Abstract
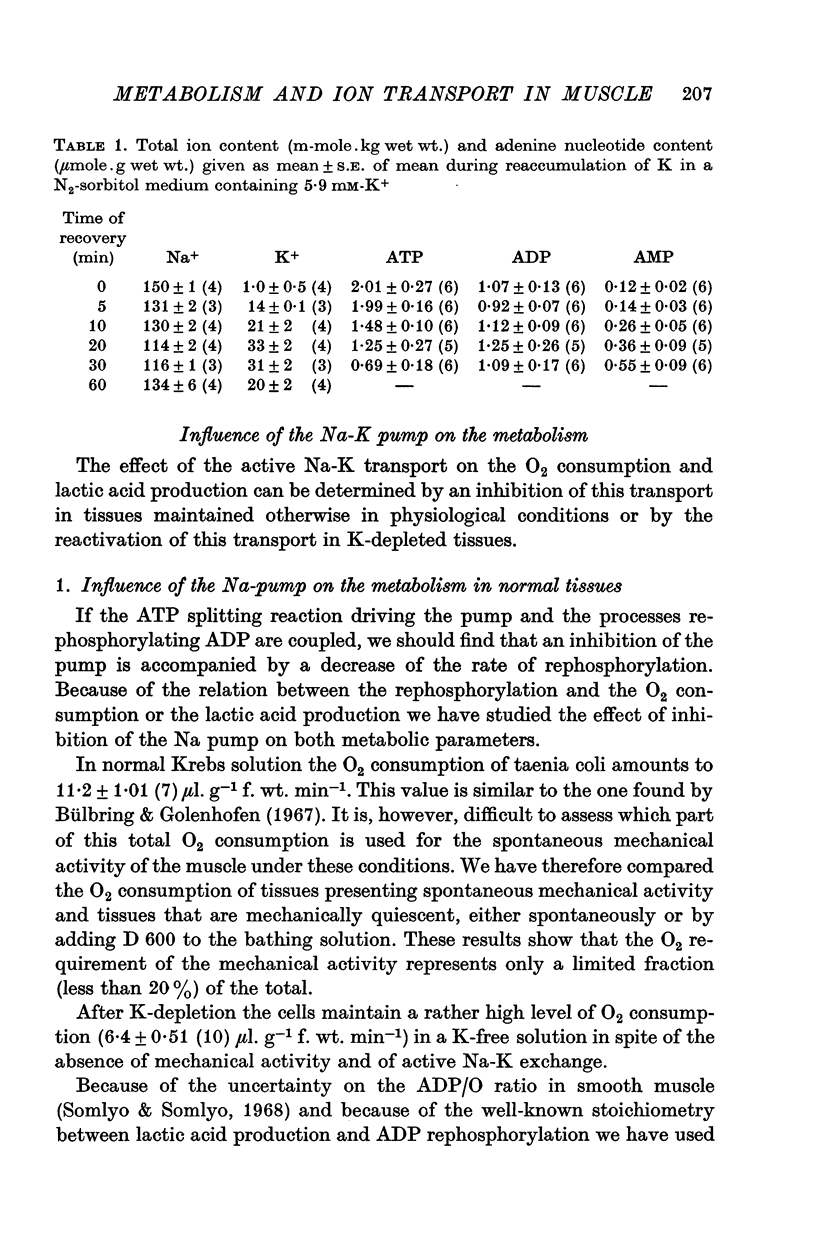
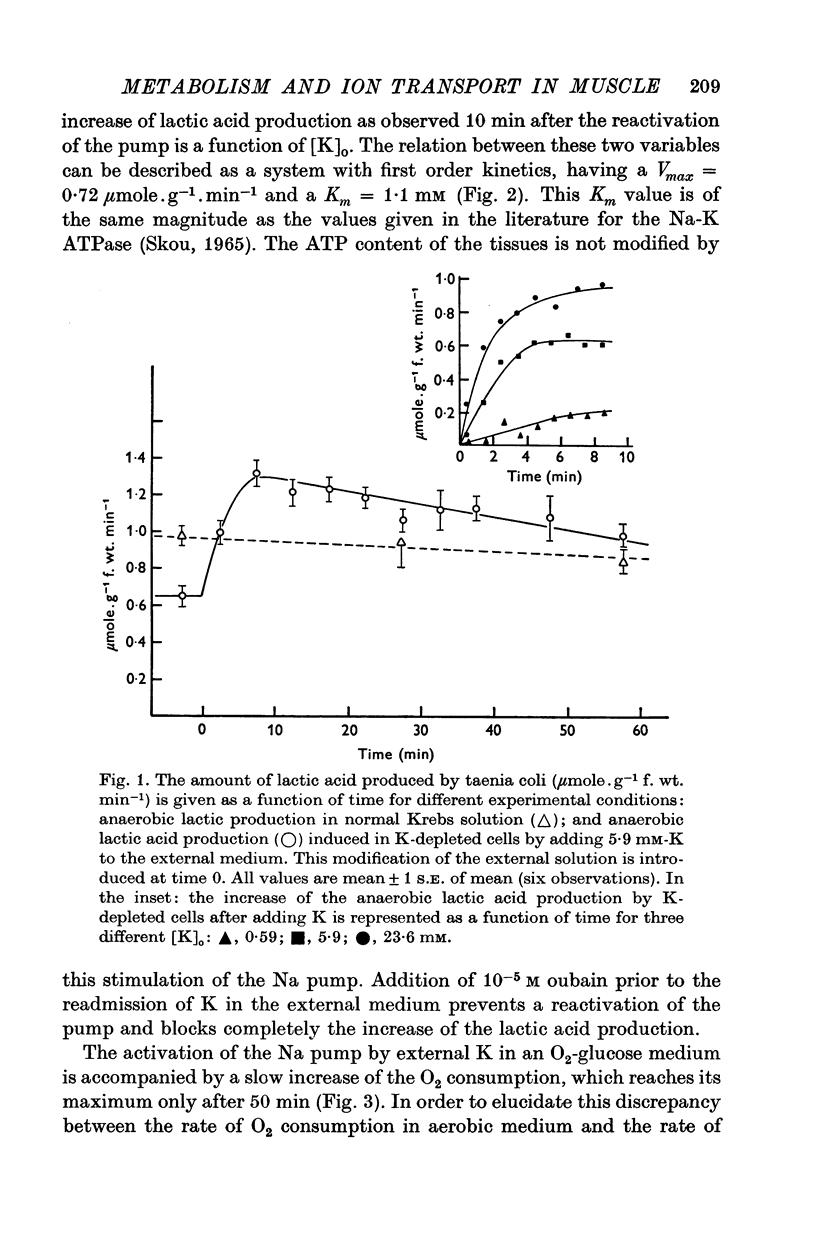
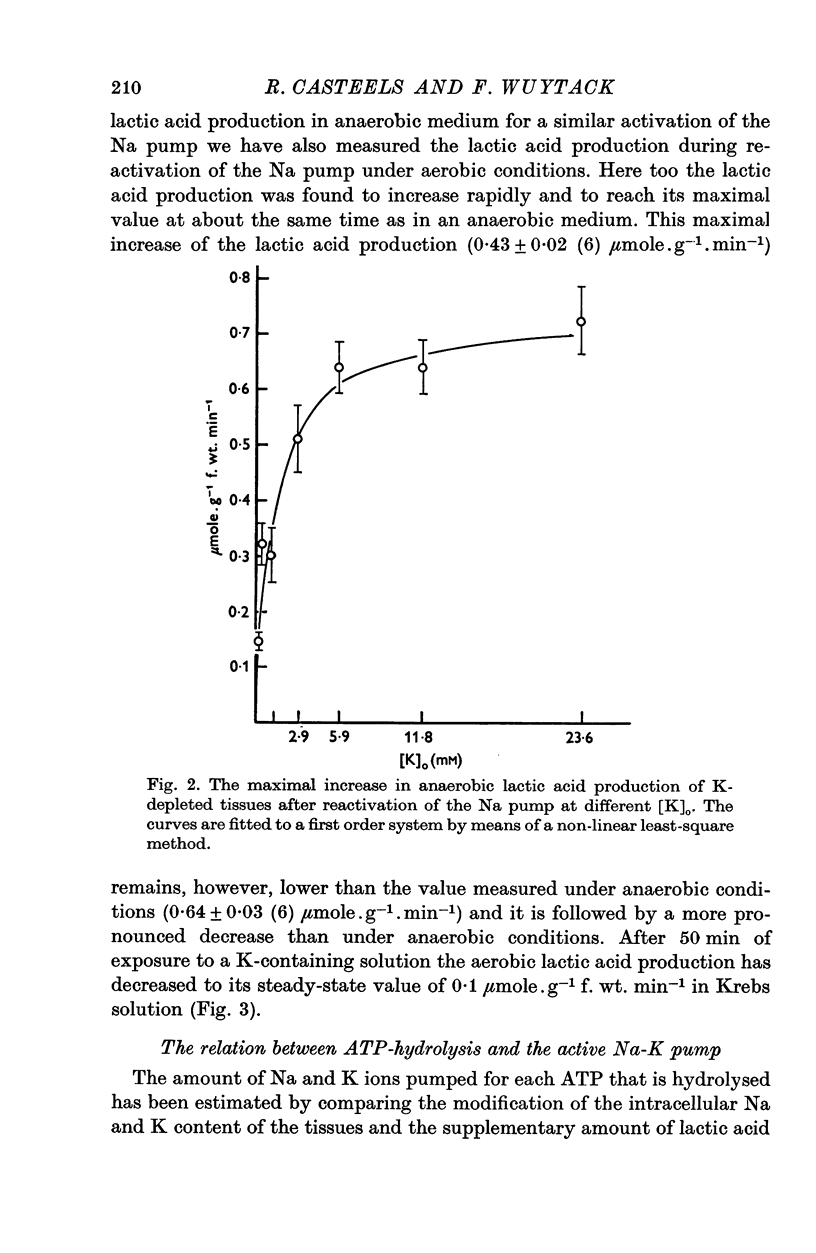
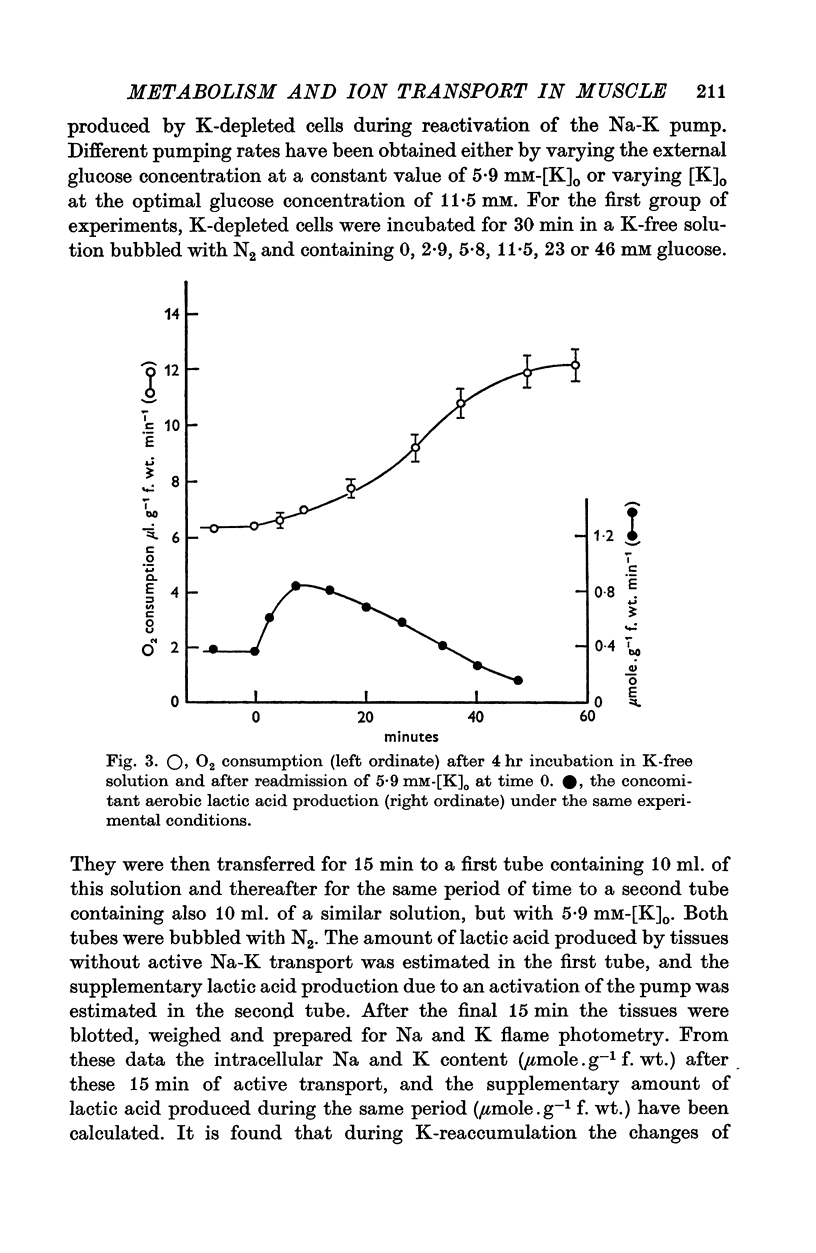
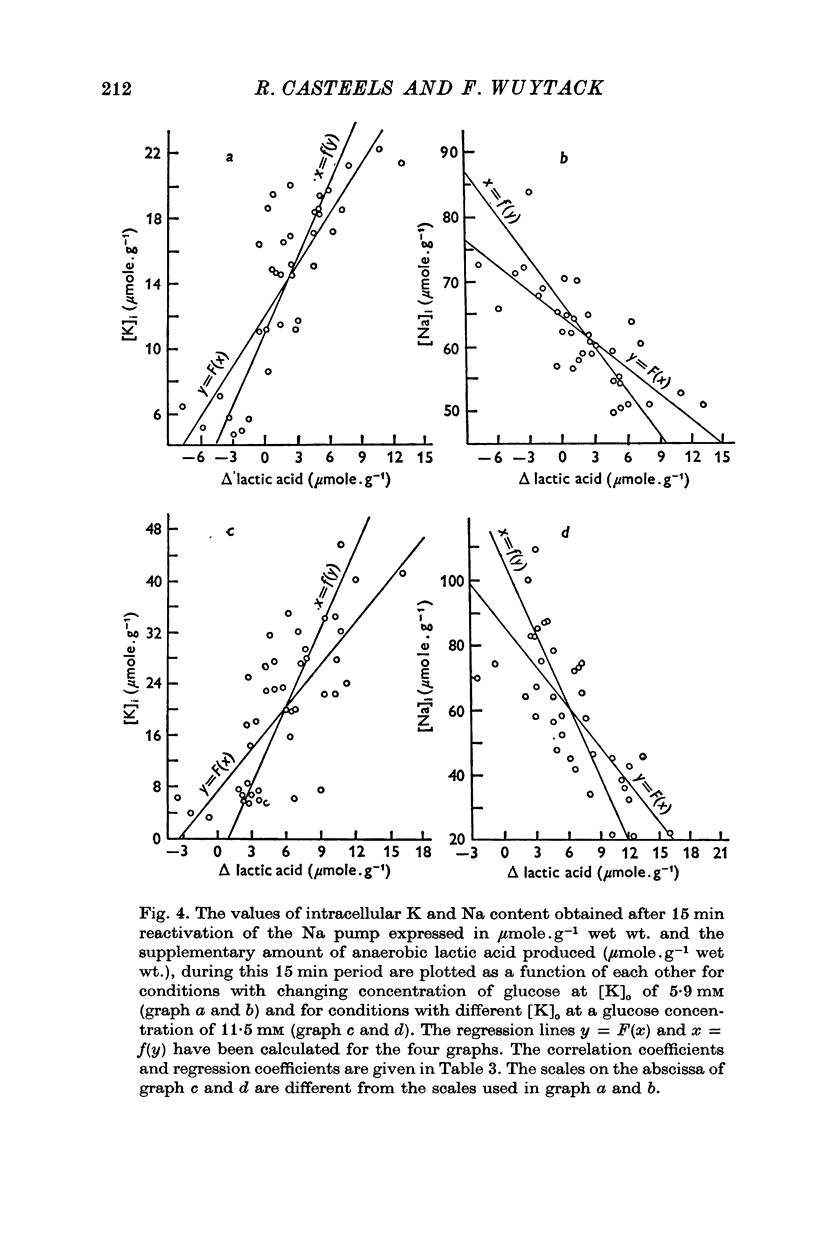
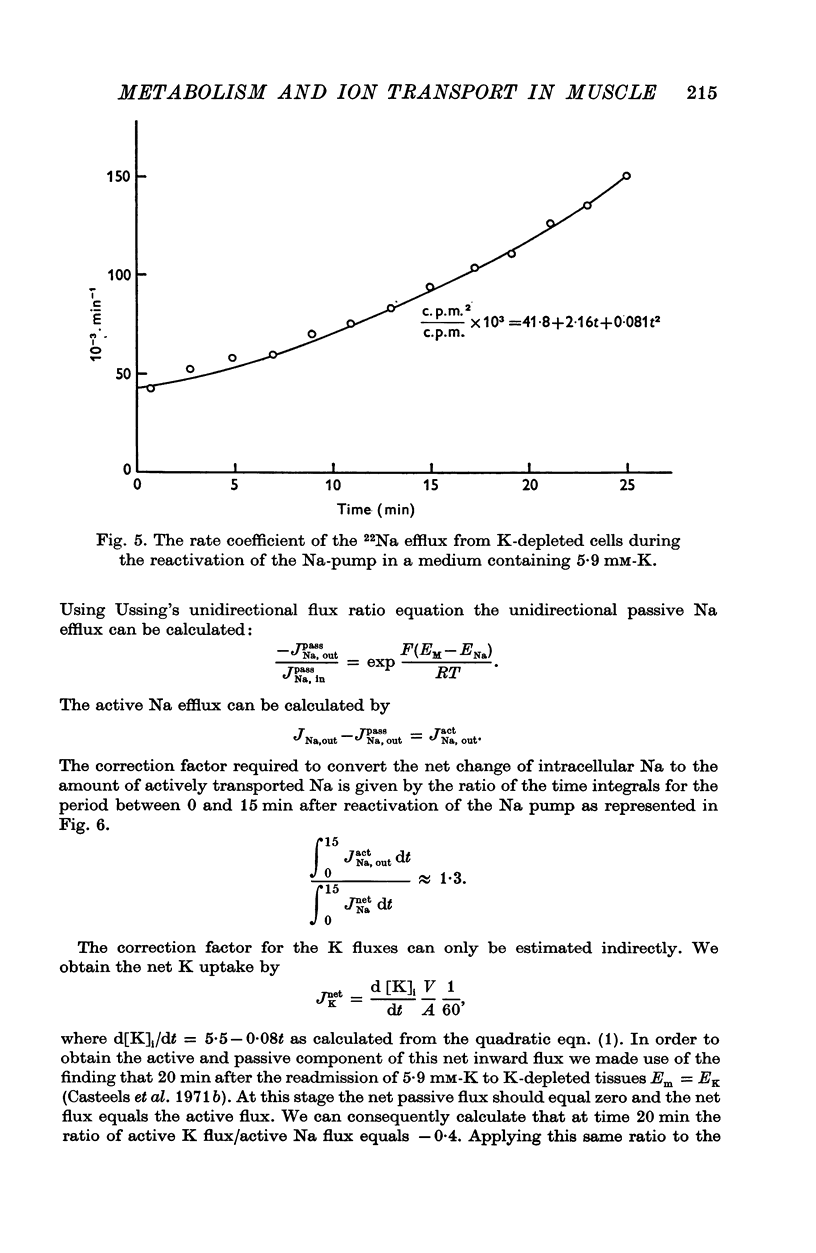
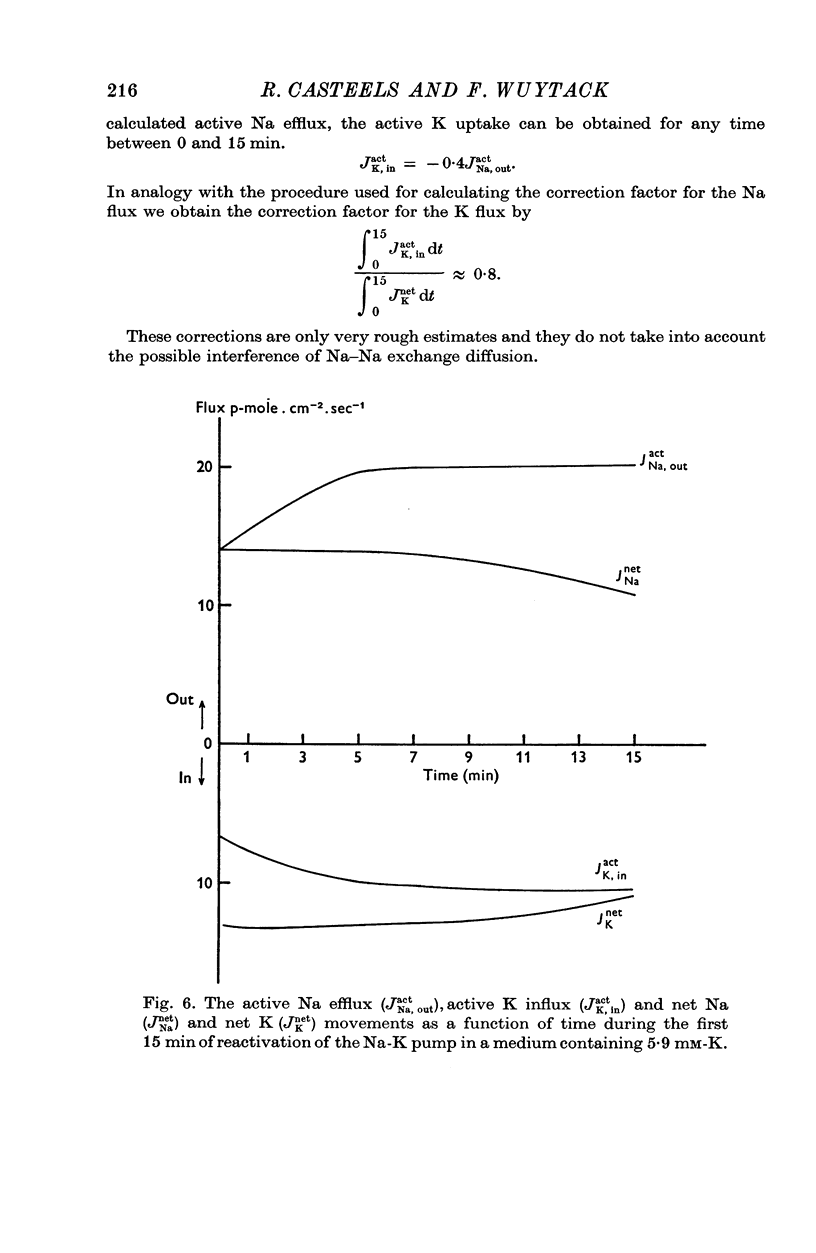
1. The O2 consumption and lactic acid production of the guinea-pig's taenia coli have been studied in relation to the active Na-K transport, in order to estimate the ratio: active Na extrusion/active K uptake/ATP hydrolysis. 2. By applying different procedures of partial metabolic ingibition, it was found that a reactivation of the active Na-K transport in K-depleted tissues could occur in an anaerobic medium, provided glucose was present and in an aerobic medium free of added metabolizable substrate. The active Na-K transport was rapidly blocked in an anaerobic-substrate free medium. 