Abstract
The PKC1-associated mitogen-activated protein (MAP) kinase pathway of Saccharomyces cerevisiae regulates cell integrity by controlling the actin cytoskeleton and cell wall synthesis. Activation of PKC1 occurs via the GTPase RHO1 and the kinase pair PKH1 and PKH2. Here we report that YPK1 and YPK2, an essential pair of homologous kinases and proposed downstream effectors of PKH and sphingolipids, are also regulators of the PKC1-controlled MAP kinase cascade. ypk mutants display random distribution of the actin cytoskeleton and severely reduced activation of the MAP kinase MPK1. Upregulation of the RHO1 GTPase switch or the PKC1 effector MAP kinase pathway suppresses the growth and actin defects of ypk cells. ypk lethality is also suppressed by overexpression of an uncharacterized gene termed TUS1. TUS1 is a novel RHO1 exchange factor that contributes to cell wall integrity-mediated modulation of RHO1 activity. Thus, TUS1 and the YPKs add to the growing complexity of RHO1 and PKC1 regulation in the cell integrity signaling pathway. Furthermore, our findings suggest that the YPKs are a missing link between sphingolipid signaling and the cell integrity pathway.
Spatial control of cell growth in the budding yeast Saccharomyces cerevisiae involves cell wall synthesis and organization of the actin cytoskeleton. A polarized actin cytoskeleton targets secretion to the growth, or bud, site and is thus essential for the establishment and the maintenance of polarized growth (11, 40). The yeast cell wall, composed of mannoproteins, β-1,6-glucan, β-1,3-glucan, and chitin, determines the cell shape and provides rigidity. Both the actin cytoskeleton and the cell wall are dynamic structures that are constantly remodeled and reorganized, in response to growth signals or environmental stress, to ensure the integrity of the yeast cell (15, 33, 52, 57).
In yeast, protein kinase C (PKC1) activates a mitogen-activated protein (MAP) kinase cascade composed of BCK1 (MAPKKK), the redundant MKK1 and MKK2 proteins (MAPKK), and MPK1 (MAPK) (22, 24). Activation of this cascade occurs in response to hypotonic or heat shock, but also during periods of polarized growth (14, 31, 67). This MAP kinase cascade ultimately controls actin organization and transcription of cell wall biosynthesis genes (26, 27, 43, 68). Since both of these readouts function to maintain cellular integrity under normal as well as stress conditions, the pathway(s) controlling and comprising PKC1 and its effector MAP kinase cascade is often termed the cell integrity signaling pathway.
The organization of the yeast actin cytoskeleton and thus polarized growth is also regulated by a family of Rho-like GTPases (52). Members of the Rho family of small GTPases are also key regulators of the actin cytoskeleton in mammalian cells (23, 30). In yeast, the Rho family includes CDC42 and RHO1 through -4 (52, 59). RHO1 and its nonessential homologue RHO2 control bud growth via five downstream effectors (9, 21, 52). RHO1 binds and activates PKC1, which in turn controls the above-mentioned MAP kinase cascade (32, 44). RHO1 also binds and activates the integral plasma membrane protein FKS1 (β-1,3-glucan synthase) to control cell wall synthesis directly (17, 49). Furthermore, RHO1 binds to BNI1, a formin family member; to SKN7, a two-component signaling protein; and to SEC3, a spatial landmark for polarized exocytosis (1, 21, 36).
The cycling between the inactive (GDP-bound) and active (GTP-bound) states of Rho type GTPases is catalyzed by three types of proteins: GDP/GTP exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (GDIs). Exchange factors activate Rho proteins by exchanging GDP for GTP. Most Rho GEFs display a characteristic architecture, a tandem motif consisting of a Dbl homology (DH) domain followed by a pleckstrin homology (PH) domain (65). The catalytic DH domain binds the nucleotide-free form of the GTPase, thereby facilitating the GDP-to-GTP exchange. The polyphosphoinositide-binding PH domain may contribute to the localization of the GEF (41, 70).
RHO1 is activated, via the exchange factor ROM2, by at least two separate inputs, the essential TOR2 kinase and cell wall defects (7, 51). tor2 mutant cells display random distribution of the actin cytoskeleton and a severe decrease in exchange activity toward RHO1 (25, 51, 53). Cell wall defects are sensed and signaled to ROM2 by plasma membrane proteins of the WSC family (15, 20, 48, 61).
PKH2 and possibly PKH1, the yeast homologues of mammalian 3"-phosphoinositide-dependent kinase 1 (PDK1), phosphorylate and thereby activate PKC1 (28). It remains to be determined whether PKHs activate PKC1 in conjunction with or independently of RHO1. PKH1 and PKH2 have also been proposed to act upstream of the essential pair of homologous kinases YPK1 and YPK2 (yeast protein kinases 1 and 2) (10, 28). The functionally redundant YPK genes were originally isolated on the basis of their sequence homology to PKC isozymes (12). The YPKs are homologues of mammalian serum-and glucocorticoid-induced kinase (SGK) and display substrate specificity similar to that of protein kinase B (PKB) (10). The cellular function of the YPKs is unknown, although YPK1 has recently been linked to sphingolipid signaling (58) (see Discussion).
In mammalian cells, the PDK1-PKB signaling cascade downstream of phosphatidylinositol 3-kinase mediates the physiological effects of insulin and other growth factors. PDK1 has been shown to phosphorylate a conserved residue within the activation loop of several members of the AGC subfamily of protein kinases, including PKB, PKC isozymes, p70 S6 kinase, and SGK (35, 46, 60). SGK is controlled at the transcriptional level by serum and glucocorticoid hormones (64) and is activated by cyclic AMP and insulin (47). SGK may be implicated in the regulation of sodium homeostasis and in the response to hyperosmotic stress (5, 13). Recent findings indicate that SGK acts in concert with PKB to mediate the readouts of phosphatidylinositol 3-kinase signaling, such as cell survival and cell cycle progression (8).
Here we present evidence that the YPKs are activators of the PKC1 effector MAP kinase cascade. We also identify and characterize TUS1, an exchange factor for RHO1. Thus, YPK kinases and TUS1 are novel components of yeast cell integrity signaling.
MATERIALS AND METHODS
Strains, plasmids, and media.
The S. cerevisiae strains and plasmids used in this study are listed in Tables 1 and 2, respectively. All strains, except EGY191 (see “Two-hybrid assays”), are isogenic derivatives of JK9-3da or TB50a. Rich media (yeast extract-peptone-dextrose [YPD] or YPGal/Gly) or synthetic minimal media (SD, SGal/Gly, or SGal/Raff) complemented with the appropriate nutrients for plasmid maintenance were as described previously (4, 55). Sodium dodecyl sulfate (SDS) was added to the medium to a final concentration of 0.005% from a stock of 10% in water (7).
TABLE 1.
