether its HAT activity is regulated during the cell cycle. A549 cells were synchronized at the G1/S boundary by a double-thymidine block, released into the cell cycle, and then assayed for the expression of Hbo1 by immunoblotting and Hbo1 HAT activity in anti-Hbo1 immunoprecipitates (Fig. 4A). HAT activity was assayed as the transfer of [3H]acetate from acetyl-CoA to purified chicken histones as substrate (Materials and Methods). Cell cycle progression of the cultures was monitored by flow cytometry (Fig. 4B) and by assays of the H1 kinase activity of anti-cyclin B1 immunoprecipitates (Fig. 4C). The levels of total Hbo1 protein did not change significantly during the cell cycle (Fig. 4A). In contrast, fluorograms of the HAT assays revealed that the activity of Hbo1 was low during S phase, rose during G2/M, and remained active through G1 (Fig. 4A). Thus, Hbo1 HAT activity is cell cycle regulated and is most active during the time of pre-RC assembly and licensing.
FIG. 4.
The enzymatic activity of Hbo1 during the cell cycle. (A) A549 cells were synchronized by a double-thymidine block and release, and whole-cell extracts were prepared at the indicated time points. The whole lysates were probed with antiactin (Actin) as a loading control and anti-Hbo1 antibody (Hbo1). Anti-Hbo1 immunoprecipitates were assayed for HAT activity by the transfer of [3H]acetate to purified chicken histones (Fluorogram). The input histone levels were monitored by Coomassie staining (CBB Stain). (B) The cell cycle synchrony of the culture used for the data shown in panel A was followed by flow cytometry. At the times indicated, cells were sampled and stained with propidium iodide. Histograms of DNA fluorescence are shown. The histogram of the starting asynchronous culture is also shown (async). (C) The cell cycle progression of the culture was also followed by assaying the Cdc2-cyclin B1 kinase activity of cyclin-B1 immunoprecipitates using histone H1 as a substrate.
Identification of Xenopus XHbo1.
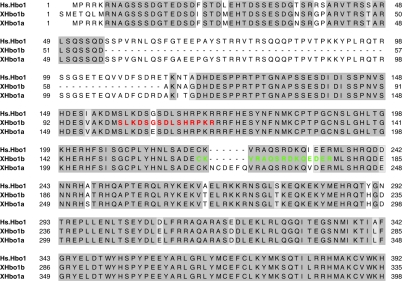
To examine the role of Hbo1 in regulating pre-RC assembly more directly, we exploited the well-characterized cell-free system of Xenopus eggs. A search of the public databases for Xenopus homologues of Hbo1 (XHbo1) revealed nucleotide sequences for at least two Hbo1 variants (Fig. 5). Polyclonal antibodies were generated against full-length XHbo1a and against two peptide sequences (Materials and Methods). The sequence of peptide 1 (residues 158 to 172) is conserved between the two variants, while that of peptide 2 (residues 220 to 234) is conserved between human Hbo1 and XHbo1b (Fig. 5). Affinity-purified antibody against peptide 2 recognized two protein bands of approximately 124 kDa and 75 kDa; both peptide antibodies were able to immunoprecipitate p124 from extracts. The antibody against peptide 1 efficiently immunodepleted p124 from the extracts; all the antibodies, including that raised against the full-length protein, recognized p124 as being highly enriched in the immunoprecipitates (Fig. 6A and data not shown). Thus, multiple antibodies raised against different sequences of XHbo1b protein all recognized the same protein of 124 kDa.
FIG. 5.
Sequence alignments of human Hbo1 and Xenopus homologues. Xenopus cDNAs encoding putative Hbo1 homologues were cloned by the I.M.A.G.E. Consortium. The amino-terminal regions of these proteins were aligned with the sequence of human Hbo1. The highly conserved MYST family histone acetyltransferase domains are further C terminal and are not included in the figure. Boxed regions indicate identical (dark gray shading) or similar (light gray shading) amino acids. We refer to the sequence represented by AAH72987 as XHbo1a and that represented by AAH77173 as XHbo1b. The peptide sequences used for producing antibodies are colored with red for peptide 1 and green for peptide 2 (see Materials and Methods).
