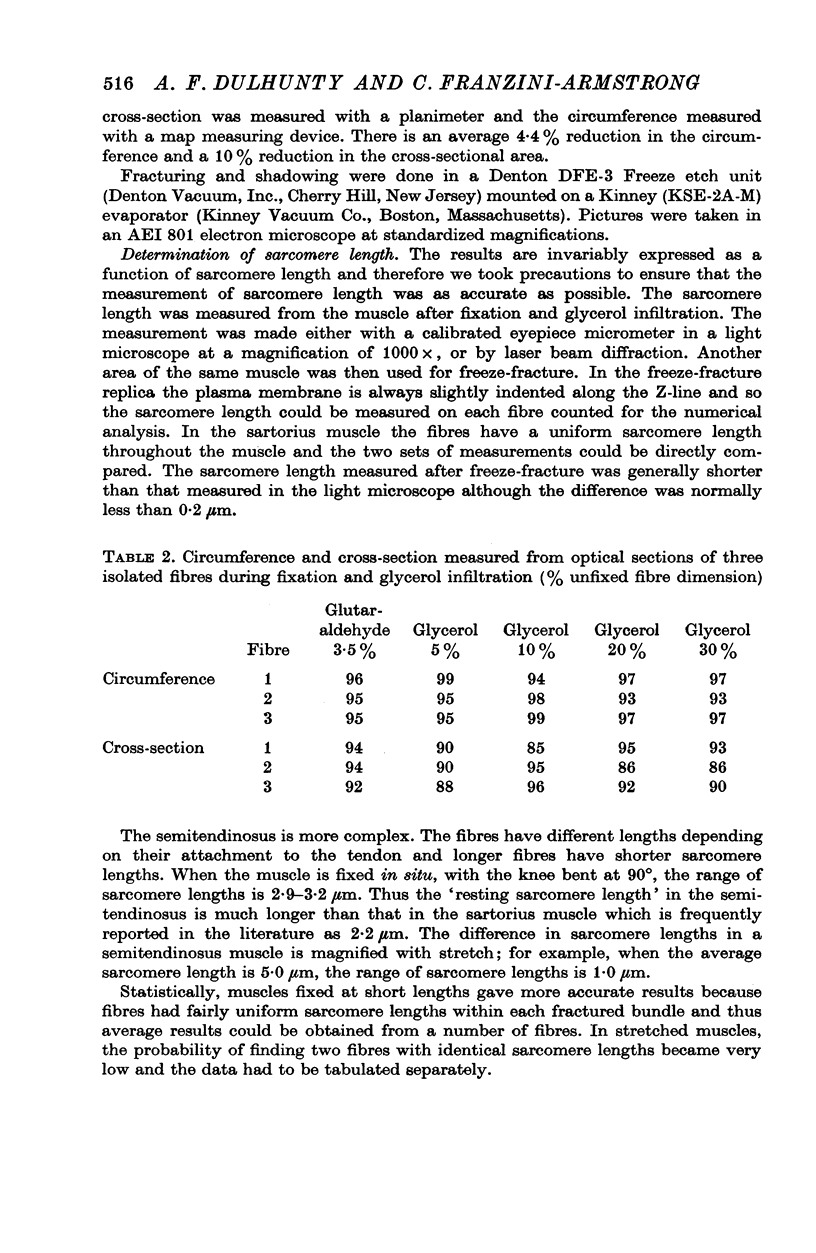
Abstract