Strains used in this study
| Strain | Genotype |
|---|---|
| JK9-3da/α | MATa/MATα leu2-3,112/leu2-3,112 ura3-52/ura3-52 trp1/trp1 his4/his4 rme1/rme1 HMLa/HMLa |
| JK9-3da | MATaleu2-3,112 ura3-52 trp1 his4 rme1 HMLa |
| TB50a | MATaleu2-3,112 ura3-52 trp1 his3 rme1 HMLa |
| MB119 | JK9-3daade2 tor2::ADE2-3/YCplac33::tor2-21tscwh41::kanMX2 |
| PA109-1C | TB50abck1::HIS3MX6 |
| SD40-1A | TB50arom2::HIS3MX6 |
| SH121 | JK93daade2 tor2::ADE2-3/YCplac111::tor2-21ts |
| TS24-6D | TB50atus1::HIS3MX6 |
| TS27-4C | JK9-3daade2 tor2::ADE2-3/YCplac111::tor2-21tstus1::kanMX4 |
| TS38-1C | TB50aypk1::kanMX4 |
| TS40-5B | TB50arho2::kanMX4 |
| TS43-2B | TB50arho2::kanMX4 tus1::HIS3MX6 |
| TS45-1A | TB50ampk1::TRP1 |
| TS48-3A | TB50ampk1::TRP1 tus1::HIS3MX6 |
| TS49 | JK9-3da/α tus1::kanMX4/tus1::kanMX4 |
| TS57-2B | TB50aypk1::kanMX4 ypk2::HIS3MX6/pGal-YPK1 (pTS72) |
| TS63-2B | TB50arom2::HIS3MX6 tus1::kanMX4 |
| TS80-6D | TB50acwh41::kanMX2 tus1::HIS3MX6 |
| TS92-2D | TB50aade2 tor2::ADE2-3/YCplac111::tor2-21tscwh41::kanMX2 tus1::HIS3MX6 |
| TS99-5C | TB50aMPK1-HA3-kanMX6 |
| TS100-1B | TB50abck1::HIS3MX6 tus1::kanMX4 |
| TS105-1C | TB50aMPK1-HA3-kanMX6 ypk1::HIS3MX6 |
| TS147-3B | TB50α MPK1-HA3-kanMX6 ypk1::kanMX4 ypk2::HIS3MX6/pGal-YPK1 (pTS72) |
TABLE 2.
Plasmids used in this study
| Plasmid | Description (reference) |
|---|---|
| pGal-YPK1 | pTS72; expresses YPK1 under the control of the GAL1 promoter. Cloned as a 2.5-kb SalI fragment into YCplac111::GAL1 promoter (CEN LEU2) |
| pTUS1 | pTS38; 5.3-kb PstI-EcoRI fragment containing TUS1 in YEplac195 (2μm; URA3) |
| pROM2 | pAS30; ROM2 in YEplac195 (2μm; URA3) (51) |
| pRHO2 | pC-186; RHO2 (2μm; URA3) (39) |
| pPKC1∗ | PKC1R398P in YCp50 (CEN URA3) (44) |
| pBCK1∗ | BCK1-20 in pRS316 (CEN URA3) (37) |
| pMKK1∗ | MKK1S386P in YCplac33 (CEN URA3) (63) |
| pYPK3 | pTS84; 2-kb EcoRI-Asp718 fragment containing YPK3 in YEplac 195 (2μm; URA3) |
| pEG202::TUS1ΔN | pTS46; 2.9-kb EcoRI insert containing a fragment of TUS1 (TUS1 starting at Asn420; TUS1ΔN) in pEG202 (ADH1 promoter; 2μm; HIS3) |
| pJG4-5::RHO1 | pETE25; RHO1C206S in pJG4-5 (GAL1 promoter; 2μm; TRP1) (51) |
| pJG4-5::RHO1Q68H | pETE27; RHO1Q68H C206S in pJG4-5 (51) |
| pJG4-5::RHO1G22A | pAS92; RHO1G22A C206S in pJG4-5. Mutation changing Gly22 to Ala was introduced by PCR and verified by sequencing. RHO1G22A was cloned as a 0.7-kb EcoRI-XhoI fragment into pJG4-5. |
| pTS49 | 0.65-kb insert containing a fragment of TUS1 (Asn420 to Asp631) in pEG202; made by BamHI deletion of pTS46 |
| pTS50 | 1.1-kb insert containing a fragment of TUS1 (Asn420 to Glu773) in pEG202, made by XhoI deletion of pTS46 |
| pTS56 | 0.9-kb BglII fragment containing TUS1 DH domain plus 50 amino acids on each side (Gly425 to Thr721) in pEG203 (pEG202 derivative) |
| pGST-RHO1 | pAS53; RHO1 in pEG-KT (2μm; URA3 Leu2-d) (7) |
| pGST-TUS1DH | pTS55; 0.9-kb BglII fragment containing TUS1 DH domain plus 50 amino acids on each side (Gly425 to Thr721) in pGEX-2T (Amersham Pharmacia Biotech) |
Genetic techniques.
Restriction enzyme digests and ligations were performed according to standard methods. All enzymes and buffers were obtained commercially (Roche Diagnostics). Escherichia coli strains MH1 and DH5α were used for propagation and isolation of plasmids. Plasmid DNA was isolated as described previously (50). Yeast transformation was performed by the lithium acetate procedure (29). The entire open reading frames of TUS1, YPK1, and YPK2 were replaced by PCR-generated kanMX4 or HIS3MX6 cassettes, as described previously (38, 62). Disruptions were verified by PCR.
Actin staining.
Logarithmically growing cells were fixed in formaldehyde (3.7%) and potassium phosphate buffer (100 mM; pH 6.5) and stained with tetramethyl rhodamine isothiocyanate (TRITC)-phalloidin (Sigma) to visualize actin, as described previously (6).
Two-hybrid assays.
The interaction trap two-hybrid system was used (19). Bait (pEG202-based) and prey (pJG4-5-based) plasmids are described in Table 2. To assess β-galactosidase activities, strain EGY191 (MATα trp1 his3 ura3 2×lexAop-LEU2) containing the lexAop-lacZ reporter plasmid pSH18-34 was cotransformed with either pEG202 or a pEG202-derived plasmid expressing a LexA-BD (DNA binding domain) fusion protein and with either pJG4-5 or a pJG4-5-derived plasmid expressing an AD (activation domain) fusion protein. Several independent transformants of each combination were streaked onto SD-Ura-Trp-His plates and replica plated onto SGal/Raff-Ura-Trp-His/5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) plates.
GEF activity assay.
Glutathione S-transferase (GST)-RHO1 was prepared as described previously (7). GST and GST-TUS1DH were prepared as follows. GST- or GST-TUS1DH-expressing E. coli cells (XL1-Blue transformed with pGEX-2T or pGST-TUS1DH) were grown in ampicillin-containing Luria-Bertani medium, and protein expression was induced for 3 h by addition of isopropyl-β-d-thiogalactopyranoside (IPTG) (final concentration, 0.1 mM). Cells were harvested by centrifugation for 10 min at 3,000 × g, resuspended in ice-cold phosphate-buffered saline (PBS, comprising 53 mM Na2HPO4, 13 mM NaH2PO4, and 75 mM NaCl) containing 1 mM phenylmethylsulfonyl fluoride (PMSF), and lysed by three cycles (30 s each) of sonication (Sonicator Ultrasonic Processor XL; MISONIX Inc.). Cell debris was removed by centrifugation at 3,000 × g for 10 min, and Triton X-100 was added to the supernatant to a final concentration of 1%. The GST or GST-TUS1DH protein was isolated by incubating the extract with glutathione-Sepharose beads (Amersham Pharmacia Biotech) for 2 h. Beads were then washed five times with PBS containing 0.1% Triton X-100. Glutathione-Sepharose beads with bound GST-RHO1 (purified from yeast) were first washed with, and then resuspended in, reaction buffer (20 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 2.5 mM EDTA, 1 mM dithiothreitol, 1 mM PMSF) and incubated for 20 min at 25°C in the presence of 0.75 mM l-α-dimyristoylphosphatidylcholine (Sigma) and 10 μM GDP (69). Beads with bound GST or GST-TUS1DH were also washed and then resuspended in reaction buffer. Following the preloading with GDP, GST-RHO1 was aliquoted and beads with bound GST or GST-TUS1DH were added to the GTPase aliquots. Reactions were started by addition of 3 μM [35S]GTPγS (Amersham Pharmacia Biotech) and incubated for 5, 10, or 15 min. Reactions were stopped by adding 1 ml of ice-cold stop buffer (20 mM Tris-HCl [pH 7.5], 25 mM MgCl2, 100 mM NaCl). The diluted mixture was centrifuged and washed twice with stop buffer. Bound radioactivity was quantified by scintillation counting. As a control, GST or GST-TUS1DH was incubated by following the same protocol, but in the absence of GST-RHO1. Neither GST nor GST-TUS1DH bound any radioactivity under these conditions (data not shown).
