Abstract
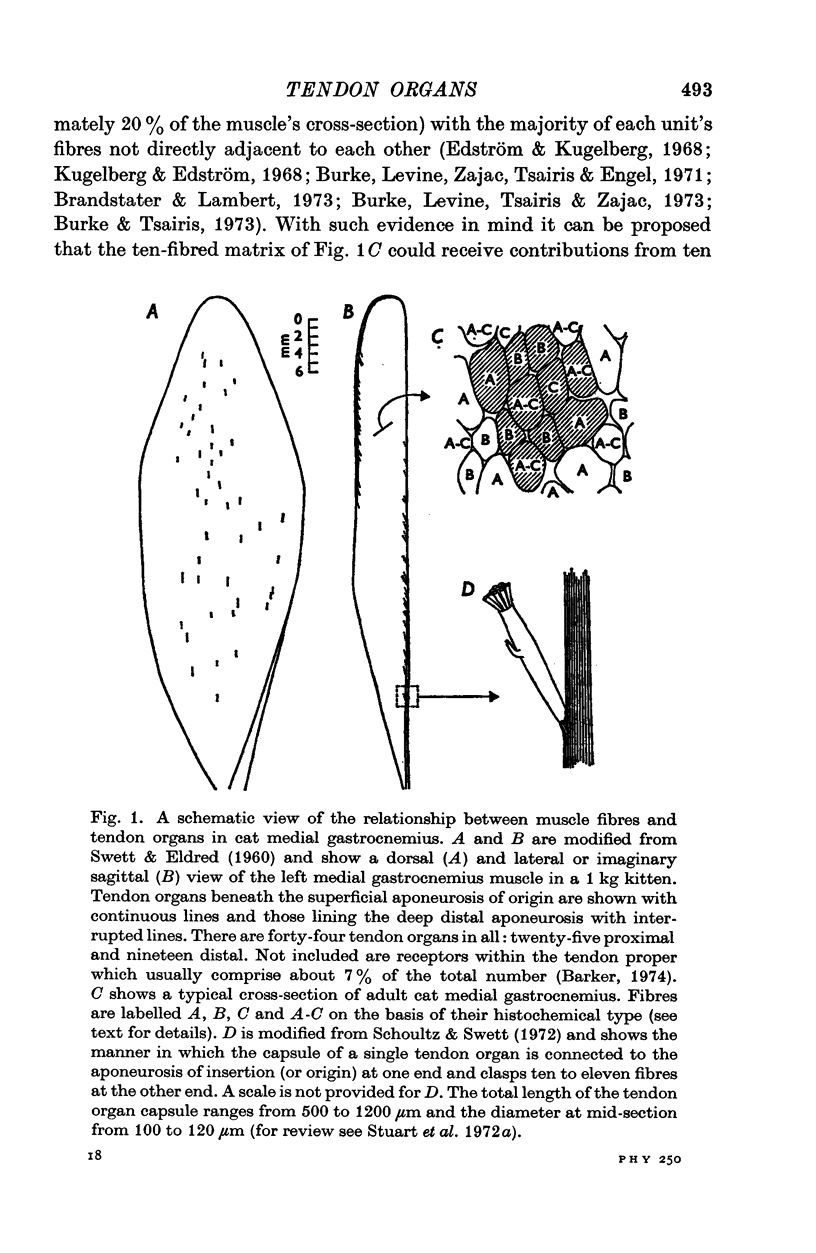
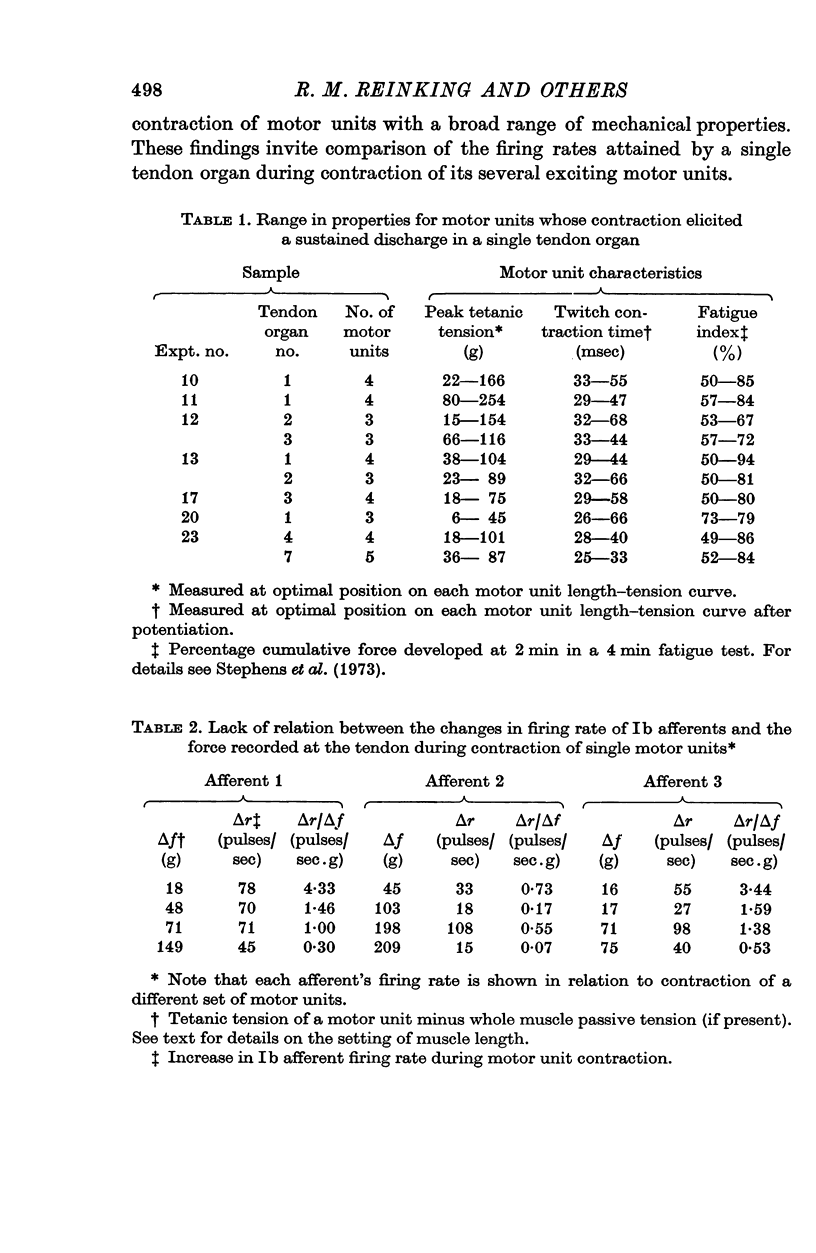
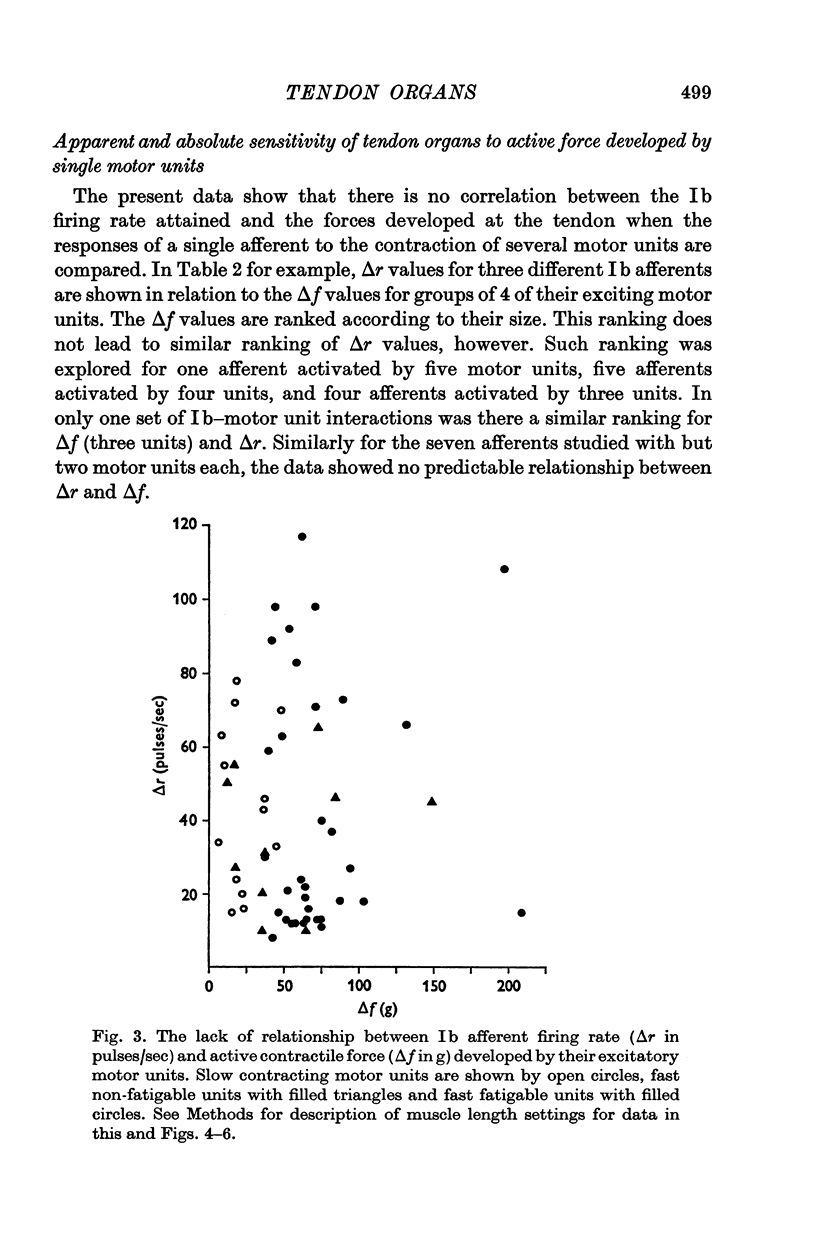
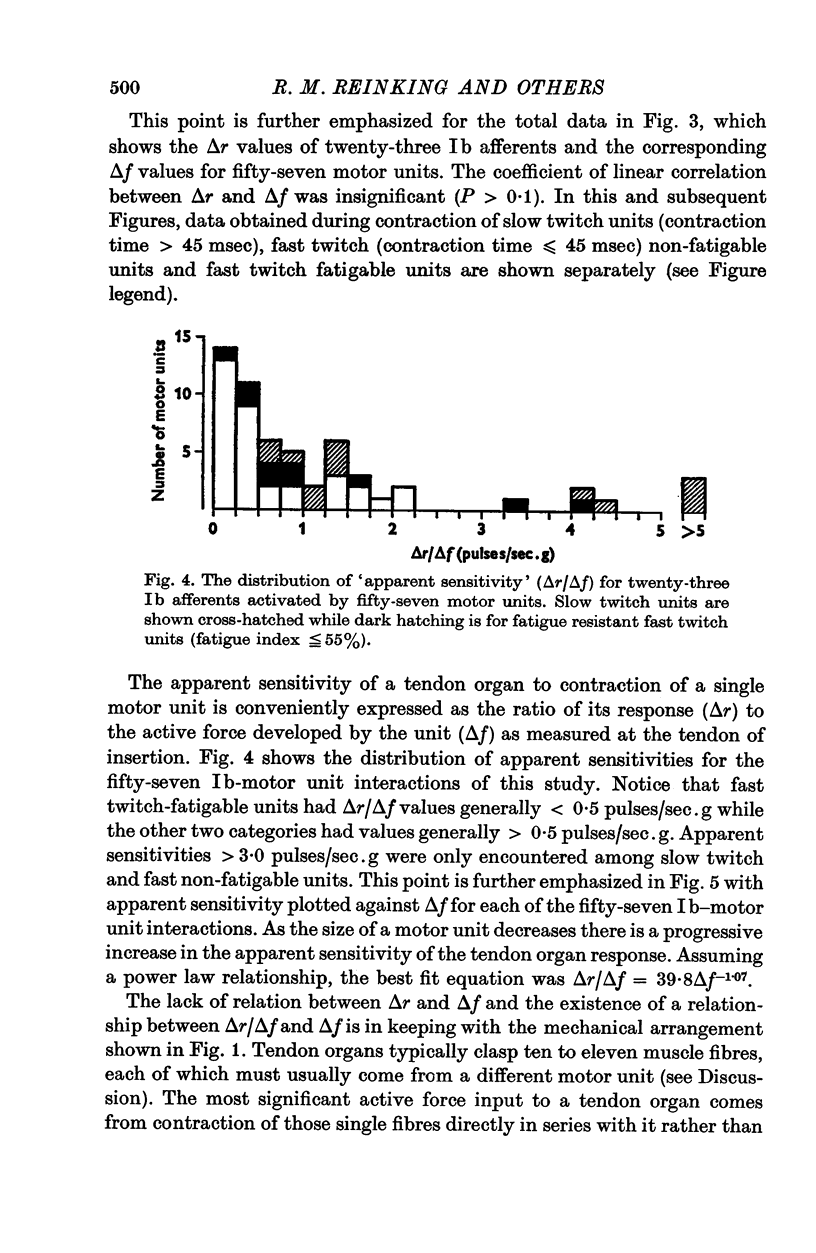
1. Histological and histochemical studies suggest that each tendon organ in a mixed mammalian muscle should be particularly responsive to the contraction of a discrete number of motor units (ca. ten to fifteen), each with differing mechanical properties. This report describes physiological experiments that demonstrate this arrangement for the tendon organs of cat medial gastrocnemius. 2. No correlations could be found between the intensity of discharge of a single tendon organ and the contraction strengths of motor units whose contraction excited the receptor. Tendon organs were found to be as responsive to contraction of small slow twitch units as they were to contraction of larger fast twitch units. Taking the data as a whole, the apparent sensitivity of the receptors during motor unit contractions (pps/force recorded at the tendon) was inversely related to the contraction strengths of the motor units. 3. These findings are discussed in relation to recent evidence on the territory of single motor units in medial gastrocnemius and the force producing capabilities of their individual muscle fibres. It is concluded that in general each motor