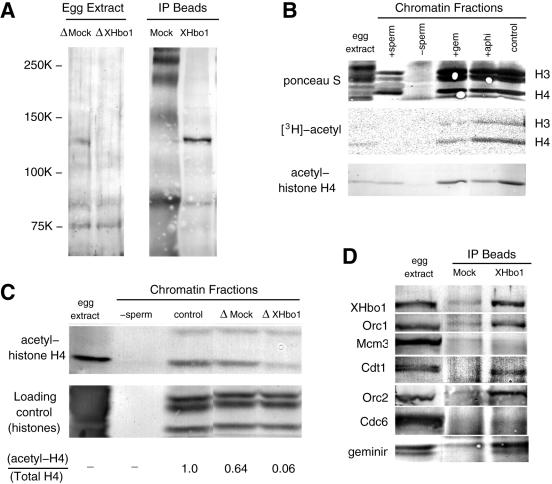
FIG. 6.
Xenopus egg extracts contain an Hbo1 protein (XHbo1) that is required for chromatin-bound histone H4 acetylation and is associated with Orc1, Orc2, Cdt1, and geminin. (A) Immunoprecipitation and immunodepletion of a 124-kDa protein in egg extracts with anti-XHbo1 peptide antibody. Interphase egg extracts were treated with preimmune (ΔMock) or anti-XHbo1 peptide 1 (ΔXHbo1) antibodies conjugated to recombinant protein A beads. The depleted extracts were then subjected to sodium dodecyl sulfate (SDS)-PAGE (egg extract). Similarly, the immunoprecipitates from the preimmune (Mock) and anti-peptide 1 (Xhbo1) beads were also subjected to SDS-PAGE (IP Beads). XHbo1 was detected by probing immunoblots with anti-XHbo1 peptide 2 antibody. (B) Acetylation of histone H4 bound to chromatin in Xenopus egg extracts. Sperm chromatin was incubated with 4.5 MBq of [3H]acetyl-coenzyme A in the absence (control) or the presence of GFP-geminin (+gem) or aphidicolin (+aphi). After 60 min, chromatin fractions were isolated and subjected to SDS-PAGE, followed by autoradiography to detect tritium-labeled proteins. A negative control for the chromatin factions was obtained by incubating the extracts in the absence of sperm chromatin (−sperm). Sperm chromatin was also analyzed for acetylation of histone H4 bound to sperm (+sperm). The acetylation of histone H4 was detected by immunoblotting with anti-acetyl-histone H4 antiserum. (C) Sperm chromatin was incubated in untreated (control), mock-depleted (ΔMock), or XHbo1-depleted (ΔXHbo1) extracts in the presence of aphidicolin for 45 min, and chromatin fractions were separated. A negative control for the chromatin factions is shown (−sperm). Histone H4 acetylation was detected by immunoblotting (acetyl-histone H4) and protein staining of histones with Ponceau S is shown as loading control. Both immunostaining and protein-staining intensities were quantified, and the relative amount of acetylated histone H4 in total histone H4 is shown (acetyl H4/total H4). (D) Treated extracts, prepared as in panel A, were immunoblotted with antibody against XHbo1, Orc1, Mcm3, Cdt1, Orc2, Cdc6, and geminin.
We reasoned that if p124 is a Xenopus Hbo1, then its immunodepletion should decrease the histone acetyltransferase activity of the egg extracts. First, we examined the state of histone acetylation in normal egg extracts and found that chromatin-bound histone H4, but not H3, was hyperacetylated before DNA replication even in the presence of geminin, showing that the acetylation of H4 precedes the assembly of the pre-RC (Fig. 6B). When XHbo1 was then immunodepleted from extracts, the total acetylation of histone H4 was also diminished (Fig. 6C). Thus, these results are consistent with our assumption that the depletion of p124 actually depletes XHbo1 and its histone acetylation activity.
An authentic Xenopus Hbo1 protein would also be predicted to coimmunoprecipitate with Orc1 or other components of the pre-RC, since human Hbo1 coimmunoprecipitates with Orc1 and Mcm2. Consistent with this prediction, we found that Orc1 and Orc2 are coimmunoprecipitated with XHbo1 antibody (Fig. 6D and data not shown). In addition, Cdt1 and geminin are also coimmunoprecipitated with p124 (Fig. 6D). Thus, the composition of the immunoprecipitates is also consistent with p124 representing XHbo1.
