Abstract
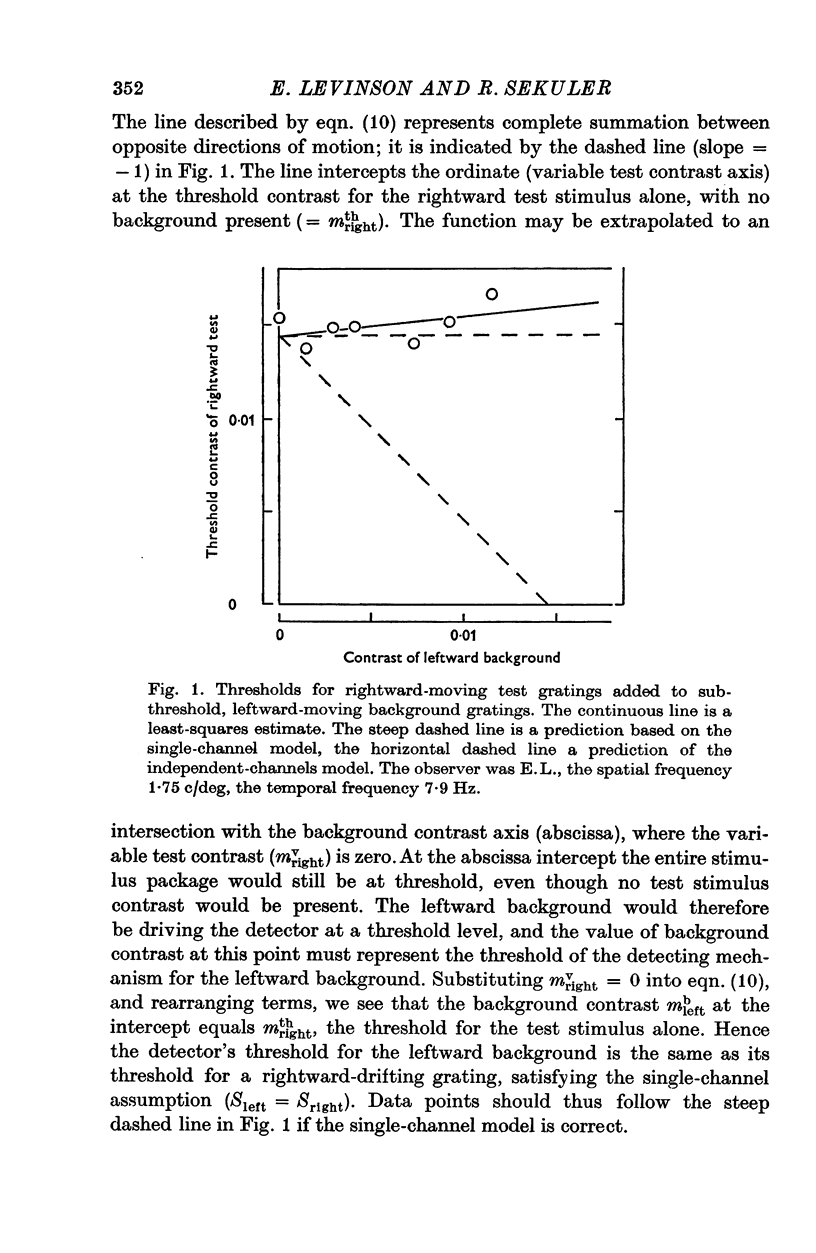
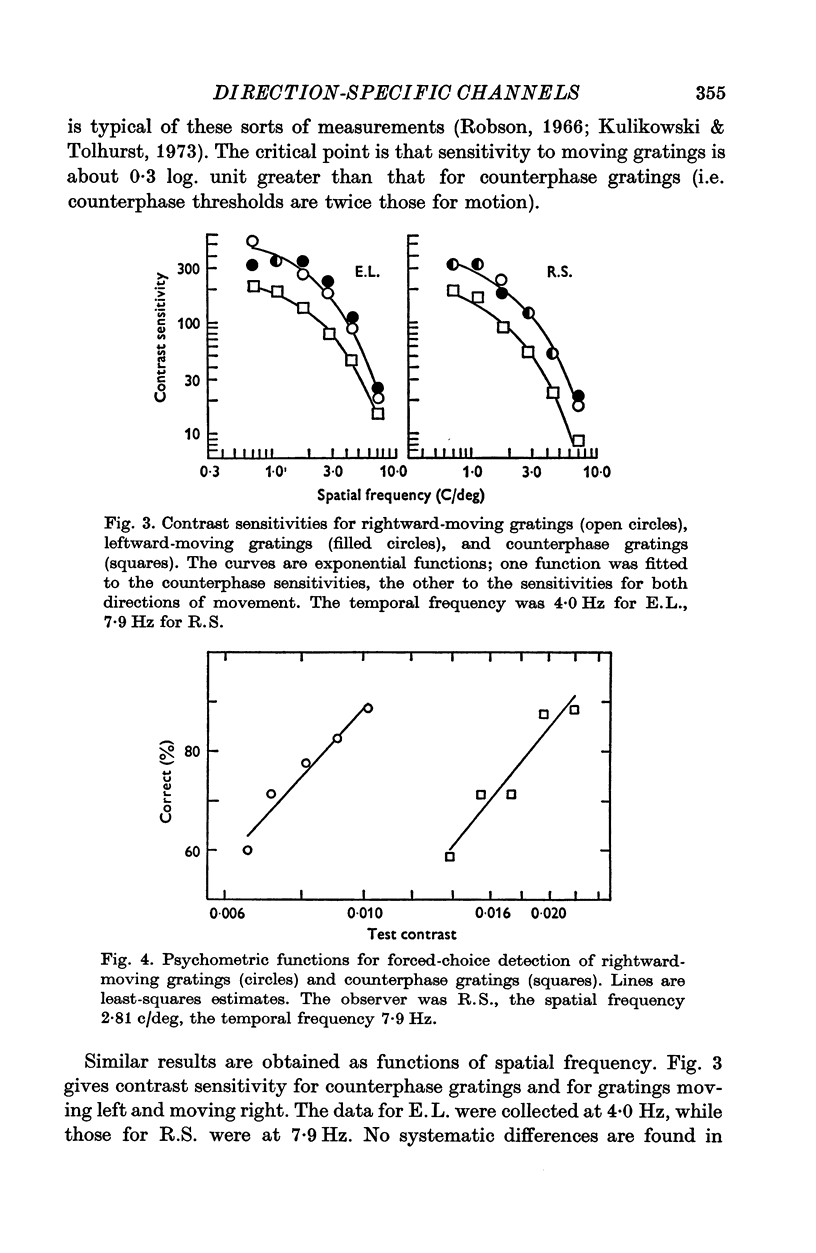
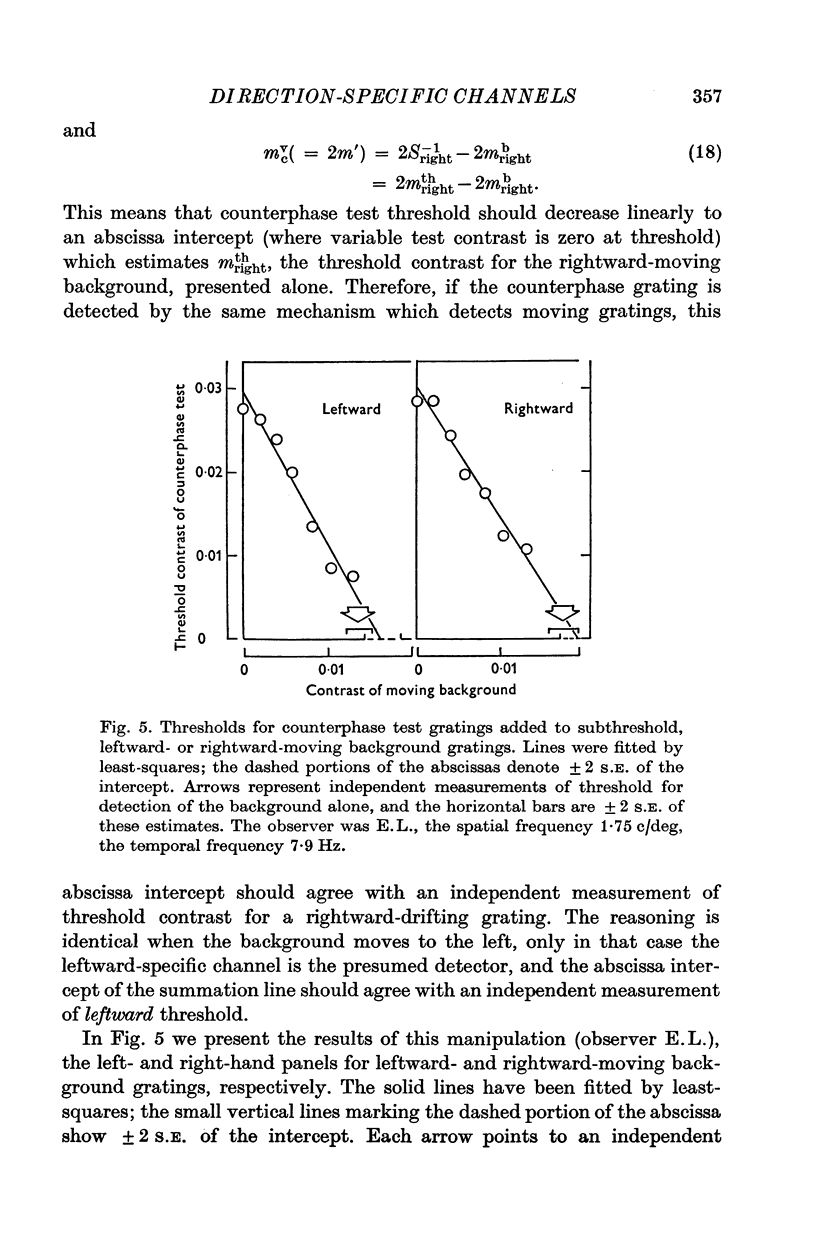
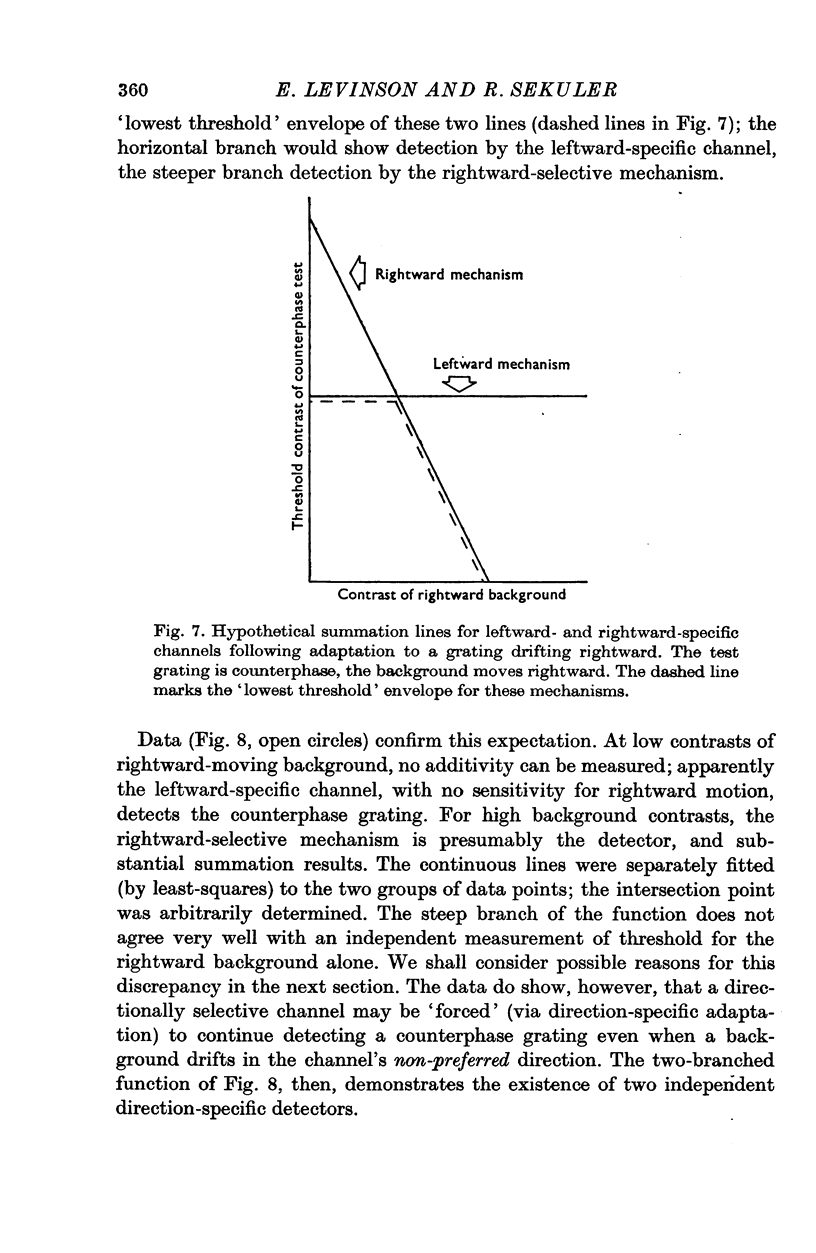
1. Human visual selectivity for direction of movement was determined using a subthreshold summation technique. 2. The threshold contrast for detecting a drifting sinusoidal grating was found to be independent of the contrast of an added subthreshold grating which moved in the opposite direction. 3. The detection threshold for a counterphase flickering grating is twice that for a moving grating, suggesting that the visual system analyses a counterphase grating as the sum of two half-contrast gratings which move in opposite directions. 4. Threshold for a counterphase grating may be linearly reduced by the addition of subthreshold background gratings drifting in either direction. Additivity between counterphase grating and moving background is complete. 5. After adaptation to a drifting grating, the behaviour of counterphase detection threshold as a function of the contrast of a moving subthreshold background depends upon the direction of background movement. When the background moves in a direction opposite that of the adaptation stimulus, complete linear