MAP kinase activation assay.
To monitor activation and expression of the MAP kinase MPK1, a strain expressing C-terminally triple hemagglutinin (HA)-tagged MPK1 (MPK1-HA3) under the control of its own promoter was created. Tagging of chromosomal MPK1 was performed as described previously (38) and verified by PCR. The MPK1-HA3 was functional (data not shown).
MAP kinase activation assays were performed as described previously (42), with minor modifications. Cultures of exponentially growing cells (in YPD at 24°C) were shifted to 39°C for the indicated time. Harvest, lysis of cells with glass beads, and clearing of cell extracts were performed as described previously (42). Protein concentrations of extracts were determined by using the Bio-Rad microassay. Samples were denatured by addition of 6× SDS-polyacrylamide gel electrophoresis (PAGE) sample loading buffer and boiling at 95°C for 5 min. A total of 40 μg of protein (for anti-HA detection) or 80 μg of protein (for anti-activated MAPK detection) was loaded per lane for standard SDS-PAGE (10% acrylamide) and Western analysis (3). For detection, a mouse anti-HA antibody (clone 16B12, 1:10,000 dilution; BabCO) and mouse anti-MAP kinase, activated (clone MAPK-YT, 1:2,500 dilution; Sigma) were used. The anti-MAPK (YT) antibody was verified to specifically recognize activated MPK1 under heat stress conditions (data not shown). Primary antibodies were detected using a horseradish peroxidase-conjugated anti-mouse immunoglobulin G antibody (1:10,000 dilution; Amersham Pharmacia Biotech) with the ECL detection system (Amersham Pharmacia Biotech).
RESULTS
Activation of the RHO1 GTPase switch or the PKC1 effector MAP kinase cascade suppresses a YPK deficiency.
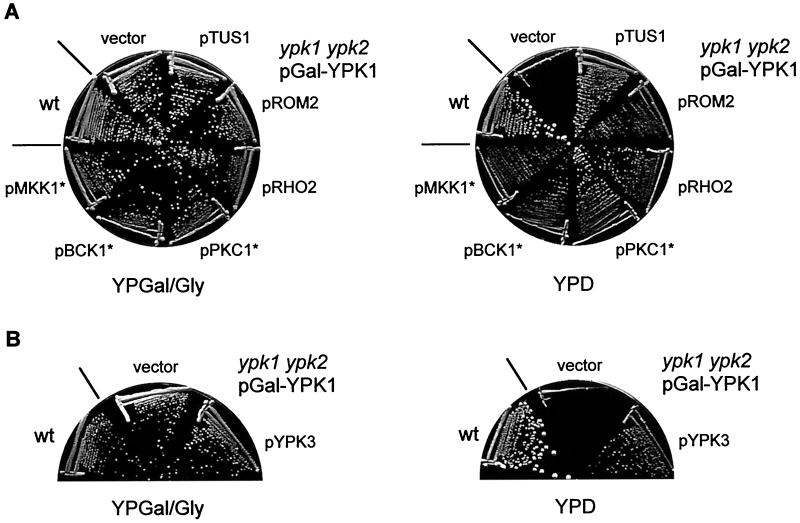
To investigate the essential cellular role of the two YPK kinases, a mutant was constructed lacking the genomic copies of YPK1 and YPK2 and containing a plasmid-borne copy of YPK1 expressed under the control of the glucose-repressible and galactose-inducible GAL1 promoter. This conditional mutant, referred to below simply as ypk, was able to grow only on a galactose-containing medium. Cells lacking YPK1 or YPK2 alone are viable, although ypk1 mutants have a severe growth defect (10, 12; also data not shown). Because the YPKs have been linked to the PKHs (10) which in turn have been linked to PKC1 (28), we first examined if upregulation of the PKC1 effector MAP kinase cascade or the RHO1 GTPase switch could suppress the growth defect of ypk cells. As shown in Fig. 1A, activation of the PKC1 effector MAP kinase cascade by expression of constitutively active PKC1, BCK1, or MKK1 (encoded by PKC1*, BCK1*, and MKK1*, respectively) suppressed the growth defect of ypk cells on glucose (YPD) medium. Similarly, activation of the RHO1 GTPase switch by overexpression of the GEF ROM2 or the RHO1-related GTPase RHO2 efficiently restored growth of ypk cells (Fig. 1A). We also found that overexpression of an uncharacterized gene termed TUS1 suppressed the ypk mutation (Fig. 1A). TUS1 (open reading frame YLR425w), encoding a protein of unknown function, had previously been isolated in our laboratory as a multicopy suppressor of a tor2 mutation (hence the name TUS1, for TOR2 unique function suppressor 1) (25; S. B. Helliwell, I. Howald, and M. N. Hall, unpublished data). As described below, TUS1 is a novel exchange factor (GEF) for RHO1. The growth defect of cells lacking only YPK1 (ypk1) was also suppressed by overexpression of TUS1 or by expression of PKC1* (data not shown). The above findings strongly suggest that the essential cellular role of the YPKs is to activate the cell integrity signaling pathway.
FIG. 1.
Activation of the RHO1 GTPase switch or the PKC1 effector MAP kinase cascade suppresses the growth defect of ypk cells. (A) Wild-type (wt) (TB50a) cells and ypk1 ypk2/pGal-YPK1 (TS57-2B) cells transformed with either empty vector, pTUS1, pROM2, pRHO2, pPKC1*, pBCK1*, or pMKK1* were streaked onto YPGal/Gly (galactose) and YPD (glucose) media and incubated at 37°C. (B) Overexpression of YPK3 restores growth of ypk cells. wt (TB50a) cells and ypk1 ypk2/pGal-YPK1 (TS57-2B) cells transformed with empty vector or pYPK3 were streaked onto YPGal/Gly and YPD media and incubated at 30°C.
Examination of the yeast genome sequence revealed a predicted Ser/Thr protein kinase homologous to YPK1 and YPK2 (approximately 40% identity in catalytic domains) that we termed YPK3 (open reading frame YBR028c). Overexpression of YPK3 also suppressed the ypk growth defect (Fig. 1B). Disruption of YPK3 did not confer a growth defect under standard growth conditions. Furthermore, ypk1 ypk3 and ypk2 ypk3 double-mutant cells grew like the single ypk1 or ypk2 mutant, respectively (data not shown).
TUS1 interacts genetically with components of the RHO1 GTPase switch and the PKC1 effector MAP kinase cascade.