Taken together, these results argue that our antibodies against XHbo1 are detecting and immunodepleting a Xenopus Hbo1-like protein with histone H4 acetyltransferase activity. The predicted amino acid sequence of XHbo1a would express a protein of about 70 kDa, which is smaller than 124 kDa. Thus, based on the immunoreactivity of the different antibodies and the properties of the immunoprecipitates, we presume p124 to be a variant or modified form of Xenopus Hbo1.
Depletion of XHbo1 prevents pre-RC assembly.
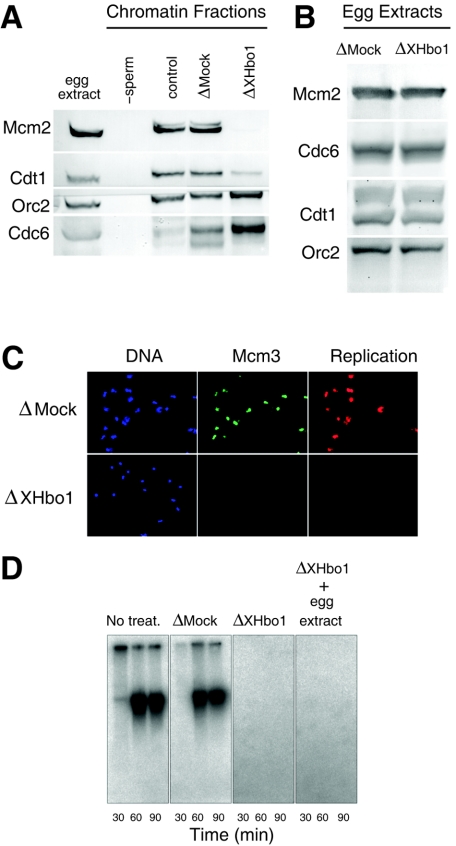
We next examined the chromatin binding of pre-RC components in extracts depleted of XHbo1, compared with extracts that were mock depleted (Fig. 7). Consistent with the results of inhibiting Hbo1 expression in human cells, we found that the chromatin binding of Mcm2 was diminished in XHbo1-depleted extracts. But the binding of Orc2 was slightly increased in the depleted extracts, and the amounts of histones bound to the chromatin fractions were essentially unchanged by the depletion of XHbo1 (Fig. 6C). In addition, there was an increase in the binding of Cdc6 (Fig. 7A). The lack of Mcm2 binding was not due to a decrease in pre-RC components, since the amounts of Mcm2, Orc2, Cdc6, and Cdt1 remained similar in both mock- and XHbo1-depleted extracts (Fig. 7B). Interestingly, we also found that the chromatin binding of Cdt1 was specifically decreased in XHbo1-depleted extracts (Fig. 7A), suggesting that Hbo1 might act through Cdt1 to regulate Mcm2-7 loading in Xenopus eggs. This differs from the mammalian cell results, where we observed a strong inhibition of Mcm2 and Mcm6 loading without a significant decrease in chromatin-bound Cdt1 (Fig. 1B; see Discussion).
FIG. 7.
Depletion of XHbo1 inhibits the chromatin binding of Mcm2 and DNA replication. (A) Sperm chromatin was incubated in mock-depleted (ΔMock) or XHbo1-depleted (ΔXHbo1) extracts in the presence of aphidicolin for 45 min, and chromatin fractions were separated and analyzed by immunoblotting with specific antibodies for Mcm2, Cdt1, Orc2, and Cdc6. A negative control for the chromatin fractions was obtained by incubating the extracts in the absence of sperm chromatin (−sperm). (B) Pre-RC components remain in the egg extracts after immunodepletion of XHbo1. Mock-depleted (ΔMock) and XHbo1-depleted (ΔXHbo1) extracts prepared as described in the legend to Fig. 6 were analyzed for Mcm2, Cdc6, Cdt1, and Orc2 by immunoblotting. (C) Sperm chromatin was incubated with Cy3-dCTP in mock-depleted (ΔMock) or XHbo1-depleted (ΔXHbo1) extracts for 60 min, and chromatin fractions were isolated in the presence of 0.25% NP-40. After fixation, samples were visualized by indirect immunofluorescence with Hoechst (DNA), anti-XMcm3 antibody (Mcm3), or Cy3 (Replication). (D) The replication activity was measured as the incorporation of [α-32P]dCTP into sperm DNA with various extracts: untreated (No treat.), mock depleted (ΔMock), XHbo1 depleted (ΔXHbo1), or XHbo1 depleted plus a 1/10 volume of untreated extracts (ΔXhbo1 + egg extract). Replication products were subjected to agarose gel electrophoresis, followed by autoradiography.