3. Readmission of K to K-depleted tissues under aerobic conditions stimulates both O2 consumption and lactic acid production. While the O2 consumption creeps up slowly and requires 50 min to reach control values, the aerobic lactic acid production increases to a maximum within 10 min and decreases again during the next 50 min to its steady-state value. 4. A reactivation of the Na-pump in K-depleted cells in a N2-glucose medium causes an immediate increase of the lactic acid production, which decreases to its control value after 60 min. The maximal increase in anaerobic lactic acid production during reactivation of the Na-K pump is a function of [K]O. The system can be cescribed with first order kinetics having a Vmax = 0-72 mumole.g-1 f. wt. min-1 and a Km = 1-1 mM. 5. By varying the glucose concentration of [K]O during reactivation of the Na-K pump, different Na-K pumping rates can be obtained. The ratios net Na extrusion/ATP or net K accumulation/ATP amount to -1-32 +/- 0-19 (36) and 1-02 +/- 0-11 (36), in the experiments with different glucose concentrations. Taking into account the interference by net passive fluxes, one can estimate a ratio:active Na transport/active K transport/ATP, of 1-7/0-8/1. This ratio is not very different from the values observed in other tissues.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUEDING E., HAWKINS J. T. ENZYMIC DEGRADATION AND MICRODETERMINATION OF GLYCOGEN. Anal Biochem. 1964 Jan;7:26–36. doi: 10.1016/0003-2697(64)90116-2. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Connelly C. M. Some properties of the external activation site of the sodium pump in crab nerve. J Physiol. 1966 Jul;185(2):270–297. doi: 10.1113/jphysiol.1966.sp007987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blond D. M., Whittam R. Effects of sodium and potassium ions on oxidative phosphorylation in relation to respiratory control by a cell-membrane adenosine triphosphatase. Biochem J. 1965 Nov;97(2):523–531. doi: 10.1042/bj0970523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueding E., Bülbring E., Gercken G., Hawkins J. T., Kuriyama H. The effect of adrenaline on the adenosine otriphosphate and creatine phosphate content of intestinal smooth muscle. J Physiol. 