As described above, the uncharacterized gene TUS1 was identified as a multicopy suppressor of the ypk growth defect (Fig. 1A). The TUS1 gene encodes a protein of 1,307 amino acids, with a predicted molecular size of approximately 150 kDa. To characterize the cellular function(s) of TUS1 and the mechanism by which multiple copies of TUS1 restore the growth of ypk cells, a mutant lacking TUS1 was created by replacing the entire TUS1 open reading frame with a PCR-generated marker cassette. Haploid tus1 cells displayed only a very mild growth defect and only at an elevated temperature. However, homozygous diploid tus1/tus1 cells were severely affected in growth at a high temperature (39°C) (Fig. 2A and B). It has been reported previously that components of the cell integrity pathway (e.g., WSC1) are more important in diploid cells (15).
FIG. 2.
Genetic interaction of TUS1 with components of the cell integrity pathway. (A) The tus1/tus1 mutant is defective in cell integrity signaling. Wild-type (wt) (JK9-3da/α) cells and tus1/tus1 (TS49) cells transformed with either empty vector, pROM2, pRHO2, or pPKC1* were streaked out onto YPD medium and incubated at 30 and 39°C. (B) Osmotic stabilization suppresses the growth defect of the tus1/tus1 mutant. wt (JK9-3da/α) and tus1/tus1 (TS49) cells were streaked out onto YPD medium and YPD medium containing 1 M sorbitol at 39°C. (C) wt (TB50a), tus1 (TS24-6D), rom2 (SD40-1A), tus1 rom2 (TS63-2B), rho2 (TS40-5B), tus1 rho2 (TS43-2B), bck1 (PA109-1C), tus1 bck1 (TS100-1B), mpk1 (TS45-1A), and tus1 mpk1 (TS48-3A) cells were streaked out onto YPD medium and incubated at 37°C. Strains were also streaked out onto YPD medium containing 1 M sorbitol at 37°C.
To investigate if tus1 cells are defective in cell integrity signaling, we performed a suppression analysis of tus1/tus1 diploid cells. Overexpression of ROM2, RHO1 (pGAL-HA-RHO1) (51), or RHO2, or expression of constitutively active PKC1, suppressed the growth defect of the tus1/tus1 mutant at 39°C (Fig. 2A; also data not shown), whereas expression of an activated allele of RHO3 (pRHO3*, RHO3Val25) or CDC42 (pCDC42*, CDC42Val12), or overexpression of RHO4 (pRHO4) (51), did not suppress the growth defect (data not shown). Lack of functionality of the cell integrity pathway often leads to cell lysis, which can be prevented by osmotic stabilization. Osmotic stabilization of tus1/tus1 cells by addition of 1 M sorbitol to the medium restored almost wild-type-like growth at 39°C (Fig. 2B). These findings indicate that tus1/tus1 cells are defective in cell integrity, most likely in the activation of RHO1 or the RHO1 effectors.
We also examined the effect of a tus1 mutation in combination with mutations affecting components of the cell integrity pathway, such as ROM2, RHO2, the PKC1 effector BCK1, and the PKC1-controlled MAP kinase MPK1. Whereas mutants with deletions of TUS1, ROM2, RHO2, BCK1, or MPK1 alone showed no or only mild growth defects, tus1 rom2, tus1 bck1, and tus1 mpk1 cells were unable to grow at the examined temperatures of 30 and 37°C (Fig. 2C; also data not shown). tus1 rho2 mutant cells grew poorly only at an elevated temperature (37°C) (Fig. 2C). The growth defect of double mutants was rescued, at least in part, by addition of an osmotic stabilizer (1 M sorbitol) to the growth medium (Fig. 2C). We also analyzed a genetic interaction between TUS1 and the ROM2 homologue ROM1, another RHO1 exchange factor. However, unlike tus1 rom2 mutants, no synthetic interaction was observed between tus1 and rom1 at any temperature (data not shown). The synthetic interactions between tus1 and rom2, rho2, bck1, and mpk1 further suggest that TUS1 is involved in cell integrity.
TUS1 is a novel exchange factor for RHO1.
TUS1 displays limited homology to the yeast ROM1 and ROM2 (45) and the mammalian NET1 Rho GEFs (2). The homology is restricted to the regions encoding the catalytic DH domain and the PH domain. This finding, in combination with the results described above, prompted us to investigate if TUS1 is an exchange factor for RHO1.
Using the yeast two-hybrid system, we investigated whether TUS1 interacts with RHO1. Different RHO1 variants were examined. Wild-type RHO1, RHO1 trapped in its GTP-bound form (RHO1Q68H), and RHO1 in its nucleotide-free form (RHO1G22A), all lacking the C-terminal CAAX-box to prevent prenylation and thus to facilitate nuclear localization, were fused to the prey transcriptional activation domain (see Materials and Methods). TUS1 starting at residue N420 (TUS1ΔN), including putative DH and PH domains, was fused to the bait DNA-binding domain. TUS1ΔN strongly interacted with nucleotide-free RHO1 (G22A) but not with activated RHO1 (Q68H) or with wild-type RHO1 (Fig. 3A). This specific interaction is characteristic for proteins that act as exchange factors (45), since GEFs interact with the nucleotide-free form of GTPases to facilitate the GDP-to-GTP exchange. Because a tus1 mutation is suppressed by overexpression of the RHO1-related GTPase RHO2 (see above), we also examined if TUS1 interacts with RHO2. We failed to detect, by two hybrid analysis, an interaction between TUS1ΔN and wild-type or nucleotide-free (G19A) RHO2 (data not shown).
FIG. 3.
TUS1 is an exchange factor for RHO1. (A) TUS1 interacts with nucleotide-free RHO1 in the two-hybrid system. Yeast strain EGY191 was transformed with pEG202::TUS1ΔN and pJG4-5 (vector), pJG4-5::RHO1, pJG4-5::RHO1Q68H, or pJG4-5::RHO1G22A and streaked out onto SGal/Raff-Ura-Trp-His/X-Gal plates at 30°C. Blue color (seen here as dark color) indicates a specific interaction in the two-hybrid system. (B) The TUS1 DH domain mediates the TUS1-RHO1 interaction. Yeast strain EGY191 was transformed with pJG4-5::RHO1G22A and pEG202 (vector), pEG202::TUS1ΔN, or TUS1 fragments in pEG202 (pTS49, pTS50, and pTS56) and incubated on SGal/Raff-Ura-Trp-His/X-Gal plates at 30°C. A schematic representation of the TUS1 bait constructs is shown on the right; numbers represent amino acid positions. Blue color (seen here as dark color) indicates a specific interaction in the two-hybrid system. (C) TUS1 has GEF activity toward RHO1. GST-RHO1 was preincubated with GDP and subsequently mixed with [35S]GTPγS and GST-TUS1DH (circles) or GST (squares). Reactions were stopped after 5, 10, and 15 min of incubation, and reaction products were washed. [35S]GTPγS bound to GST-RHO1 was quantified by scintillation counting (see Materials and Methods). A representative curve obtained from one of at least three experiments is shown.
To determine which domain of TUS1 is required for interaction with RHO1, additional deletion mutants of TUS1 were constructed and assayed for interaction with nucleotide-free RHO1. The deletion variants contained the following regions of TUS1: (i) the putative DH domain and the N-terminal one-third of the PH domain (Asn420 to Glu773; pTS50), (ii) the DH domain lacking a small C-terminal fragment (Asn420 to Asp631, pTS49), and (iii) the DH domain plus 50 amino acids on each side (Gly425 to Thr721; pTS56) (Fig. 3B). Only the deletion variants containing an intact DH domain (pTS50 and pTS56) interacted with RHO1G22A. The binding was independent of the TUS1 PH domain (Fig. 3B). These results demonstrate that the TUS1 DH domain mediates the TUS1-RHO1 interaction, and they further indicate that TUS1 may be a RHO1 exchange factor.