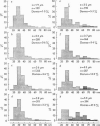
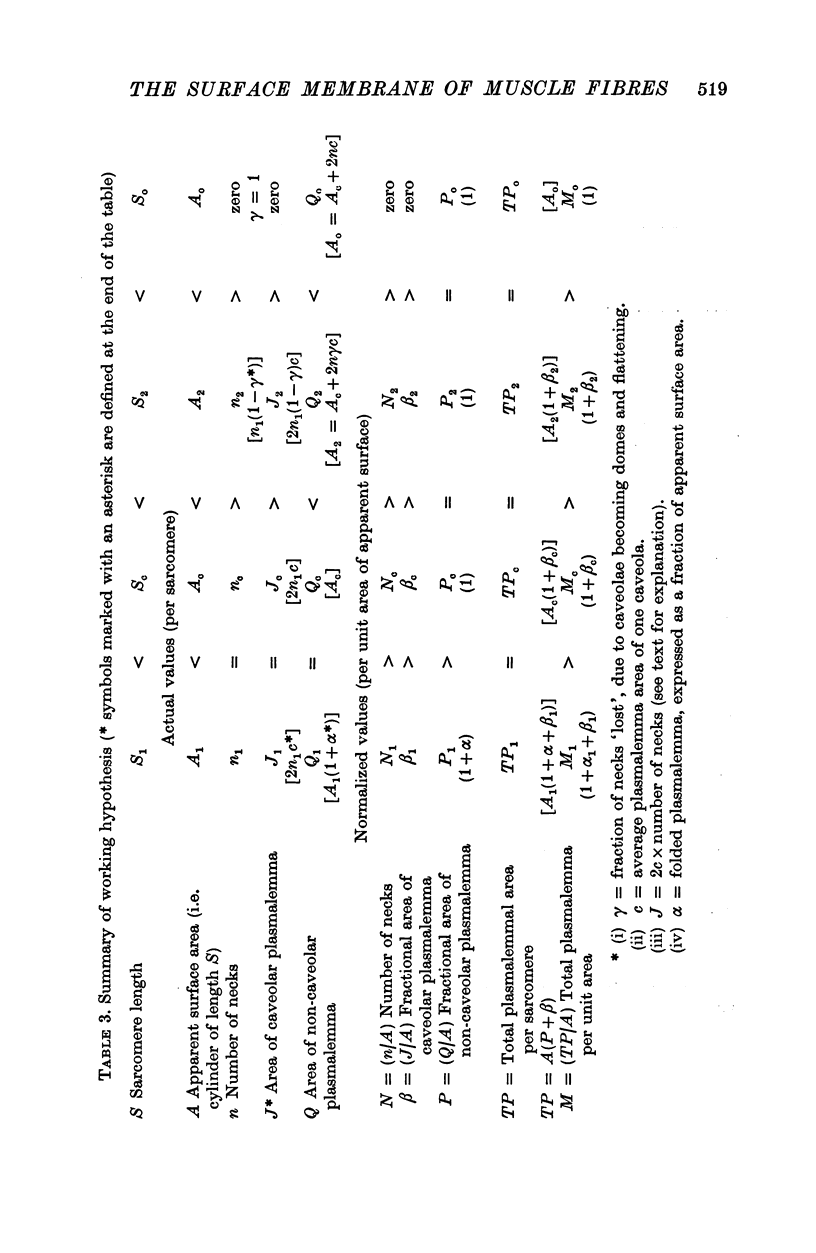