As a consequence of failing to load Mcm2-7, the DNA replication activity of XHbo1-depleted extracts was almost completely lost, as monitored by the incorporation of either Cy3-dCTP or [32P]dCTP into chromatin DNA (Fig. 7C and D). The depletion of XHbo1 did not affect the formation of nuclei in the extracts (data not shown), which is a critical step for the initiation reaction. We also found that replication activity could not be restored by the addition of a 1/10 volume of untreated egg extracts to the depleted ones (Fig. 7D). This result suggests that the lack of replication activity was not simply due to the depletion of some essential factor in the extracts, but that depleting XHbo1 from the extracts switched off licensing activity. Assuming that the acetylation of some protein is required for maintaining licensing activity, a threshold concentration of Hbo1 may be required for counteracting deacetylation activities in the extracts.
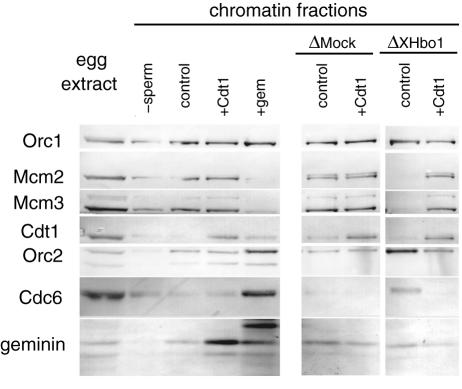
Cdt1 and geminin, but not Mcm3 or Cdc6, were coimmunoprecipitated with XHbo1 in the extracts (Fig. 6D). Reasoning that this might affect the activity of these proteins in the extracts, we tested whether an excess amount of recombinant Cdt1 could rescue the deficiency in the licensing activity of Hbo1-depleted extracts. Figure 8 demonstrates that the addition of recombinant Cdt1 rescued licensing activity, as measured by the chromatin binding of Mcm2 and Mcm3. At the same time, the chromatin binding of Cdc6 was suppressed by the loading of Mcm2-7 onto chromatin. These results suggest that Cdt1 by itself, or together with other interacting proteins, may be inactivated in Hbo1-depleted extracts.
FIG. 8.
Recombinant Cdt1 restores the chromatin binding of Mcm2-7 in the absence of XHbo1. Sperm chromatin was incubated in untreated, mock-depleted (ΔMock), or Hbo1-depleted (ΔXHbo1) extracts with addition of buffer alone (control), recombinant Xenopus Cdt1 (+Cdt1) or GFP-tagged Xenopus geminin (+gem). The chromatin fractions were isolated and immunoblotted as described in the legend to Fig. 6D.
Hbo1 acetylates mammalian pre-RC components in vitro.
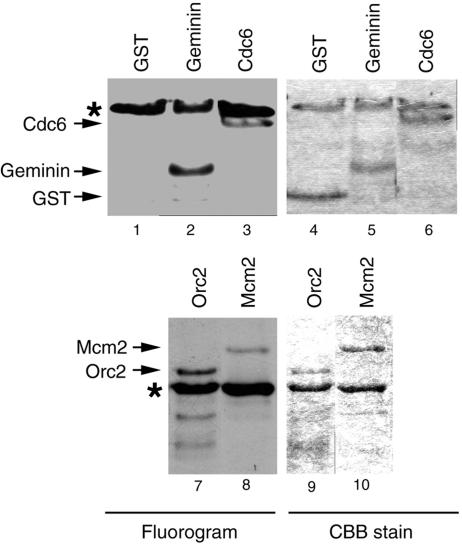
Previous studies have found that human Hbo1 physically interacts with Orc1 (20), Orc2 (our unpublished results), and Mcm2 (10); in the present work, we found that Cdt1 and geminin are coimmunoprecipitated with XHbo1. Together, these results raise the possibility that one or more of the pre-RC components and their regulators may be physiological targets of Hbo1 acetylation. To test the feasibility of this hypothesis, we assayed the ability of recombinant human Hbo1 to acetylate bacterially expressed human Orc2, Mcm2, Chinese hamster Cdc6, and human geminin. As shown in Fig. 9, Hbo1 acetylated Orc2 (lanes 7 and 9), Mcm2 (lanes 8 and 10), geminin (lanes 2 and 5), and Cdc6 (lanes 3 and 6), in addition to autoacetylating itself. An equivalent amount of control GST (lanes 1 and 4) was not acetylated. The in vitro acetylation of Orc2, Mcm2, Cdc6, and geminin supports the intriguing possibility that acetylation of these factors by Hbo1 might regulate the initiation of DNA replication.