To determine if TUS1 indeed encodes a RHO1 exchange factor, we investigated if TUS1 can catalyze the GDP-to-GTP exchange on RHO1 in vitro. The putative catalytic DH domain of TUS1 was fused to GST, and the resulting fusion protein was purified from bacteria. GST-RHO1 (purified from yeast) was preloaded with GDP, and purified GST-TUS1DH or GST alone and [35S]GTPγS were added to start the exchange reaction (see Materials and Methods). Whereas GST-RHO1 incubated with GST alone did not significantly incorporate radioactive GTP over time, GST-TUS1DH strongly promoted incorporation of labeled GTP into GST-RHO1 (Fig. 3C). GST-TUS1DH alone did not bind labeled GTP (data not shown). These findings demonstrate that the TUS1 DH domain can catalyze the GDP-to-GTP exchange on RHO1. We conclude that TUS1 encodes a novel exchange factor (GEF) for RHO1. Furthermore, the above suppression and interaction analyses suggest that TUS1 is an exchange factor specifically for RHO1. The suppression of ypk (or tor2) mutations by multiple copies of TUS1 can be explained by TUS1-mediated upregulation of RHO1 activity.
Signaling of cell wall defects to RHO1 requires TUS1.
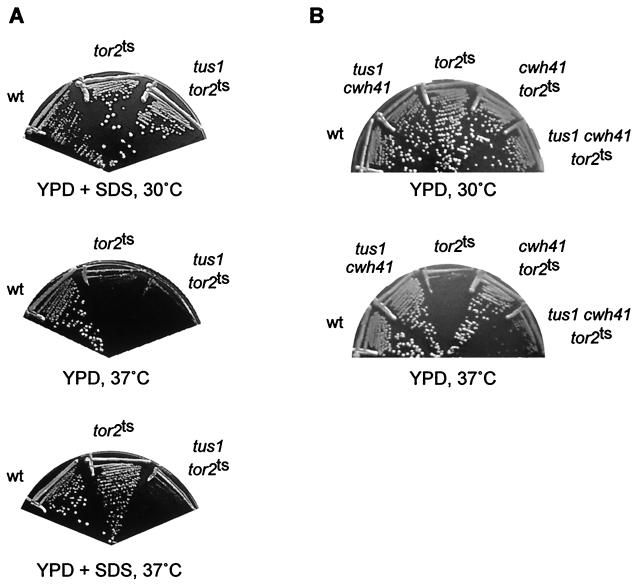
RHO1 is activated in response to cell wall defects (7, 15, 48). RHO1 in turn activates cell wall synthesis via its effectors FKS1 and the PKC1-associated MAP kinase cascade. We investigated if TUS1 is required to activate RHO1 in response to cell wall damage. Cell wall defects, induced by addition of small amounts of SDS to the growth medium or deletion of the CWH41 gene encoding endoplasmic reticulum-glucosidase I, have been demonstrated to suppress a tor2 mutation by restoring RHO1 activity (7). Thus, we tested if 0.005% SDS or deletion of CWH41 could still restore growth in tor2 mutants when TUS1 is deleted. tus1 tor2ts cells, in contrast to tor2ts cells, were not able to form colonies when incubated in the presence of SDS at a nonpermissive temperature (37°C), indicating that TUS1 contributes to the activation of RHO1 upon cell wall stress (Fig. 4A). tus1 cells did not display a growth defect on SDS-containing medium (data not shown). Suppression of a tor2 mutation by deletion of CWH41 was also strongly diminished in the absence of TUS1 (Fig. 4B). The almost wild-type growth rate of tus1 cwh41 cells (Fig. 4B) indicates that the decrease in the cwh41-mediated suppression of a tor2 mutation is not due to additive growth defects, but rather to a defect in signal transmission to RHO1. Taken together, these results suggest that TUS1, probably in conjunction with ROM2 (7), participates in activating RHO1 in response to cell wall stress.
FIG. 4.
Activation of RHO1 in response to cell wall defects requires TUS1. (A) Wild-type (wt) (JK9-3da) cells, tor2ts (SH121) cells, and tus1 tor2ts (TS27-4C) cells were streaked onto YPD medium and onto YPD medium containing 0.005% SDS and were incubated at 37°C. As a control, strains were also streaked onto YPD medium containing 0.005% SDS and incubated at 30°C. (B) wt (JK9-3da), tus1 cwh41 (TS80-6D), tor2ts (SH121), cwh41 tor2ts (MB119), and tus1 cwh41 tor2ts (TS92-2D) cells were streaked onto YPD medium and incubated at 30 and 37°C. A tus1 mutation prevents the suppression of the tor2 mutation by cell wall defects (induced by SDS or cwh41).
YPKs are required for organization of the actin cytoskeleton.
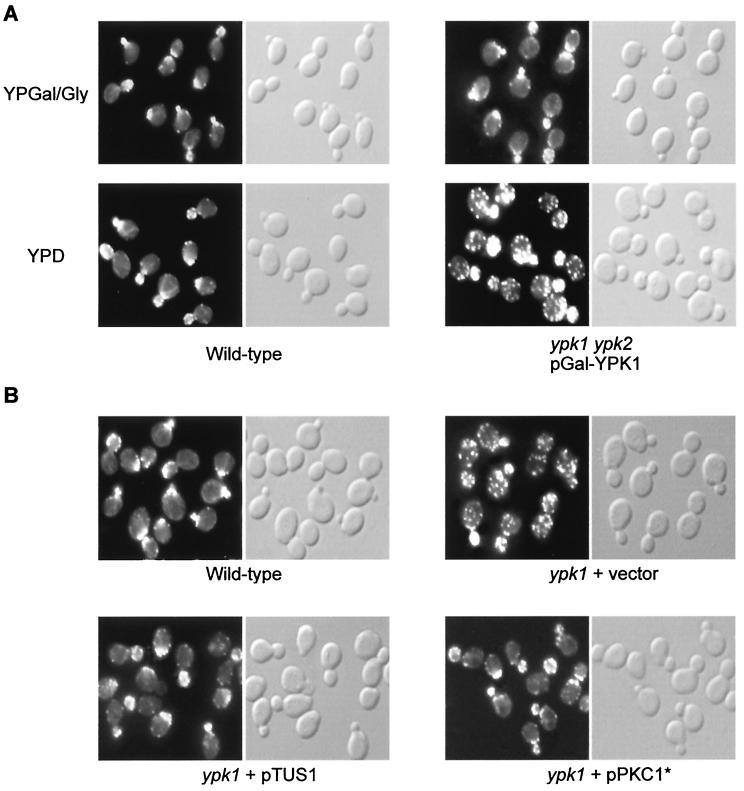
The above described suppression of the ypk growth defect by activation of RHO1 or the PKC1-controlled MAP kinase cascade prompted us to investigate if ypk cells exhibit a defect in the organization of the actin cytoskeleton, one of the readouts of the cell integrity signaling pathway. Wild-type and ypk cells grown under permissive (galactose) and nonpermissive (glucose) conditions were examined for actin distribution by phalloidin staining. Whereas wild-type cells displayed a normal polarized distribution of actin at the various stages of the cell cycle, cells lacking both YPK1 and YPK2 (glucose-grown cells) exhibited a severe defect in actin distribution. Small-budded ypk cells displayed a random distribution of actin patches in mother and daughter cells instead of a concentration of patches at the site of growth. Large-budded ypk cells failed to properly localize actin patches to the mother-bud neck (Fig. 5A).