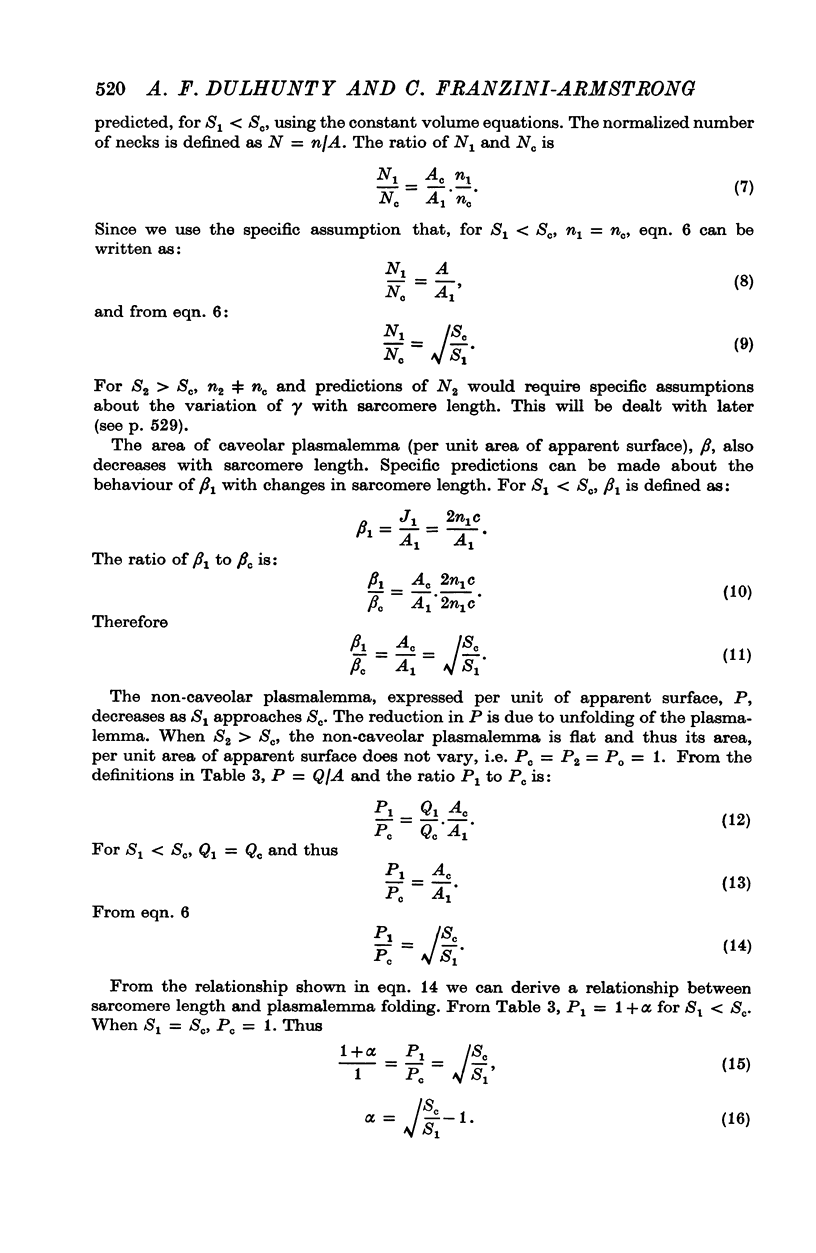