additivity results. When the background moves in the same direction as the adapting grating, counterphase threshold is constant for low background contrasts, but drops linearly for higher background contrasts. 6. The results support the hypothesis that directionally selective channels in human vision are independent contrast detectors. Counterphase gratings are detected by one or the other of these direction-specific mechanisms, whichever is momentarily the more sensitive.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benevento L. A., Creutzfeldt O. D., Kuhnt U. Significance of intracortical inhibition in the visual cortex. Nat New Biol. 1972 Jul 26;238(82):124–126. doi: 10.1038/newbio238124a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Tobin E. A. Lateral inhibition between orientation detectors in the cat's visual cortex. Exp Brain Res. 1972;15(4):439–440. doi: 10.1007/BF00234129. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Green D. G. Optical and retinal factors affecting visual resolution. J Physiol. 1965 Dec;181(3):576–593. doi: 10.1113/jphysiol.1965.sp007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dealy R. S., Tolhurst D. J. Is spatial adaptation an after-effect of prolonged inhibition? J Physiol. 1974 Aug;241(1):261–270. doi: 10.1113/jphysiol.1974.sp010652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham N., Nachmias J. Detection of grating patterns containing two spatial frequencies: a comparison of single-channel and multiple-channels models. Vision Res. 1971 Mar;11(3):251–259. doi: 10.1016/0042-6989(71)90189-1. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Dreher B. Orientation, axis and direction as stimulus parameters for striate cells. Vision Res. 1974 Sep;14(9):767–777. doi: 10.1016/0042-6989(74)90141-2. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesey U. T. Flicker and pattern detection: a comparison of thresholds. J Opt Soc Am. 1972 Mar;62(3):446–448. doi: 10.1364/josa.62.000446. [DOI] [PubMed] [Google Scholar]
- Kelly D. H. Adaptation effects on spatio-temporal sine-wave thresholds. Vision Res. 1972 Jan;12(1):89–101. doi: 10.1016/0042-6989(72)90139-3. [DOI] [PubMed] [Google Scholar]
- Kelly D. H. Theory of flicker and transient responses. II. Counterphase gratings. J Opt Soc Am. 1971 May;61(5):632–640. doi: 10.1364/josa.61.000632. [DOI] [PubMed] [Google Scholar]
- Kulikowski J. J., King-Smith P. E. Spatial arrangement of line, edge and grating detectors revealed by subthreshold summation. Vision Res. 1973 Aug;13(8):1455–1478. doi: 10.1016/0042-6989(73)90006-0. [DOI] [PubMed] [Google Scholar]