FIG. 5.
ypk cells display an actin organization defect. (A) Wild-type (TB50a) and ypk1 ypk2/pGal-YPK1 (TS57-2B) cells were grown in YPGal/Gly (galactose) medium, shifted to YPD (glucose) medium for 16 h, fixed, stained for actin with TRITC-phalloidin, and observed by fluorescence (actin, left panels) and Nomarski (right panels) microscopy. (B) Overexpression of TUS1 or expression of PKC1* suppresses the actin defect in ypk1 cells. Wild-type (TB50a) cells and ypk1 (TS38-1C) cells transformed with empty vector, pTUS1, or pPKC1* were pregrown in SD medium. Cells were then grown to early-logarithmic phase in YPD medium and processed for actin staining. Fluorescence (actin) and Nomarski microscopy images are shown in the left and right panels, respectively.
We also examined the actin cytoskeleton in ypk1 cells. Loss of YPK1 alone was sufficient to cause a defect in the polarization of the actin cytoskeleton (Fig. 5B). Consistent with the suppression of the ypk1 growth defect by TUS1 or PKC1* (see above), overexpression of TUS1 or expression of PKC1* suppressed the actin defect in ypk1 cells (Fig. 5B). Cells in which YPK2 was deleted did not display any apparent defect in actin polarization (data not shown). These findings indicate a role for the YPKs in the control of the actin cytoskeleton and further support their involvement in activation of the cell integrity signaling pathway.
ypk1 cells are defective in MAP kinase activation in response to heat shock.
To further confirm the role of YPKs in the cell integrity pathway, we examined activation of the PKC1-controlled MAP kinase MPK1 in YPK-deficient cells. We monitored the kinetics of heat stress-induced MPK1 activation in a ypk1 mutant. Activation of MPK1 was monitored with antibodies that specifically recognize the activated, dually phosphorylated form of MAP kinases. In wild-type cells, heat stress-induced activation of MPK1 was transient, with MPK1 phosphorylation peaking at 30 to 60 min after the shift to 39°C and returning to basal levels within 100 to 120 min after the shift (Fig. 6). This transient MPK1 activation parallels the previously reported transient actin depolarization upon heat shock (15). In ypk1 cells, similar kinetics of MPK1 activation were detected, but the level of activation was severely decreased in comparison to that for wild-type cells (Fig. 6). Total MPK1 (MPK1-HA3) levels were similar in wild-type and ypk1 cells at all time points, as determined by anti-HA immunoblotting (Fig. 6). The finding that MPK1 activation was severely reduced but not delayed in the slow-growing ypk1 mutant suggests that the defect in MPK1 activation is not a secondary consequence of the growth defect, but rather a direct consequence of a defect in signaling through the cell integrity pathway. The residual MPK1 phosphorylation observed in ypk1 cells is presumably due to YPK2.
FIG. 6.
YPK1 is required for MPK1 activation in response to heat stress. Wild-type (TS99-5C) and ypk1 (TS105-1C) cells, expressing MPK1-HA3, were grown to early-logarithmic phase at 24°C (time 0), shifted to 39°C for the indicated times (20, 40, 60, 80, 100, and 120 min), and processed for total protein extraction, SDS-PAGE, and Western analysis as described in Materials and Methods. The upper panel for each strain is the anti-activated MAP kinase immunoblot (phospho-MPK1); the lower panel is the anti-HA immunoblot (MPK1-HA3).
We also examined heat stress-induced MPK1 activation in cells lacking only YPK2. In accordance with the wild-type-like growth and actin organization of these cells, ypk2 mutant cells did not display any reduction in MPK1 activation in comparison to wild-type cells (data not shown). These results indicate that YPK2 plays a minor role relative to YPK1 in the activation of MPK1.
DISCUSSION
PKC1 is activated by interaction with GTP-bound RHO1 and by PKH2-mediated phosphorylation of the PKC1 activation loop. The PKC1 effector MAP kinase cascade subsequently controls the organization of the actin cytoskeleton and transcription of cell wall biosynthesis genes, i.e., cell integrity. Here we show that YPK-deficient cells are defective in cell integrity signaling. First, mutants lacking both YPKs or only YPK1 display a randomized distribution of the actin cytoskeleton. Second, cells lacking both YPKs (data not shown) or only YPK1 do not activate the PKC1-activated MAP kinase MPK1 in response to heat stress. Third, upregulation of the RHO1 GTPase switch or the PKC1 effector MAP kinase pathway suppresses the growth and actin defects of ypk cells. Thus, YPK1 and YPK2, an essential pair of homologous kinases, are novel regulators of the PKC1-controlled MAP kinase cascade.
The target(s) of the YPKs in the cell integrity pathway is unknown. The finding that ypk lethality is suppressed by RHO1, more precisely by overexpression of the RHO1 GEFs ROM2 and TUS1, suggests that the YPKs are upstream of RHO1. To determine if the YPKs control RHO1 via a GEF, we performed in vitro GEF assays with isolated RHO1 and total cellular extracts derived from wild-type and ypk mutant cells. However, for unknown reasons, these assays yielded inconclusive results. The position of the YPKs in the cell integrity signaling pathway thus remains to be determined.
Suppression of the ypk growth defect by TUS1 or PKC1 was not possible in a ypk null mutant (i.e., ypk1 ypk2 without pGal-YPK1), and the ypk growth defect was not suppressed by addition of 1 M sorbitol to the medium (data not shown). These results possibly indicate that YPKs control targets other than those involved in cell integrity signaling. It is also of interest to determine the functional relationship between the PKHs and the YPKs. PKH2 preferentially activates PKC1 (28), whereas PKH1 primarily acts on the YPKs (10). Thus, the PKHs may activate PKC1 in two different ways, directly and via the YPKs.
What is upstream of the PKHs and the YPKs? Sun et al. have recently reported that overexpression of YPK1 or PKH1 allows cells to survive sphingoid base depletion and that sphingolipid levels regulate the phosphorylation and localization of YPK1 (58). Furthermore, sphingoid base synthesis, like the PKHs and YPKs, is required for PKC1-mediated organization of the actin cytoskeleton (18, 28, 66). Finally, sphingolipids are signaling molecules specific for heat stress in yeast (16, 56), and the cell integrity signaling pathway is particularly responsive to heat stress. These findings strongly suggest that the PKHs and the YPKs act downstream of a sphingolipid-derived signal. Interestingly, sphingosine has recently been identified as an activator of PDK1 (the mammalian counterpart of PKHs), which in turn phosphorylates PAK1, an effector of the Rho GTPases Rac and Cdc42 (34). The parallel situation in yeast would be that a sphingolipid-derived signal activates the PKHs and YPKs to regulate the RHO1 effector PKC1.