FIG. 9.
Acetylation of pre-RC proteins by Hbo1. Two micrograms of recombinant human Hbo1 protein was incubated with His6-geminin (lanes 2 and 5), His6-Cdc6 (lanes 3 and 6), GST-Orc2 (lanes 7 and 9), and His6-Mcm2 (lanes 8 and 10) in the presence of [3H]acetyl-CoA; the proteins were separated on 10% SDS-PAGE gels. The gels were stained with Coomassie (CBB) stain (lanes 4 to 6, 9, and 10), followed by fluorography (Fluorogram) (lanes 1 to 3, 7, and 8). GST protein (lanes 1 and 4) served as a negative control. Bands marked by asterisks identify labeled Hbo1 proteins produced by its autoacetylation.
DISCUSSION
Histone acetylation is most prominently associated with transcription, where its roles in regulating gene expression are well known. Here, we propose that the acetylation activity of Hbo1 facilitates the chromatin recruitment and/or maintenance of the Mcm2-7 complex necessary for pre-RC assembly and replication licensing. The experiments described above show that the downregulation of Hbo1 expression in human cell lines results in a loss of Mcm2-7 loading during the time of normal pre-RC assembly in G1 phase. Furthermore, the depletion of XHbo1 from Xenopus egg extracts blocks pre-RC formation; this arrest can be overcome by the addition of excess Cdt1.
The initiation of DNA replication in human tissue culture cells and Xenopus egg cells differs in several respects. For instance, because of their rapid replication cycle, there are a greater number of active origins in Xenopus eggs than in human somatic cells. Abundant geminin protein is stored in an inactive form in interphase Xenopus eggs, whereas most geminin is subjected to degradation at G1 phase in human cells (47). Despite these differences, it is striking that Hbo1 is required for Mcm2-7 binding to chromatin in both experimental systems. This argues that Hbo1 acetylation is involved in an evolutionarily conserved pathway in replication licensing. We also detected some differences between the two systems. In Xenopus extracts, the depletion of XHbo1 partially reduced the chromatin association of Cdt1 (Fig. 7A), while in mammalian cells Mcm2-7, loading was inhibited without a major change in Cdt1 association (Fig. 1B). Thus, the regulation of pre-RC assembly by Hbo1 likely involves additional targets. Our results suggest that potential targets include both histones, as well as nonhistone components of the pre-RC.
Histone acetylation and pre-RC assembly.
In vitro, histones are preferentially acetylated by Hbo1 compared with the other nonhistone substrates tested (M. Iizuka and M. M. Smith, unpublished data). Several lines of evidence support a role for histone acetylation in replication licensing. In budding yeast, shifting the balance of histone modification toward hyperacetylation by deleting the histone deacetylase Rpd3 accelerates the timing of replication initiation, consistent with a positive role for acetylation in licensing (4, 46). Conversely, mutation of the acetylated lysines in histone H4 results in a prolonged S phase, also consistent with a positive role for acetylation and a direct link with histone modification (30). Previous findings also link acetylation to origin activity (1, 38, 41). Here, we found that the depletion of XHbo1 from Xenopus egg extracts results in the deacetylation of histone H4 in the chromatin (Fig. 6C). Furthermore, downregulation of Hbo1 in mammalian cells by antisense RNA expression or siRNA also leads to hypoacetylation of histone H4 in mammalian cell lines (Iizuka and Smith, unpublished). There are several mechanisms by which histone acetylation could facilitate pre-RC assembly. First, acetylation could be important in establishing a chromatin conformation conducive to the recruitment of Mcm2-7 or its assembly into the pre-RC. Alternatively, acetylation could facilitate direct protein-protein interactions between Mcm2-7 or other pre-RC components and the histone tails. In either case, excess Cdt1 might drive pre-RC assembly in the face of an unfavorable chromatin context.