unit, whose contraction excites a given receptor, contributes one muscle fibre to the receptor capsule. Further, it appears that the various excitatory effects of those muscle fibres inserting into a given receptor capsule are not simply related to their relative contraction strengths but also depend on the details of the mechanical coupling between each fibre and the Ib afferent receptor endings. 4. The results of an ensemble analysis show that despite the lack of correlation between the intensity of tendon organ discharge and the force developed at the tendon during contraction of different motor units, a correlation does appear when the responses of several tendon organs and the forces developed by the motor units which excite them are summed progressively. This finding has implications for the recruitment order of motor units in that the profile of the collective Ib response is shown to differ according to whether motor unit forces are summed randomly or in order of increasing contraction strengths.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- BULLER A. J., LEWIS D. M. FURTHER OBSERVATIONS ON THE DIFFERENTIATION OF SKELETAL MUSCLES IN THE KITTEN HIND LIMB. J Physiol. 1965 Feb;176:355–370. doi: 10.1113/jphysiol.1965.sp007555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust J., Knott S., Lewis D. M., Luck J. C., Westerman R. A. Isometric contractions of motor units in a fast twitch muscle of the cat. J Physiol. 1973 May;231(1):87–104. doi: 10.1113/jphysiol.1973.sp010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman C. F. The structure of tendon organs in the cat: a proposed mechanism for responding to muscle tension. Anat Rec. 1968 Oct;162(2):209–220. doi: 10.1002/ar.1091620208. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Zajac F. E., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971 Nov 12;174(4010):709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Anatomy and innervation ratios in motor units of cat gastrocnemius. J Physiol. 1973 Nov;234(3):749–765. doi: 10.1113/jphysiol.1973.sp010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Edström L., Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslow G. E., Jr, Reinking R. M., Stuart D. G. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol. 1973 Sep;141(1):1–41. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Grillner S. The role of muscle stiffness in meeting the changing postural and locomotor requirements for force development by the ankle extensors. Acta Physiol Scand. 1972 Sep;86(1):92–108. doi: 10.1111/j.1748-1716.1972.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Houk J. C., Singer J. J., Goldman M. R. An evaluation of length and force feedback to soleus muscles of decerebrate cats. J Neurophysiol. 1970 Nov;33(6):784–811. doi: 10.1152/jn.1970.33.6.784. [DOI] [PubMed] [Google Scholar]