We also present a novel RHO1 exchange factor, TUS1, as a new component of the cell integrity pathway. TUS1 was identified as a multicopy suppressor of a ypk mutation and is the third exchange factor identified for RHO1. Why does RHO1 have multiple GEFs, i.e., ROM1, ROM2, and TUS1? A similar situation has been described for mammalian cells, where the number of Rho GEFs (approximately 35 members) far exceeds the number of Rho type GTPases (approximately 15 members). An attractive hypothesis is that the GEFs determine the downstream signaling specificity of Rho GTPases, as suggested for Rac and Cdc42 signaling in mammalian cells (54, 71). Specific GEFs may, therefore, activate specific RHO1 effector pathways. In agreement with such a hypothesis, we find that the heat shock-induced activation of the PKC1-controlled MAP kinase MPK1 is reduced in rom2 mutant cells but not in tus1 (or tus1/tus1) mutant cells (data not shown). TUS1 may thus activate RHO1 for other effector functions involved in cell wall integrity (e.g., glucan synthase), explaining the requirement of TUS1 for RHO1 activation in response to cell wall defects. Moreover, multiple copies of TUS1 were not sufficient to rescue the synthetic rom1 rom2 lethality (data not shown), possibly indicating that ROM2 and ROM1 perform unique, essential functions in the cell that TUS1 cannot provide. Different GEFs could also be required to activate RHO1 in response to different stimuli.
We have also identified a novel protein kinase, YPK3, that functions as a multicopy suppressor of the ypk growth defect and exhibits homology to YPK1 and YPK2. YPK3 may be able to act on the same substrates as YPK1 and YPK2 when overexpressed, but YPK1 and YPK2 apparently play a more crucial role under physiological growth conditions. A similar situation has been reported by Inagaki et al. for the PDK1 homologues PKH1, PKH2, and PKH3 (28).
Acknowledgments
We thank Sylvane Desrivières, David Levin, Kunihiro Matsumoto, and Anja Schmidt for strains and plasmids, and José Luis Crespo, Estela Jacinto, Robbie Loewith, and Anja Lorberg for helpful discussions and comments on the manuscript.
This work was supported by the Boehringer Ingelheim Fonds (T.S.) and by grants from the Canton of Basel and the Swiss National Science Foundation (to M.N.H.).
REFERENCES
- 1.Alberts, A. S., N. Bouquin, L. H. Johnston, and R. Treisman. 1998. Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. J. Biol. Chem. 273:8616-8622. [DOI] [PubMed] [Google Scholar]
- 2.Alberts, A. S., and R. Treisman. 1998. Activation of RhoA and SAPK/JNK signalling pathways by the RhoA-specific exchange factor mNET1. EMBO J. 17:4075-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1998. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 4.Beck, T., A. Schmidt, and M. N. Hall. 1999. Starvation induces vacuolar targeting and degradation of the tryptophan permease in yeast. J. Cell Biol. 146:1227-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell, L. M., M. L. Leong, B. Kim, E. Wang, J. Park, B. A. Hemmings, and G. L. Firestone. 2000. Hyperosmotic stress stimulates promoter activity and regulates cellular utilization of the serum- and glucocorticoid-inducible protein kinase (Sgk) by a p38 MAPK-dependent pathway. J. Biol. Chem. 275:25262-25272. [DOI] [PubMed] [Google Scholar]
- 6.Benedetti, H., S. Raths, F. Crausaz, and H. Riezman. 1994. The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol. Biol. Cell 5:1023-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bickle, M., P. A. Delley, A. Schmidt, and M. N. Hall. 1998. Cell wall integrity modulates RHO1 activity via the exchange factor ROM2. EMBO J. 17:2235-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunet, A., J. Park, H. Tran, L. S. Hu, B. A. Hemmings, and M. E. Greenberg. 2001. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol. Cell. Biol. 21:952-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabib, E., J. Drgonova, and T. Drgon. 1998. Role of small G proteins in yeast cell polarization and wall biosynthesis. Annu. Rev. Biochem. 67:307-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casamayor, A., P. D. Torrance, T. Kobayashi, J. Thorner, and D. R. Alessi. 1999. Functional counterparts of mammalian protein kinases PDK1 and SGK in budding yeast. Curr. Biol. 9:186-197. [DOI] [PubMed] [Google Scholar]
- 11.Chant, J. 1996. Generation of cell polarity in yeast. Curr. Opin. Cell Biol. 8:557-565. [DOI] [PubMed] [Google Scholar]
- 12.Chen, P., K. S. Lee, and D. E. Levin. 1993. A pair of putative protein kinase genes (YPK1 and YPK2) is required for cell growth in Saccharomyces cerevisiae. Mol. Gen. Genet. 236:443-447. [DOI] [PubMed] [Google Scholar]
- 13.Chen, S. Y., A. Bhargava, L. Mastroberardino, O. C. Meijer, J. Wang, P. Buse, G. L. Firestone, F. Verrey, and D. Pearce. 1999. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc. Natl. Acad. Sci. USA 96:2514-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davenport, K. R., M. Sohaskey, Y. Kamada, D. E. Levin, and M. C. Gustin. 1995. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J. Biol. Chem. 270:30157-30161. [DOI] [PubMed] [Google Scholar]
- 15.Delley, P. A., and M. N. Hall. 1999. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 147:163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickson, R. C., and R. L. Lester. 1999. Metabolism and selected functions of sphingolipids in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1438:305-321. [DOI] [PubMed] [Google Scholar]
- 17.Drgonova, J., T. Drgon, K. Tanaka, R. Kollar, G. C. Chen, R. A. Ford, C. S. Chan, Y. Takai, and E. Cabib. 1996. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science 272:277-279. [DOI] [PubMed] [Google Scholar]
- 18.Friant, S., B. Zanolari, and H. Riezman. 2000. Increased protein kinase or decreased PP2A activity bypasses sphingoid base requirement in endocytosis. EMBO J. 19:2834-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golemis, E. A., I. Serebriiskii, L. F. Russell, M. G. Kolonin, J. Gyuris, and R. Brent. 1999. Interaction trap/two-hybrid system to identify interacting proteins, p. 20.1.1-20.1.40. In F. M. Ausubel et al. (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 20.Gray, J. V., J. P. Ogas, Y. Kamada, M. Stone, D. E. Levin, and I. Herskowitz. 1997. A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 16:4924-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo, W., F. Tamanoi, and P. Novick. 2001. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 3:353-360. [DOI] [PubMed] [Google Scholar]
- 22.Gustin, M. C., J. Albertyn, M. Alexander, and K. Davenport. 1998. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62:1264-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 24.Heinisch, J. J., A. Lorberg, H. P. Schmitz, and J. J. Jacoby. 1999. The protein kinase C-mediated MAP kinase pathway involved in the maintenance of cellular integrity in Saccharomyces cerevisiae. Mol. Microbiol. 32:671-680. [DOI] [PubMed] [Google Scholar]
- 25.Helliwell, S. B., I. Howald, N. Barbet, and M. N. Hall. 1998. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics 148:99-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helliwell, S. B., A. Schmidt, Y. Ohya, and M. N. Hall. 1998. The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton. Curr. Biol. 8:1211-1214. [DOI] [PubMed] [Google Scholar]
- 27.Igual, J. C., A. L. Johnson, and L. H. Johnston. 1996. Coordinated regulation of gene expression by the cell cycle transcription factor Swi4 and the protein kinase C MAP kinase pathway for yeast cell integrity. EMBO J. 15:5001-5013. [PMC free article] [PubMed] [Google Scholar]
- 28.Inagaki, M., T. Schmelzle, K. Yamaguchi, K. Irie, M. N. Hall, and K. Matsumoto. 1999. PDK1 homologs activate the Pkc1-mitogen-activated protein kinase pathway in yeast. Mol. Cell. Biol. 19:8344-8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaibuchi, K., S. Kuroda, and M. Amano. 1999. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68:459-486. [DOI] [PubMed] [Google Scholar]