Nonhistone acetylation and pre-RC assembly.
An increasing number of nonhistone proteins are being recognized as substrates for histone acetyltransferases (48). At present, the cellular nonhistone targets of Hbo1 have not been determined, but we found at least that in vitro, Hbo1 can acetylate Orc2, Mcm2, Cdc6, and geminin. The rescue of the licensing activity of XHbo1-depleted egg extracts by recombinant Cdt1 makes geminin a particularly attractive Hbo1 target candidate. In interphase extracts, geminin is present in excess of Cdt1, but geminin by itself is inactivated through ubiquitination and deubiquitination at the exit of M phase (23). Inactivated geminin in the extracts is reactivated only after its nuclear accumulation, and its inactivation does not accompany any changes in its molecular mass (19). It is not known how geminin is inactivated, but we speculate that inactivation may be mediated by acetylation of geminin itself or of other proteins involved in geminin inactivation. Depletion of XHbo1 would then lead to the deacetylation and activation of geminin, causing a block to licensing which could be overcome by the addition of excess recombinant Cdt1. We tested this hypothesis by depleting geminin before depleting Hbo1 from the extracts; in one experiment, this restored licensing in the Hbo1-depleted extracts. However, this experiment was difficult to reproduce, since the double depletion of geminin and XHbo1 from the extracts generally led to almost complete depletion of Cdt1 from the extracts as well.
While the molecular mechanisms remain to be determined, our findings with human and Xenopus egg extracts show that Hbo1-mediated acetylation is nevertheless an important mechanism for regulating the initiation of DNA replication in vertebrates. Protein phosphorylation is well known to play a central role in regulating DNA replication (18); however, relatively little is known about other protein modifications. Thus, the identification of Hbo1 as a protein acetyltransferase critical for pre-RC assembly defines a novel and previously unrecognized pathway in DNA replication licensing. Our results have significant implications for translational research as well, including the potential development of drugs to control cell proliferation by specifically blocking Hbo1 activity.
Acknowledgments
This work was supported by grants from the National Institute of Health to M.M.S (GM60444) and Grants-in-Aid for Scientific Research on Priority Area (A) from the Ministry of Education, Science, Sports and Culture, Japan, to H.T. M.I. was supported in part by UVA P30 CA44579 and the Kincaid Charitable Trust.
We thank Bruce Stillman, David Allis, Hideo Nishitani, Mark Alexandrow, Daniel Engel, and Anindya Dutta for reagents.
REFERENCES
- 1.Aggarwal, B. D., and B. R. Calvi. 2004. Chromatin regulates origin activity in Drosophila follicle cells. Nature 430:372-376. [DOI] [PubMed] [Google Scholar]
- 2.Alexandrow, M. G., and H. L. Moses. 1998. c-myc-enhanced S phase entry in keratinocytes is associated with positive and negative effects on cyclin-dependent kinases. J. Cell. Biochem. 70:528-542. [PubMed] [Google Scholar]
- 3.Alexandrow, M. G., and J. L. Hamlin. 2004. Cdc6 chromatin affinity is unaffected by serine-54 phosphorylation, S-phase progression, and overexpression of cyclin A. Mol. Cell. Biol. 24:1614-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aparicio, J. G., C. J. Viggiani, D. G. Gibson, and O. M. Aparicio. 2004. The Rpd3-Sin3 histone deacetylase regulates replication timing and enables intra-S origin control in Saccharomyces cerevisiae. Mol. Cell. Biol. 24:4769-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell, S. P., and A. Dutta. 2002. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71:333-374. [DOI] [PubMed] [Google Scholar]
- 6.Bell, S. P., and B. Stillman. 1992. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature 357:128-134. [DOI] [PubMed] [Google Scholar]
- 7.Blow, J. J., and A. Dutta. 2005. Preventing re-replication of chromosomal DNA. Nat. Rev. Mol. Cell Biol. 6:476-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blow, J. J., and R. A. Laskey. 1988. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature 332:546-548. [DOI] [PubMed] [Google Scholar]