1967 Nov;193(1):187–212. doi: 10.1113/jphysiol.1967.sp008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave F. L. The ionic environment and metabolic control. Nature. 1967 May 13;214(5089):667–671. doi: 10.1038/214667a0. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Golenhofen K. Oxygen consumption by the isolated smooth muscle of guinea-pig taenia coli. J Physiol. 1967 Nov;193(1):213–224. doi: 10.1113/jphysiol.1967.sp008352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Calculation of the membrane potential in smooth muscle cells of the guinea-pig's taenia coli by the Goldman equation. J Physiol. 1969 Nov;205(1):193–208. doi: 10.1113/jphysiol.1969.sp008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Active ion transport and resting potential in smooth muscle cells. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):47–56. doi: 10.1098/rstb.1973.0008. [DOI] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Electrogenic sodium pump in smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Sep;217(2):297–313. doi: 10.1113/jphysiol.1971.sp009572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Membrane potential of smooth muscle cells in K-free solution. J Physiol. 1971 Sep;217(2):281–295. doi: 10.1113/jphysiol.1971.sp009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Goffin J., Raeymaekers L., Wuytack F. Calcium pumping in the smooth muscle cells of the taenia coli. J Physiol. 1973 May;231(1):19P–20P. [PubMed] [Google Scholar]
- Casteels R. The action of ouabain on the smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1966 May;184(1):131–142. doi: 10.1113/jphysiol.1966.sp007907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., van Breemen C., Wuytack F. Effect of metabolic depletion on the membrane permeability of smooth muscle cells and its modification by La 3+ . Nat New Biol. 1972 Oct 25;239(95):249–251. doi: 10.1038/newbio239249a0. [DOI] [PubMed] [Google Scholar]