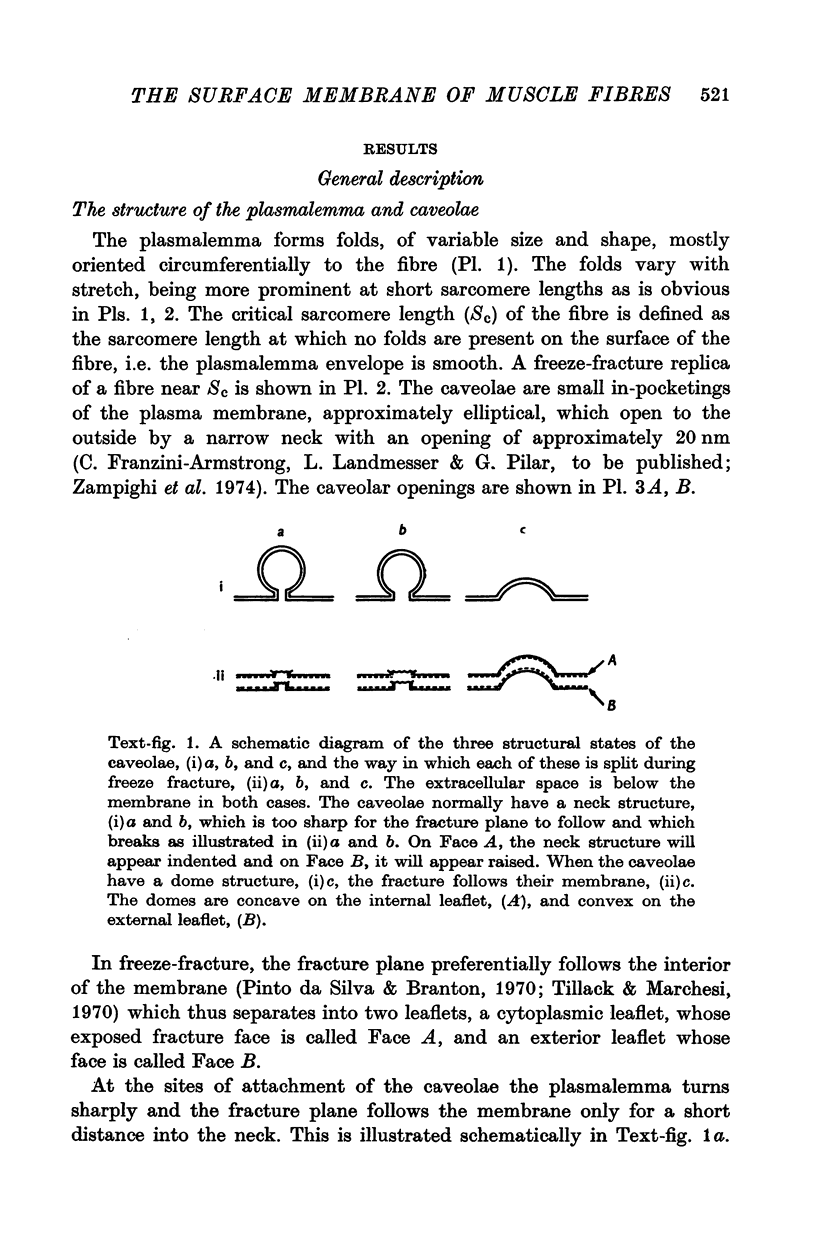
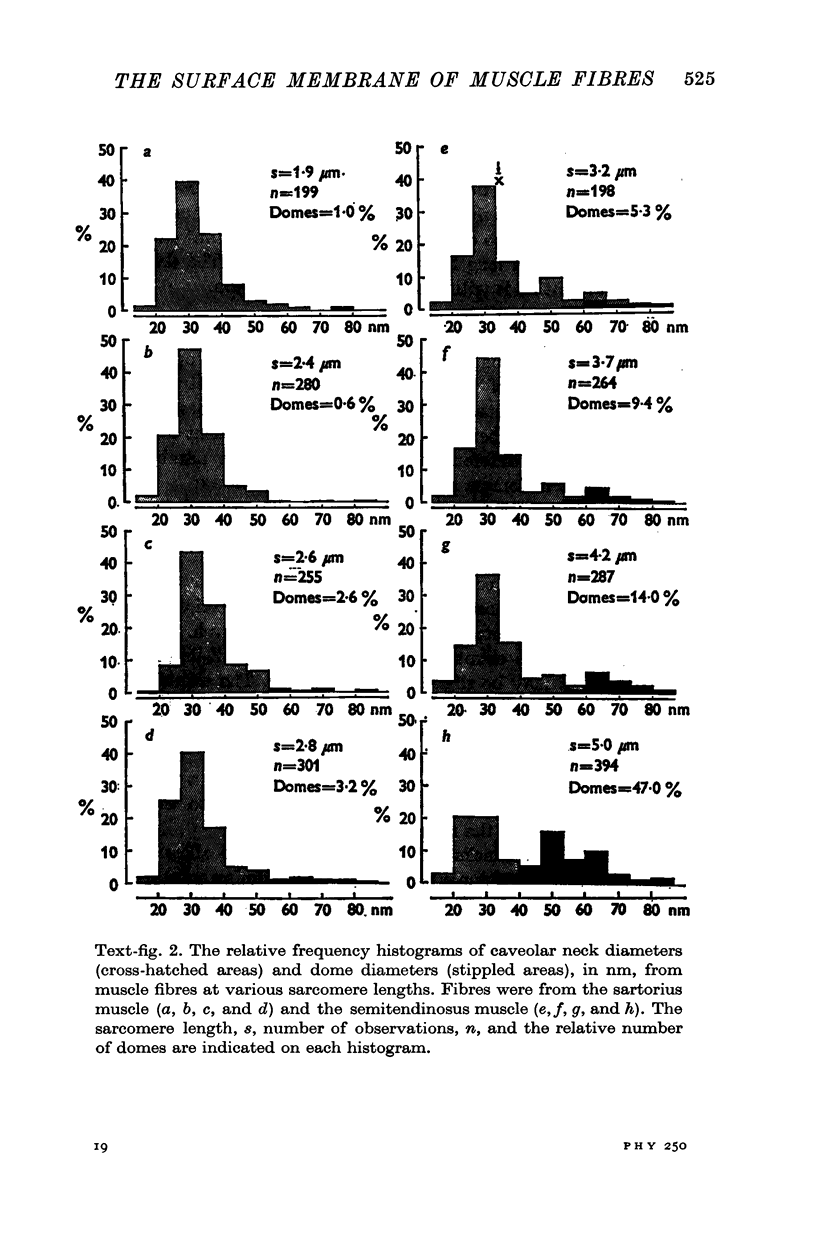