- 9.Brown, J. A., S. G. Holmes, and M. M. Smith. 1991. The chromatin structure of Saccharomyces cerevisiae autonomously replicating sequences changes during the cell division cycle. Mol. Cell. Biol. 11:5301-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke, T. W., J. G. Cook, M. Asano, and J. R. Nevins. 2001. Replication factors MCM2 and ORC1 interact with the histone acetyltransferase HBO1. J. Biol. Chem. 276:15397-15408. [DOI] [PubMed] [Google Scholar]
- 11.Coleman, T. R., P. B. Carpenter, and W. G. Dunphy. 1996. The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell 87:53-63. [DOI] [PubMed] [Google Scholar]
- 12.Danis, E., K. Brodolin, S. Menut, D. Maiorano, C. Girard-Reydet, and M. Méchali. 2004. Specification of a DNA replication origin by a transcription complex. Nat. Cell Biol. 6:721-730. [DOI] [PubMed] [Google Scholar]
- 13.Diffley, J. F. 2004. Regulation of early events in chromosome replication. Curr. Biol. 14:R778-R786. [DOI] [PubMed] [Google Scholar]
- 14.Donovan, S., J. Harwood, L. S. Drury, and J. F. Diffley. 1997. Cdc6p-dependent loading of Mcm proteins onto pre-replicative chromatin in budding yeast. Proc. Natl. Acad. Sci. USA 94:5611-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 16.Gavin, K. A., M. Hidaka, and B. Stillman. 1995. Conserved initiator proteins in eukaryotes. Science 270:1667-1671. [DOI] [PubMed] [Google Scholar]
- 17.Hardy, S., M. Kitamura, T. Harris-Stansil, Y. Dai, and M. L. Phipps. 1997. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 71:1842-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henneke, G., S. Koundrioukoff, and U. Hubscher. 2003. Multiple roles for kinases in DNA replication. EMBO Rep. 4:252-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgson, B., Li, A., S. Tada, and J. J. Blow. 2002. Geminin becomes activated as an inhibitor of Cdt1/RLF-B following nuclear import. Curr. Biol. 12:678-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iizuka, M., and B. Stillman. 1999. Histone acetyltransferase HBO1 interacts with the ORC1 subunit of the human initiator protein. J. Biol. Chem. 274:23027-23034. [DOI] [PubMed] [Google Scholar]
- 21.Kelly, T. J., and G. W. Brown. 2000. Regulation of chromosome replication. Annu. Rev. Biochem. 69:829-880. [DOI] [PubMed] [Google Scholar]
- 22.Kubota, Y., and H. Takisawa. 1993. Determination of initiation of DNA replication before and after nuclear formation in Xenopus egg cell free extracts. J. Cell Biol. 123:1321-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, A., and J. J. Blow. 2004. Non-proteolytic inactivation of geminin requires CDK-dependent ubiquitination. Nat. Cell Biol. 6:260-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, A., and J. J. Blow. 2005. Cdt1 downregulation by proteolysis and geminin inhibition prevents DNA re-replication in Xenopus. EMBO J. 24:395-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipford, J. R., and S. P. Bell. 2001. Nucleosomes positioned by ORC facilitate the initiation of DNA replication. Mol. Cell 7:21-30. [DOI] [PubMed] [Google Scholar]
- 26.Lu, M. J., C. A. Dadd, C. A. Mizzen, C. A. Perry, D. R. McLachlan, A. T. Annunziato, and C. D. Allis. 1994. Generation and characterization of novel antibodies highly selective for phosphorylated linker histone H1 in Tetrahymena and HeLa cells. Chromosoma 103:111-121. [DOI] [PubMed] [Google Scholar]
- 27.Machida, Y. J., J. L. Hamlin, and A. Dutta. 2005. Right place, right time, and only once: replication initiation in metazoans. Cell 123:13-24. [DOI] [PubMed] [Google Scholar]
- 28.Maiorano, D., J. Moreau, and M. Méchali. 2000. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature 404:622-625. [DOI] [PubMed] [Google Scholar]
- 29.McGarry, T. J., and M. W. Kirschner. 1998. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93:1043-1053. [DOI] [PubMed] [Google Scholar]
- 30.Megee, P. C., B. A. Morgan, B. A. Mittman, and M. M. Smith. 1990. Genetic analysis of histone H4: essential role of lysines subject to reversible acetylation. Science 247:841-845. [DOI] [PubMed] [Google Scholar]
