Abstract
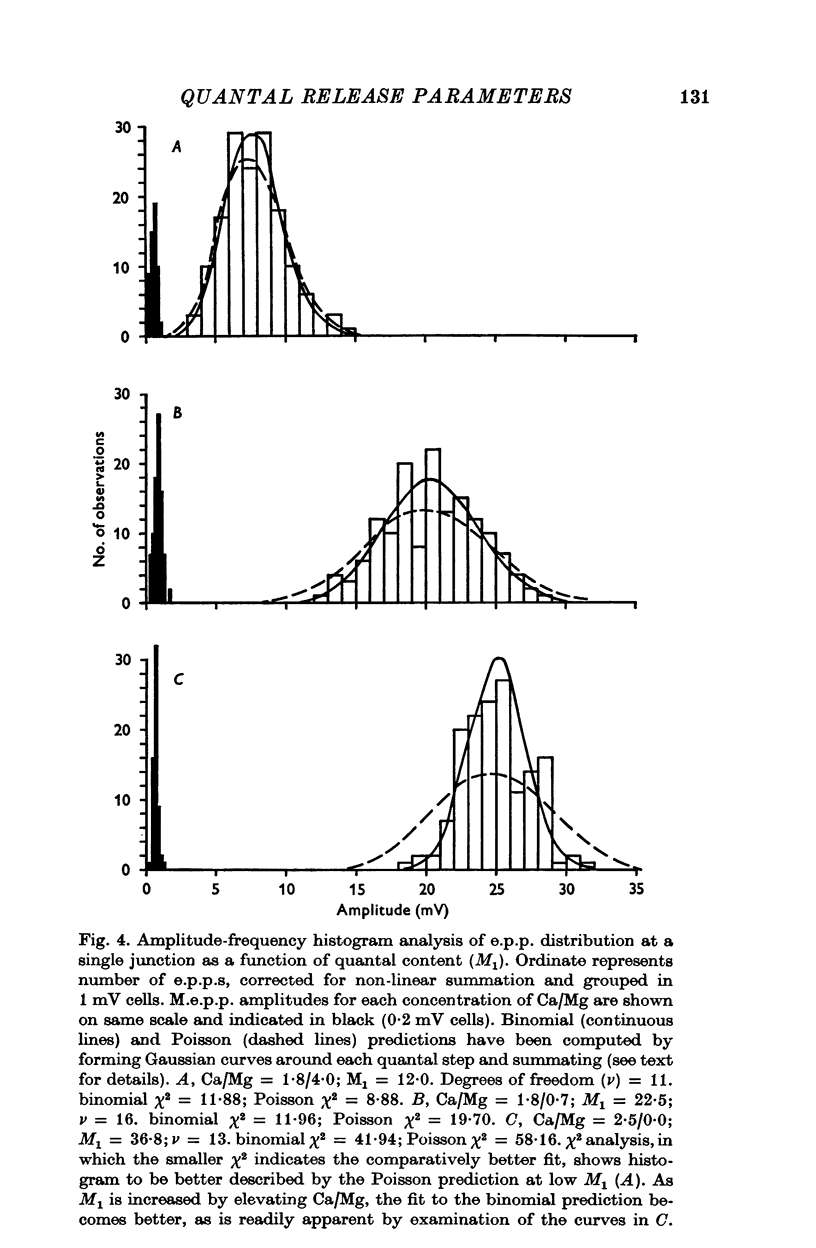
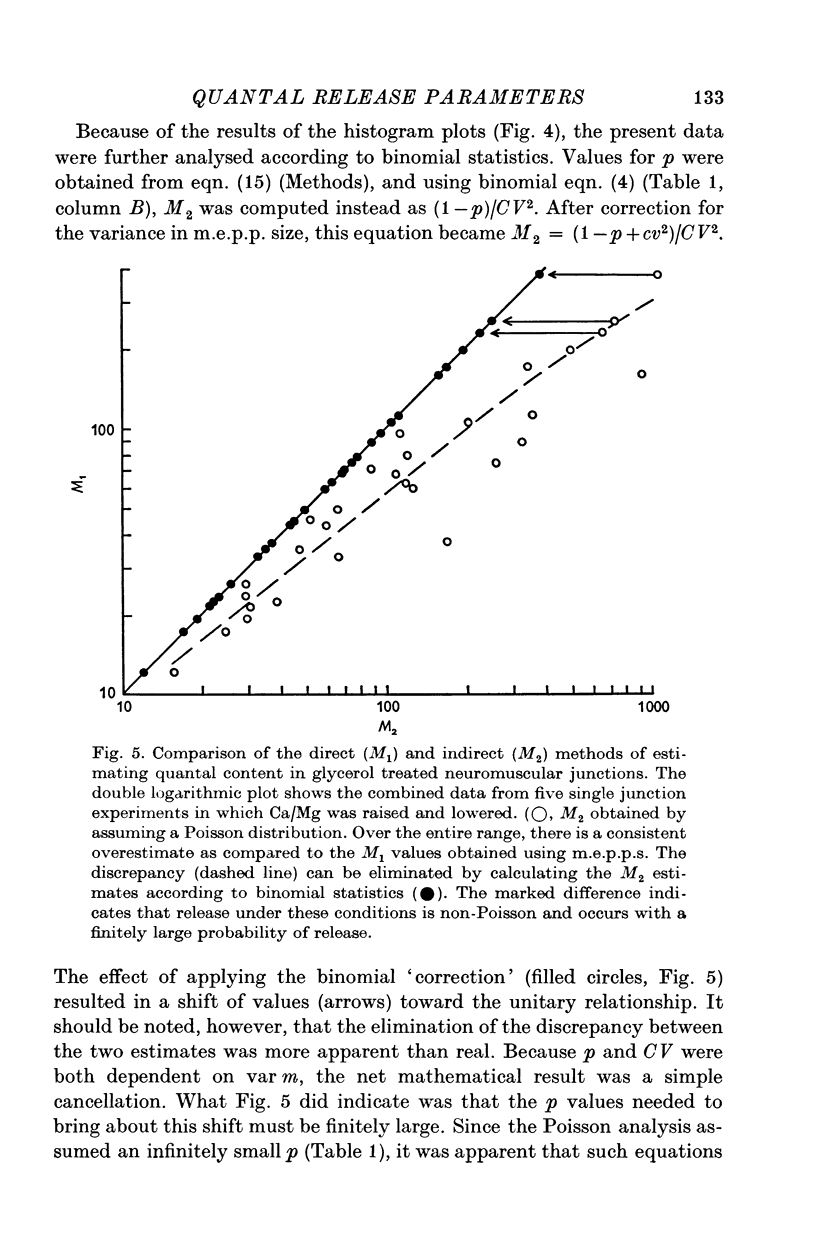
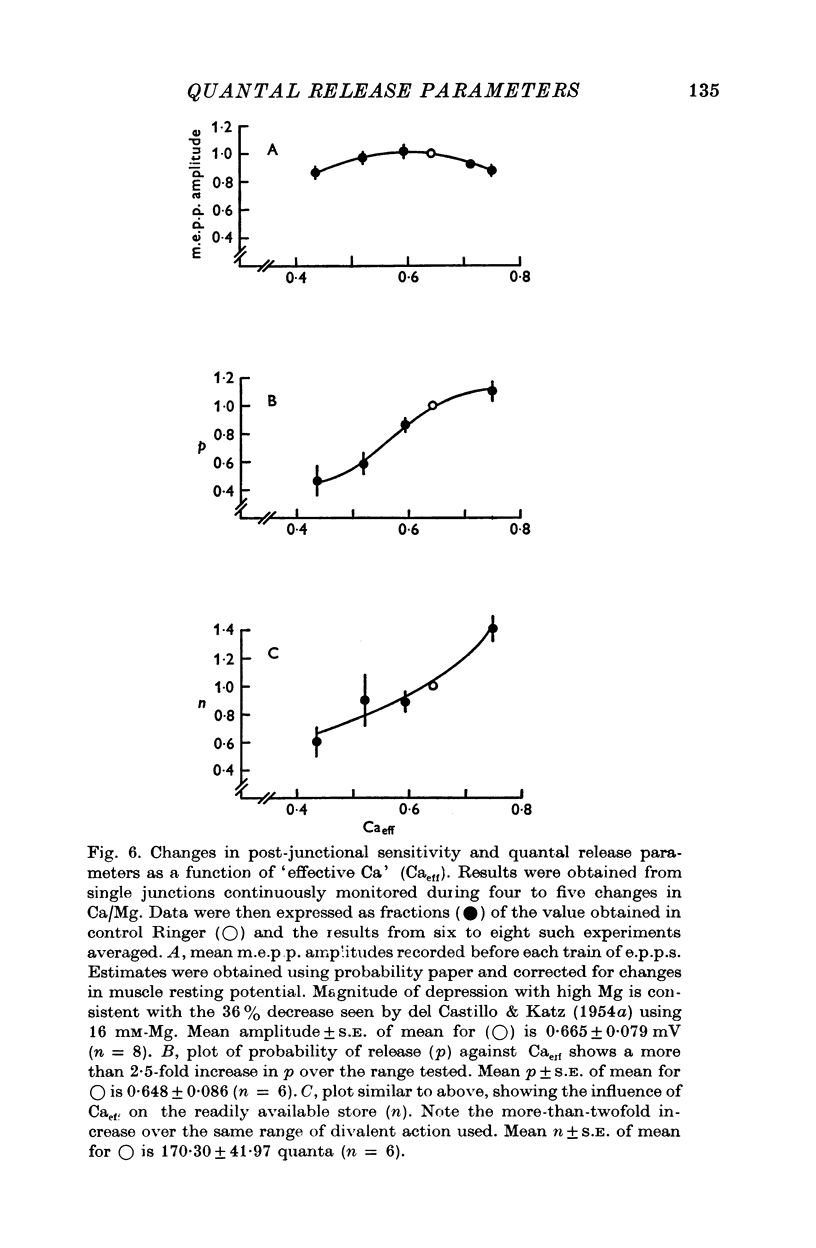
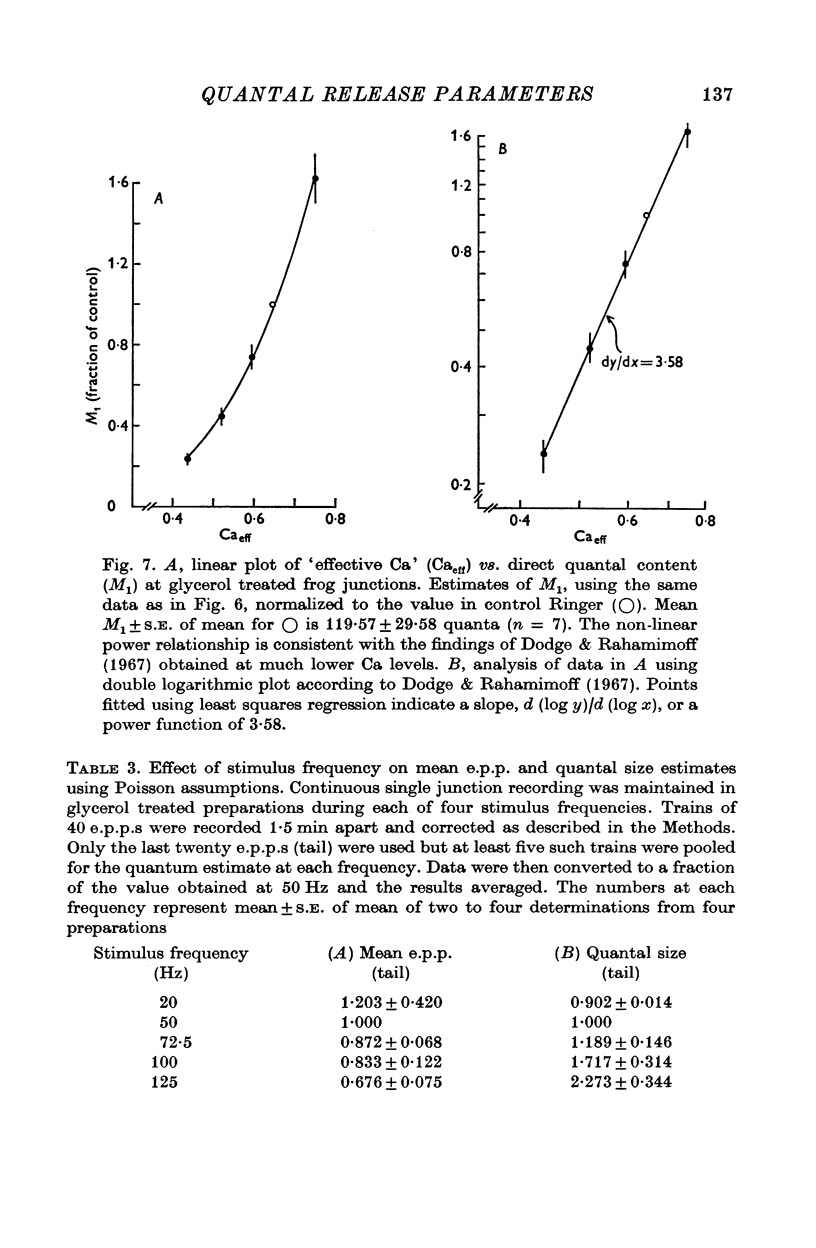
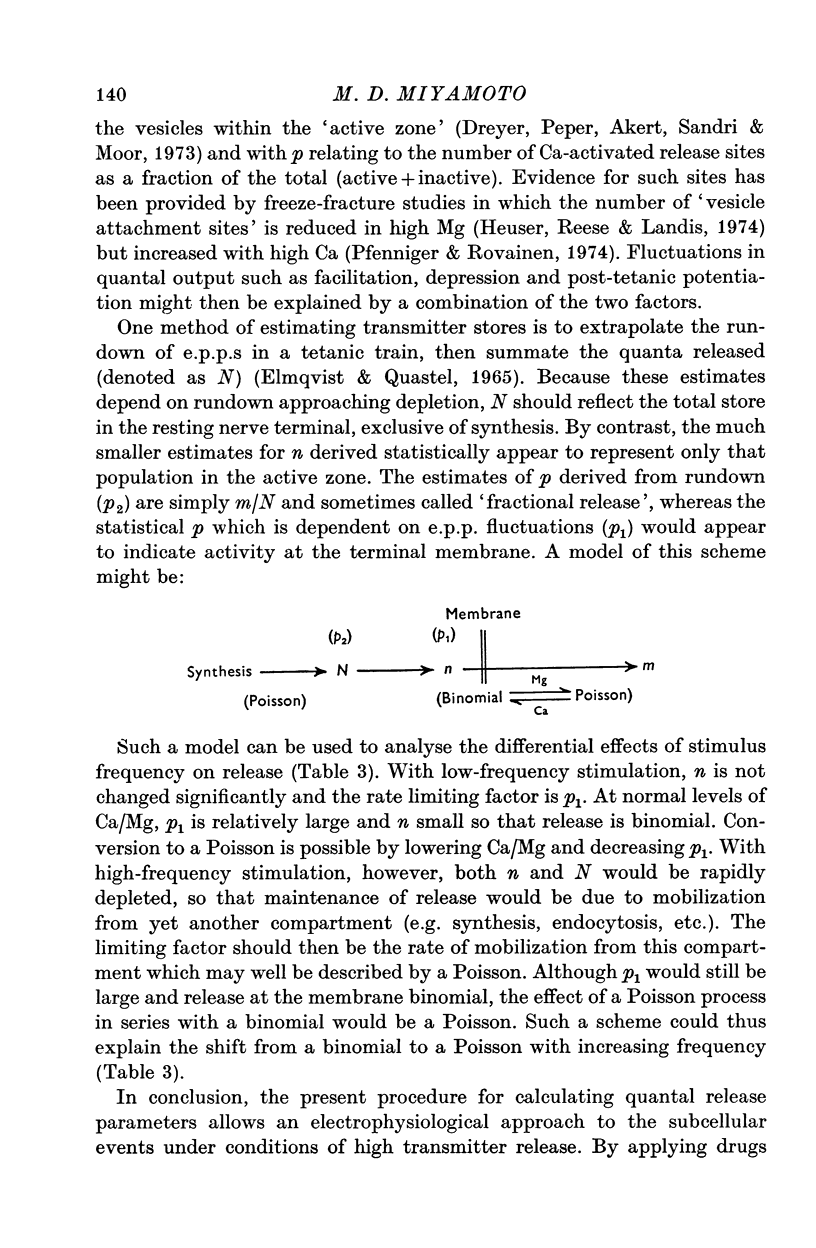
1. Transmitter release was analysed at frog neuromuscular junctions pre-treated with 400 mM glycerol Ringer. In the absence of added drugs, end-plate potentials (e.p.p.s) and miniature e.p.p.s (m.e.p.p.s) could be recorded at selected junctions. 2. E.p.p.s were unusually large and calculations of quantal content indicated a high level of release. Also recorded were anomalous action potentials resembling e.p.p.s but these could be distinguished using a summation test. 3. Plots of coefficient of variation of e.p.p.s (0-5 Hz stimulation) versus direct quantal content (M1) showed a marked deviation from Poisson expectations with high M1. Analysis of these results with amplitude-frequency histograms showed a progressively better fit to binomial predictions with increasing M1. 4. The use of binomial statistics allowed direct calculations of the mean probability of release (p) and the readily available store (n). Increasing Ca/Mg caused increases in both n and p. 5. Plots of M1 vs. Ca/Mg showed a power relationship of 3.58. Maximum m.e.p.p. amplitude occurred at control Ca/Mg. Both results were consistent with studies in muscles not treated with glycerol and indicated that glycerol treatment caused no major alterations pre- or post-junctionally. 6. Estimates of quantum size using Poisson assumptions showed an over-all increase when stimulus frequency was raised, indicating a shift from binomial to a Poisson distribution. 7. The combined findings demonstrate that the glycerol treated preparation can be used to examine the release process during high output. Such release conforms to binomial statistics and allows direct determinations of the parameters n and p.