- Houk J., Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol. 1967 May;30(3):466–481. doi: 10.1152/jn.1967.30.3.466. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Edström L. Differential histochemical effects of muscle contractions on phosphorylase and glycogen in various types of fibres: relation to fatigue. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):415–423. doi: 10.1136/jnnp.31.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS P. B. THE RESPONSE OF DE-EFFERENTED MUSCLE SPINDLE RECEPTORS TO STRETCHING AT DIFFERENT VELOCITIES. J Physiol. 1963 Oct;168:660–678. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCPHEDRAN A. M., WUERKER R. B., HENNEMAN E. PROPERTIES OF MOTOR UNITS IN A HOMOGENEOUS RED MUSCLE (SOLEUS) OF THE CAT. J Neurophysiol. 1965 Jan;28:71–84. doi: 10.1152/jn.1965.28.1.71. [DOI] [PubMed] [Google Scholar]
- NORRIS F. H., Jr, IRWIN R. L. Motor unit area in a rat muscle. Am J Physiol. 1961 May;200:944–946. doi: 10.1152/ajplegacy.1961.200.5.944. [DOI] [PubMed] [Google Scholar]
- Reinking R. M., Stuart D. G. Servo-regulated electromagnetic system for muscle stretch and vibration. Am J Phys Med. 1974 Feb;53(1):1–28. [PubMed] [Google Scholar]