- 31.Kamada, Y., U. S. Jung, J. Piotrowski, and D. E. Levin. 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9:1559-1571. [DOI] [PubMed] [Google Scholar]
- 32.Kamada, Y., H. Qadota, C. P. Python, Y. Anraku, Y. Ohya, and D. E. Levin. 1996. Activation of yeast protein kinase C by Rho1 GTPase. J. Biol. Chem. 271:9193-9196. [DOI] [PubMed] [Google Scholar]
- 33.Kapteyn, J. C., H. Van Den Ende, and F. M. Klis. 1999. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim. Biophys. Acta 1426:373-383. [DOI] [PubMed] [Google Scholar]
- 34.King, C. C., F. T. Zenke, P. E. Dawson, E. M. Dutil, A. C. Newton, B. A. Hemmings, and G. M. Bokoch. 2000. Sphingosine is a novel activator of 3-phosphoinositide-dependent kinase 1. J. Biol. Chem. 275:18108-18113. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi, T., and P. Cohen. 1999. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem. J. 339:319-328. [PMC free article] [PubMed] [Google Scholar]
- 36.Kohno, H., K. Tanaka, A. Mino, M. Umikawa, H. Imamura, T. Fujiwara, Y. Fujita, K. Hotta, H. Qadota, T. Watanabe, Y. Ohya, and Y. Takai. 1996. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15:6060-6068. [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, K. S., and D. E. Levin. 1992. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol. 12:172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longtine, M. S., A. McKenzie, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 39.Madaule, P., R. Axel, and A. M. Myers. 1987. Characterization of two members of the rho gene family from the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 84:779-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madden, K., and M. Snyder. 1998. Cell polarity and morphogenesis in budding yeast. Annu. Rev. Microbiol. 52:687-744. [DOI] [PubMed] [Google Scholar]
- 41.Manning, B. D., R. Padmanabha, and M. Snyder. 1997. The Rho-GEF Rom2p localizes to sites of polarized cell growth and participates in cytoskeletal functions in Saccharomyces cerevisiae. Mol. Biol. Cell 8:1829-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin, H., J. M. Rodriguez-Pachon, C. Ruiz, C. Nombela, and M. Molina. 2000. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 275:1511-1519. [DOI] [PubMed] [Google Scholar]
- 43.Mazzoni, C., P. Zarov, A. Rambourg, and C. Mann. 1993. The SLT2 (MPK1) MAP kinase homolog is involved in polarized cell growth in Saccharomyces cerevisiae. J. Cell Biol. 123:1821-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nonaka, H., K. Tanaka, H. Hirano, T. Fujiwara, H. Kohno, M. Umikawa, A. Mino, and Y. Takai. 1995. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 14:5931-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozaki, K., K. Tanaka, H. Imamura, T. Hihara, T. Kameyama, H. Nonaka, H. Hirano, Y. Matsuura, and Y. Takai. 1996. Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15:2196-2207. [PMC free article] [PubMed] [Google Scholar]
- 46.Park, J., M. L. Leong, P. Buse, A. C. Maiyar, G. L. Firestone, and B. A. Hemmings. 1999. Serum- and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J. 18:3024-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perrotti, N., R. A. He, S. A. Phillips, C. Renfrew Haft, and S. I. Taylor. 2001. Activation of serum- and glucocorticoid-induced protein kinase (SGK) by cyclic AMP and insulin. J. Biol. Chem. 276:9406-9412. [DOI] [PubMed] [Google Scholar]
- 48.Philip, B., and D. E. Levin. 2001. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol. Cell. Biol. 21:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qadota, H., C. P. Python, S. B. Inoue, M. Arisawa, Y. Anraku, Y. Zheng, T. Watanabe, D. E. Levin, and Y. Ohya. 1996. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science 272:279-281. [DOI] [PubMed] [Google Scholar]
- 50.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 51.Schmidt, A., M. Bickle, T. Beck, and M. N. Hall. 1997. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell 88:531-542. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt, A., and M. N. Hall. 1998. Signaling to the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 14:305-338. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt, A., J. Kunz, and M. N. Hall. 1996. TOR2 is required for organization of the actin cytoskeleton in yeast. Proc. Natl. Acad. Sci. USA 93:13780-13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scita, G., P. Tenca, E. Frittoli, A. Tocchetti, M. Innocenti, G. Giardina, and P. P. Di Fiore. 2000. Signaling from Ras to Rac and beyond: not just a matter of GEFs. EMBO J. 19:2393-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 56.Skrzypek, M. S., M. M. Nagiec, R. L. Lester, and R. C. Dickson. 1999. Analysis of phosphorylated sphingolipid long-chain bases reveals potential roles in heat stress and growth control in Saccharomyces. J. Bacteriol. 181:1134-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smits, G. J., J. C. Kapteyn, H. van den Ende, and F. M. Klis. 1999. Cell wall dynamics in yeast. Curr. Opin. Microbiol. 2:348-352. [DOI] [PubMed] [Google Scholar]
- 58.Sun, Y., R. Taniguchi, D. Tanoue, T. Yamaji, H. Takematsu, K. Mori, T. Fujita, T. Kawasaki, and Y. Kozutsumi. 2000. Sli2 (Ypk1), a homologue of mammalian protein kinase SGK, is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast. Mol. Cell. Biol. 20:4411-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka, K., and Y. Takai. 1998. Control of reorganization of the actin cytoskeleton by Rho family small GTP-binding proteins in yeast. Curr. Opin. Cell Biol. 10:112-116. [DOI] [PubMed] [Google Scholar]
- 60.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346:561-576. [PMC free article] [PubMed] [Google Scholar]
- 61.Verna, J., A. Lodder, K. Lee, A. Vagts, and R. Ballester. 1997. A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94:13804-13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wach, A., A. Brachat, R. Pohlmann, and P. Philippsen. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10:1793-1808. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe, Y., K. Irie, and K. Matsumoto. 1995. Yeast RLM1 encodes a serum response factor-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 15:5740-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webster, M. K., L. Goya, Y. Ge, A. C. Maiyar, and G. L. Firestone. 1993. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol. Cell. Biol. 13:2031-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whitehead, I. P., S. Campbell, K. L. Rossman, and C. J. Der. 1997. Dbl family proteins. Biochim. Biophys. Acta 1332:F1-F23. [DOI] [PubMed]
- 66.Zanolari, B., S. Friant, K. Funato, C. Sutterlin, B. J. Stevenson, and H. Riezman. 2000. Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J. 19:2824-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zarzov, P., C. Mazzoni, and C. Mann. 1996. The SLT2 (MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J. 15:83-91. [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao, C., U. S. Jung, P. Garrett-Engele, T. Roe, M. S. Cyert, and D. E. Levin. 1998. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol 18:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng, Y., R. Cerione, and A. Bender. 1994. Control of the yeast bud-site assembly GTPase Cdc42. Catalysis of guanine nucleotide exchange by Cdc24 and stimulation of GTPase activity by Bem3. J. Biol. Chem. 269:2369-2372. [PubMed] [Google Scholar]
- 70.Zheng, Y., D. Zangrilli, R. A. Cerione, and A. Eva. 1996. The pleckstrin homology domain mediates transformation by oncogenic dbl through specific intracellular targeting. J. Biol. Chem. 271:19017-19020. [DOI] [PubMed] [Google Scholar]
- 71.Zhou, K., Y. Wang, J. L. Gorski, N. Nomura, J. Collard, and G. M. Bokoch. 1998. Guanine nucleotide exchange factors regulate specificity of downstream signaling from Rac and Cdc42. J. Biol. Chem. 273:16782-16786. [DOI] [PubMed] [Google Scholar]