Full text
PDF





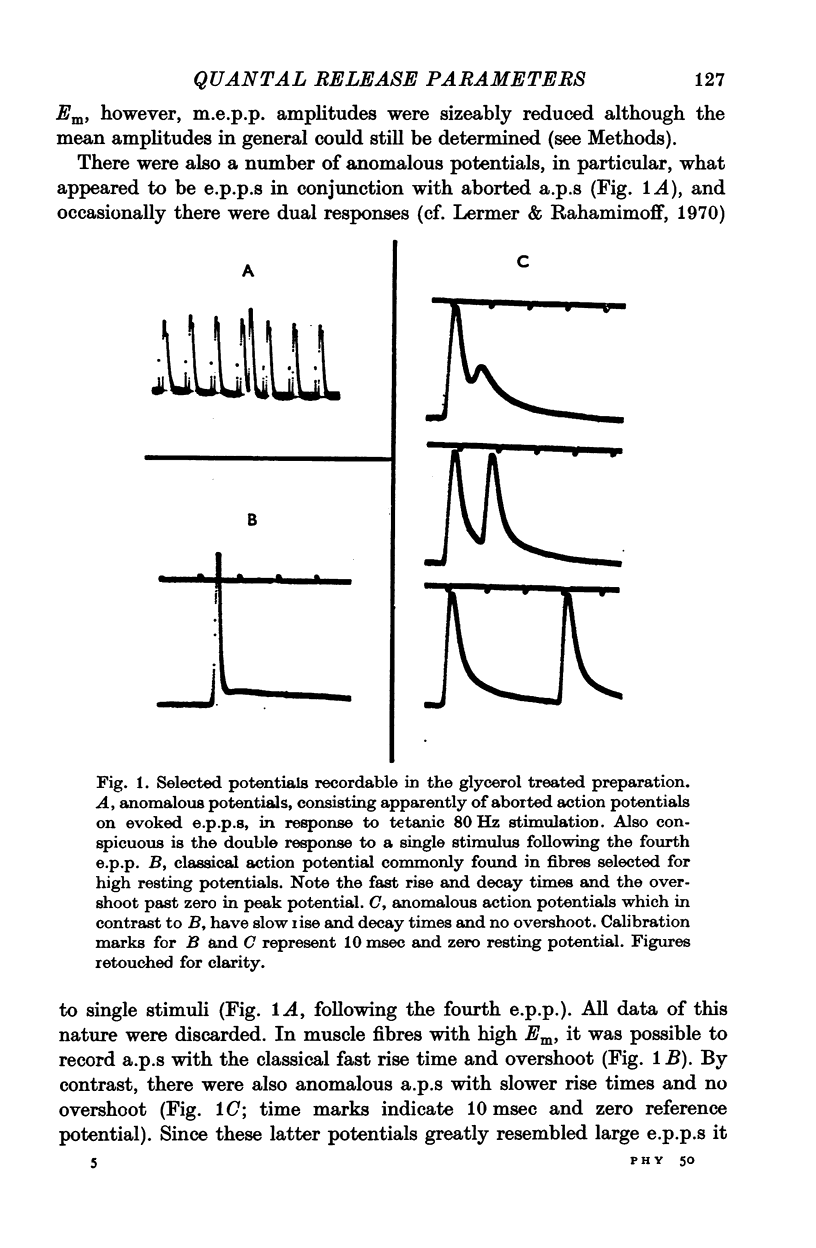
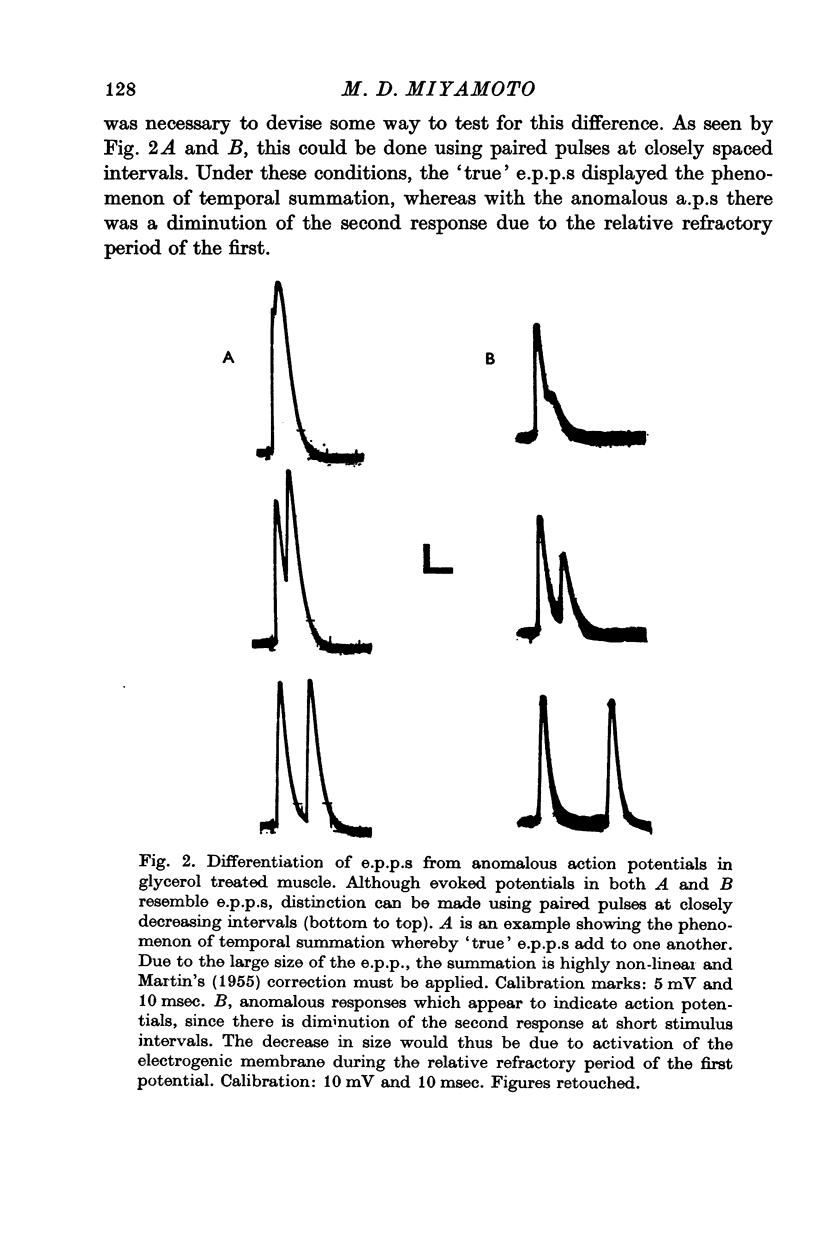
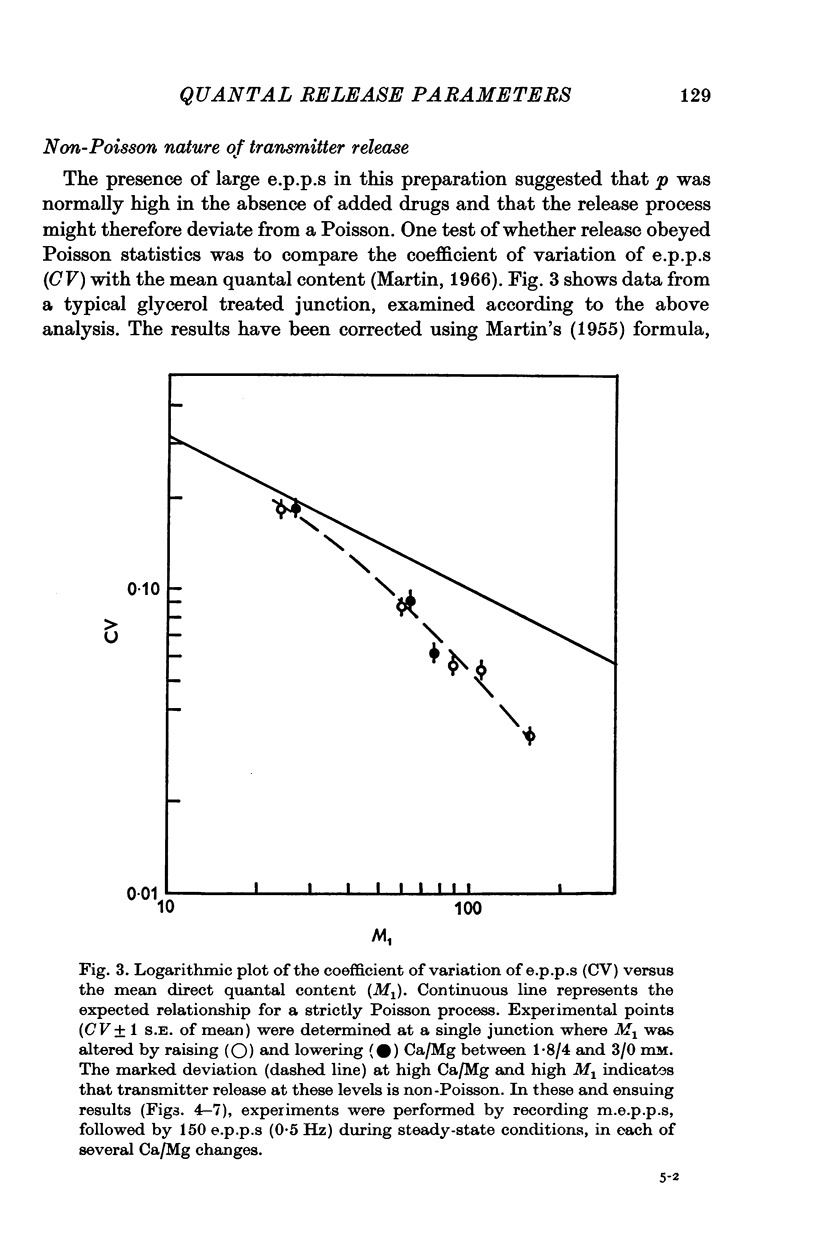
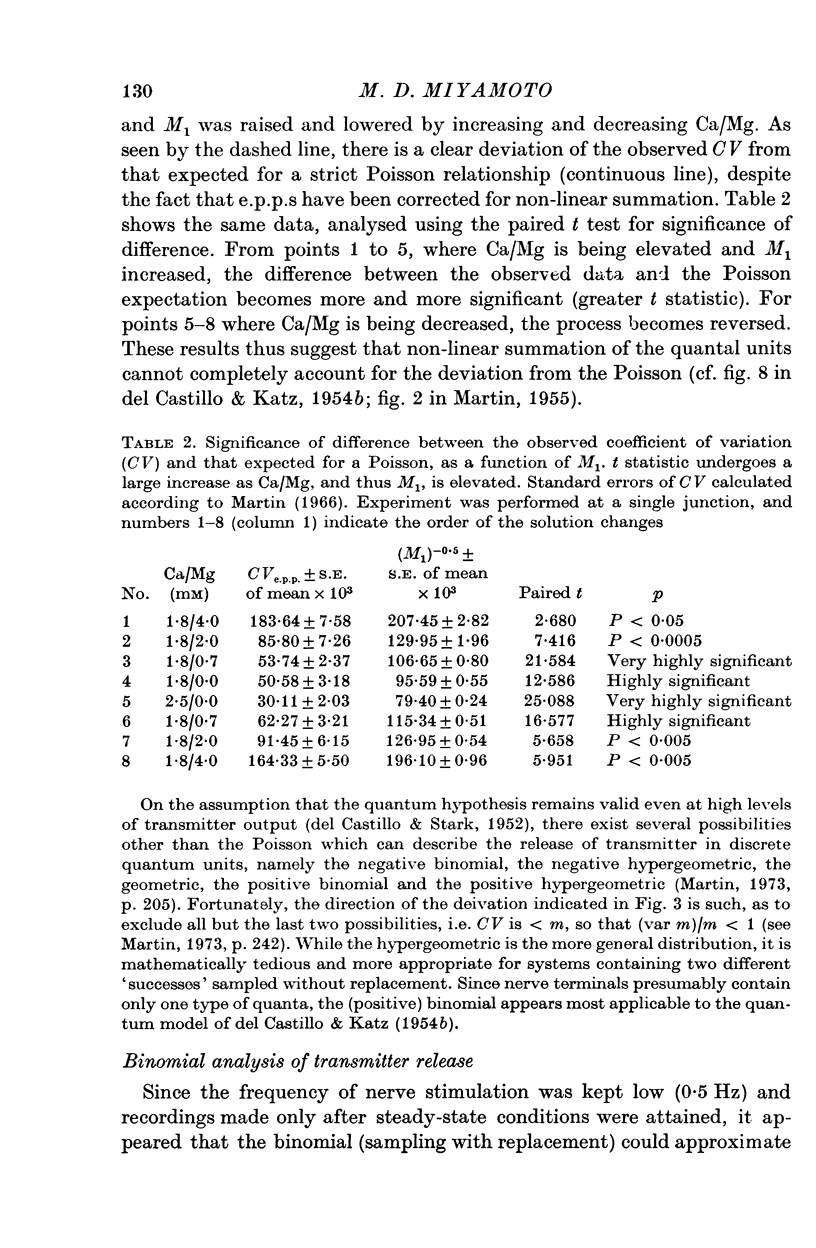







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. R., Florin T. A statistical analysis of the release of acetylcholine at newly formed synapses in striated muscle. J Physiol. 1974 Apr;238(1):93–107. doi: 10.1113/jphysiol.1974.sp010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaer L. C. The effect of facilitatory concentrations of decamethonium on the storage and release of transmitter at the neuromuscular junction of the cat. J Pharmacol Exp Ther. 1970 Dec;175(3):664–672. [PubMed] [Google Scholar]
- Christensen B. N., Martin A. R. Estimates of probability of transmitter release at the mammalian neuromuscular junction. J Physiol. 1970 Nov;210(4):933–945. doi: 10.1113/jphysiol.1970.sp009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., STARK L. Local responses in single medullated nerve fibres. J Physiol. 