- Kulikowski J. J., Tolhurst D. J. Psychophysical evidence for sustained and transient detectors in human vision. J Physiol. 1973 Jul;232(1):149–162. doi: 10.1113/jphysiol.1973.sp010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson E., Sekuler R. Inhibition and disinhibition of direction-specific mechanisms in human vision. Nature. 1975 Apr 24;254(5502):692–694. doi: 10.1038/254692a0. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A., Bisti S. Neural correlate of perceptual adaptation to gratings. Science. 1973 Dec 7;182(4116):1036–1038. doi: 10.1126/science.182.4116.1036. [DOI] [PubMed] [Google Scholar]
- Pantle A., Sekuler R. Contrast response of human visual mechanismsensitive to orientation and direction of motion. Vision Res. 1969 Mar;9(3):397–406. doi: 10.1016/0042-6989(69)90087-x. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Daniels J. D. Gamma-aminobutyric acid antagonism in visual cortex: different effects on simple, complex, and hypercomplex neurons. Science. 1973 Oct 5;182(4107):81–83. doi: 10.1126/science.182.4107.81. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Poggio G. F. Spatial properties of neurons in striate cortex of unanesthetized macaque monkey. Invest Ophthalmol. 1972 May;11(5):368–377. [PubMed] [Google Scholar]