- 31.Melixetian, M., A. Ballabeni, L. Masiero, P. Gasparini, R. Zamponi, J. Bartek, J. Lukas, and K. Helin. 2004. Loss of geminin induces rereplication in the presence of functional p53. J. Cell Biol. 165:473-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Méndez, J., and B. Stillman. 2000. Chromatin association of human origin recognition complex, Cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol. Cell. Biol. 20:8602-8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mihaylov, I. S., T. Kondo, L. Jones, S. Ryzhikov, J. Tanaka, J. Zheng, L. A. Higa, N. Minamino, L. Cooley, and H. Zhang. 2002. Control of DNA replication and chromosome ploidy by geminin and cyclin A. Mol. Cell. Biol. 22:1868-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nevins, J. R., J. DeGregori, L. Jakoi, and G. Leone. 1997. Functional analysis of E2F transcription factor. Methods Enzymol. 283:205-219. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen, V. Q., Co, C., and J. J. Li. 2001. Cyclin-dependent kinases prevent DNA re-replication through multiple mechanism. Nature 411:1068-1073. [DOI] [PubMed] [Google Scholar]
- 36.Nishitani, H., Z. Lygerou, T. Nishimoto, and P. Nurse. 2000. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature 404:625-628. [DOI] [PubMed] [Google Scholar]
- 37.Pagano, M., A. M. Theodoras, S. W. Tam, and G. F. Draetta. 1994. Cyclin D1-mediated inhibition of repair and replicative DNA synthesis in human fibroblasts. Genes Dev. 8:1627-1639. [DOI] [PubMed] [Google Scholar]
- 38.Pappas, D. L., Jr., R. Frisch, and M. Weinreich. 2004. The NAD(+)-dependent Sir2p histone deacetylase is a negative regulator of chromosomal DNA replication. Genes Dev. 18:769-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxena, S., P. Yuan, S. K. Dhar, T. Senga, D. Takeda, H. Robinson, S. Kornbluth, K. Swaminathan, and A. Dutta. 2004. A dimerized coiled-coil domain and an adjoining part of geminin interact with two sites on Cdt1 for replication inhibition. Mol. Cell 15:245-258. [DOI] [PubMed] [Google Scholar]
- 40.Simpson, R. T. 1990. Nucleosome positioning can affect the function of a cis-acting DNA element in vivo. Nature 343:387-389. [DOI] [PubMed] [Google Scholar]
- 41.Stedman, W., Z. Deng, F. Lu, and P. M. Lieberman. 2004. ORC, MCM, and histone hyperacetylation at the Kaposi's sarcoma-associated herpesvirus latent replication origin. J. Virol. 78:12566-12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suomalainen, M., M. Y. Nakano, S. Keller, K. Boucke, R. P. Stidwill, and U. R. Greber. 1999. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J. Cell Biol. 144:657-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tada, S., A. Li, D. Majorano, M. Méchali, and J. J. Blow. 2001. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat. Cell Biol. 3:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The MGC Project Team. 2004. The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC). Genome Res. 14:2121-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Utley, R. T., and J. Côté. 2003. The MYST family of histone acetyltransferases. Curr. Top. Microbiol. Immunol. 274:203-236. [DOI] [PubMed] [Google Scholar]
- 46.Vogelauer, M., L. Rubbi, I. Lucas, B. J. Brewer, and M. Grunstein. 2002. Histone acetylation regulates the time of replication origin firing. Mol. Cell 10:1223-1233. [DOI] [PubMed] [Google Scholar]
- 47.Wohlschlegel, J. A., B. T. Dwyer, S. K. Dhar, C. Cvetic, J. C. Walter, and A. Dutta. 2000. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science 290:2309-2312. [DOI] [PubMed] [Google Scholar]
- 48.Yang, X. J. 2004. The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 32:959-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida, K., H. Takisawa, and Y. Kubota. 2005. Intrinsic nuclear import activity of geminin is essential to prevent re-initiation of DNA replication in Xenopus eggs. Genes Cells 10:63-73. [DOI] [PubMed] [Google Scholar]
- 50.Zhu, W., Y. Chen, and A. Dutta. 2004. Rereplication by depletion of geminin is seen regardless of p53 status and activates a G2/M checkpoint. Mol. Cell. Biol. 24:7140-7150. [DOI] [PMC free article] [PubMed] [Google Scholar]