1952 Oct;118(2):207–215. doi: 10.1113/jphysiol.1952.sp004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T., Narahashi T. Effects of procaine on ionic conductances of end-plate membranes. J Pharmacol Exp Ther. 1971 Feb;176(2):423–433. [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Characteristics of transmitter release at regenerating frog neuromuscular junctions. J Physiol. 1974 Jun;239(3):571–594. doi: 10.1113/jphysiol.1974.sp010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Akert K., Sandri C., Moor H. Ultrastructure of the "active zone" in the frog neuromuscular junction. Brain Res. 1973 Nov 23;62(2):373–380. doi: 10.1016/0006-8993(73)90699-9. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., Howell J. N., Vaughan P. C. The maintenance of resting potentials in glycerol-treated muscle fibres. J Physiol. 1971 May;215(1):95–102. doi: 10.1113/jphysiol.1971.sp009459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Quastel D. M. A quantitative study of end-plate potentials in isolated human muscle. J Physiol. 1965 Jun;178(3):505–529. doi: 10.1113/jphysiol.1965.sp007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Action potentials without contraction in frog skeletal muscle fibers with disrupted transverse tubules. Science. 1967 Dec 29;158(3809):1702–1703. doi: 10.1126/science.158.3809.1702. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Capacitance of the surface and transverse tubular membrane of frog sartorius muscle fibers. J Gen Physiol. 1969 Mar;53(3):265–278. doi: 10.1085/jgp.53.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E. G. Potassium exchange and afterpotentials in frog sartorius muscles treated with glycerol. J Gen Physiol. 1970 Dec;56(6):692–715. doi: 10.1085/jgp.56.6.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974 Mar;3(1):109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Neuromuscular transmission in a mammalian preparation in the absence of blocking drugs and the effect of D-tubocurarine. J Physiol. 1973 Jan;228(2):307–325. doi: 10.1113/jphysiol.1973.sp010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordas M. The effect of membrane polarization on the time course of the end-plate current in frog sartorius muscle. J Physiol. 1969 Oct;204(2):493–502. doi: 10.1113/jphysiol.1969.sp008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M. Quantum aspects of central and ganglionic synaptic transmission in vertebrates. Physiol Rev. 1971 Oct;51(4):647–678. doi: 10.1152/physrev.1971.51.4.647. [DOI] [PubMed] [Google Scholar]
- Lambert D. H., Parsons R. L. Influence of polyvalent cations on the activation of muscle end plate receptors. J Gen Physiol. 1970 Sep;56(3):309–321. doi: 10.1085/jgp.56.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno T., Edwards C. Neuromuscular facilitation with low-frequency stimulation and effects of some drugs. J Neurophysiol. 1969 Sep;32(5):785–792. doi: 10.1152/jn.1969.32.5.785. [DOI] [PubMed] [Google Scholar]
- Miyamoto M., Hubbard J. I. On the inhibition of muscle contraction caused by exposure to hypertonic solutions. J Gen Physiol. 1972 Jun;59(6):689–700. doi: 10.1085/jgp.59.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A. The effects of calcium and magnesium on statistical release parameters at the crayfish neuromuscular junction. J Physiol. 1972 Nov;226(3):761–768. doi: 10.1113/jphysiol.1972.sp010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. D., Bowen J. M. Membrane capacitance and correction of end-plate potentials for nonlinear summation of quantal units. J Theor Biol. 1974 Apr;44(2):349–351. doi: 10.1016/0022-5193(74)90165-9. [DOI] [PubMed] [Google Scholar]