- SEKULER R. W., GANZ L. Aftereffect of seen motion with a stabilized retinal image. Science. 1963 Feb 1;139(3553):419–420. doi: 10.1126/science.139.3553.419. [DOI] [PubMed] [Google Scholar]
- Sachs M. B., Nachmias J., Robson J. G. Spatial-frequency channels in human vision. J Opt Soc Am. 1971 Sep;61(9):1176–1186. doi: 10.1364/josa.61.001176. [DOI] [PubMed] [Google Scholar]
- Sekuler R. Spatial vision. Annu Rev Psychol. 1974;25:195–232. doi: 10.1146/annurev.ps.25.020174.001211. [DOI] [PubMed] [Google Scholar]
- Shapley R. M., Tolhurst D. J. Edge detectors in human vision. J Physiol. 1973 Feb;229(1):165–183. doi: 10.1113/jphysiol.1973.sp010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst D. J. Separate channels for the analysis of the shape and the movement of moving visual stimulus. J Physiol. 1973 Jun;231(3):385–402. doi: 10.1113/jphysiol.1973.sp010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz R. H. Visual receptive fields of striate cortex neurons in awake monkeys. J Neurophysiol. 1969 Sep;32(5):727–742. doi: 10.1152/jn.1969.32.5.727. [DOI] [PubMed] [Google Scholar]