- SWETT J. E., ELDRED E. Distribution and numbers of stretch rceptors in medial gastrocnemius and soleus muscles of the cat. Anat Rec. 1960 Aug;137:453–460. doi: 10.1002/ar.1091370405. [DOI] [PubMed] [Google Scholar]
- Schoultz T. W., Swett J. E. The fine structure of the Golgi tendon organ. J Neurocytol. 1972 Jul;1(1):1–26. doi: 10.1007/BF01098642. [DOI] [PubMed] [Google Scholar]
- Schoultz T. W., Swett J. E. Ultrastructural organization of the sensory fibers innervating the Golgi tendon organ. Anat Rec. 1974 Jun;179(2):147–162. doi: 10.1002/ar.1091790202. [DOI] [PubMed] [Google Scholar]
- Stephens J. A., Stuart D. G. Proceedings: The responses of Golgi tendon organs to contractions of single motor units in a mixed mammalian muscle. J Physiol. 1974 Oct;242(2):62P–63P. [PubMed] [Google Scholar]
- Stephens J. A., Stuart D. G. The motor units of cat medial gastrocnemius: speed-size relations and their significance for the recruitment order of mortor units. Brain Res. 1975 Jun 27;91(2):177–195. doi: 10.1016/0006-8993(75)90542-9. [DOI] [PubMed] [Google Scholar]
- Sturart D. G., Mosher C. G., Gerlach R. I., Reinking R. M. Mechanical arrangement and transducing properties of Golgi tendon organs. Exp Brain Res. 1972;14(3):274–292. doi: 10.1007/BF00816163. [DOI] [PubMed] [Google Scholar]