- Dydynska M., Harris E. J. Consumption of high-energy phosphates during active sodium and potassium interchange in frog muscle. J Physiol. 1966 Jan;182(1):92–109. doi: 10.1113/jphysiol.1966.sp007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENNOR A. H., ROSENBERG H. The determination and distribution of phosphocreatine in animal tissues. Biochem J. 1952 Aug;51(5):606–610. doi: 10.1042/bj0510606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommaerts W. F. Energetics of muscular contraction. Physiol Rev. 1969 Jul;49(3):427–508. doi: 10.1152/physrev.1969.49.3.427. [DOI] [PubMed] [Google Scholar]
- Ruscák M., Whittam R. The metabolic response of brain slices to agents affecting the sodium pump. J Physiol. 1967 Jun;190(3):595–610. doi: 10.1113/jphysiol.1967.sp008230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Vascular smooth muscle. I. Normal structure, pathology, biochemistry, and biophysics. Pharmacol Rev. 1968 Dec;20(4):197–272. [PubMed] [Google Scholar]
- Stephens N. L., Wrogemann K. Oxidative phosphorylation in smooth muscle. Am J Physiol. 1970 Dec;219(6):1796–1801. doi: 10.1152/ajplegacy.1970.219.6.1796. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- WHITTAM R. Active cation transport as a pace-maker of respiration. Nature. 1961 Aug 5;191:603–604. doi: 10.1038/191603a0. [DOI] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. The connexion between active cation transport and metabolism in erythrocytes. Biochem J. 1965 Oct;97(1):214–227. doi: 10.1042/bj0970214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuytack F., Casteels R. The energy-rich phosphates in smooth muscle of the guinea pig taenia coli during metabolic depletion. Arch Int Physiol Biochim. 1972 Oct;80(4):829–830. [PubMed] [Google Scholar]
- Wuytack F., Raeymaekers L., Casteels R. Aerobic and anaerobic metabolism of smooth muscle cells in relation to active ion transport. Arch Int Physiol Biochim. 1973 Feb;81(1):162–163. [PubMed] [Google Scholar]
- van Breemen C., Farinas B. R., Casteels R., Gerba P., Wuytack F., Deth R. Factors controlling cytoplasmic Ca 2+ concentration. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):57–71. doi: 10.1098/rstb.1973.0009. [DOI] [PubMed] [Google Scholar]