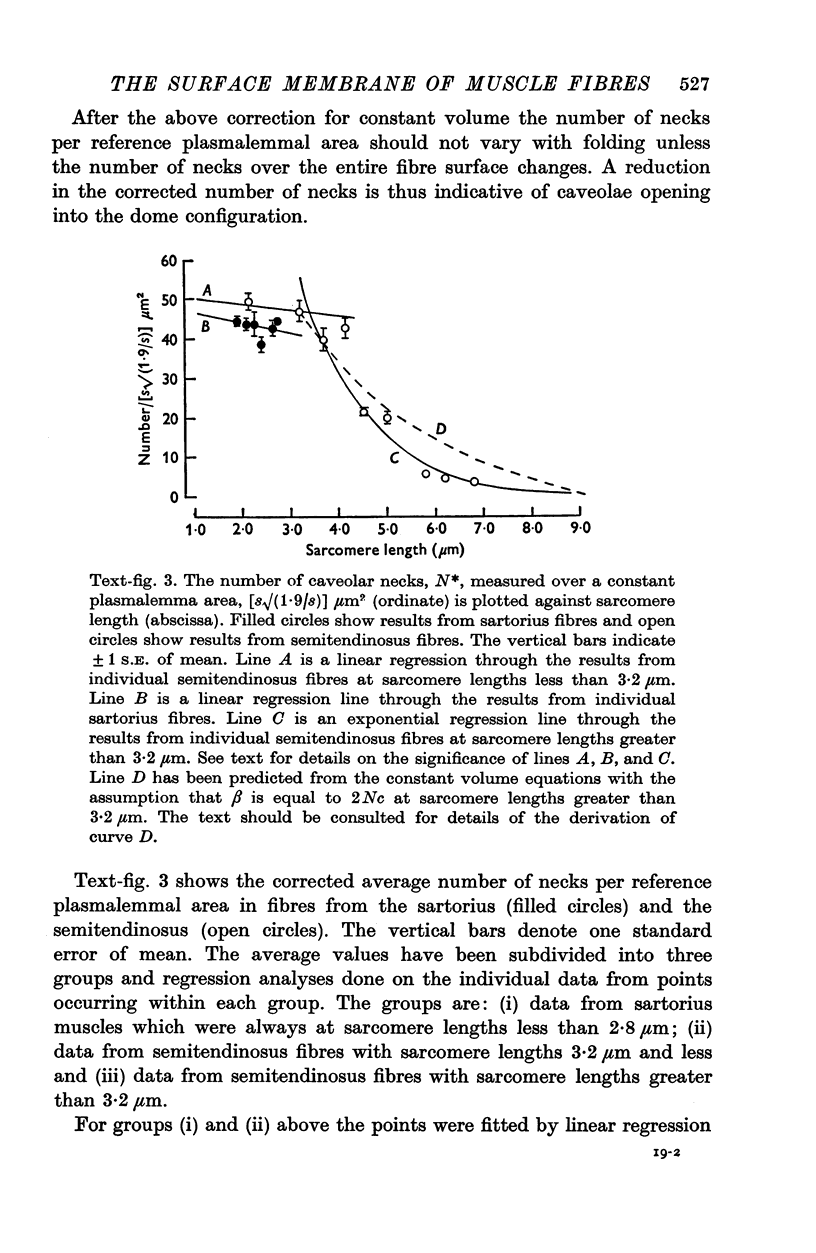
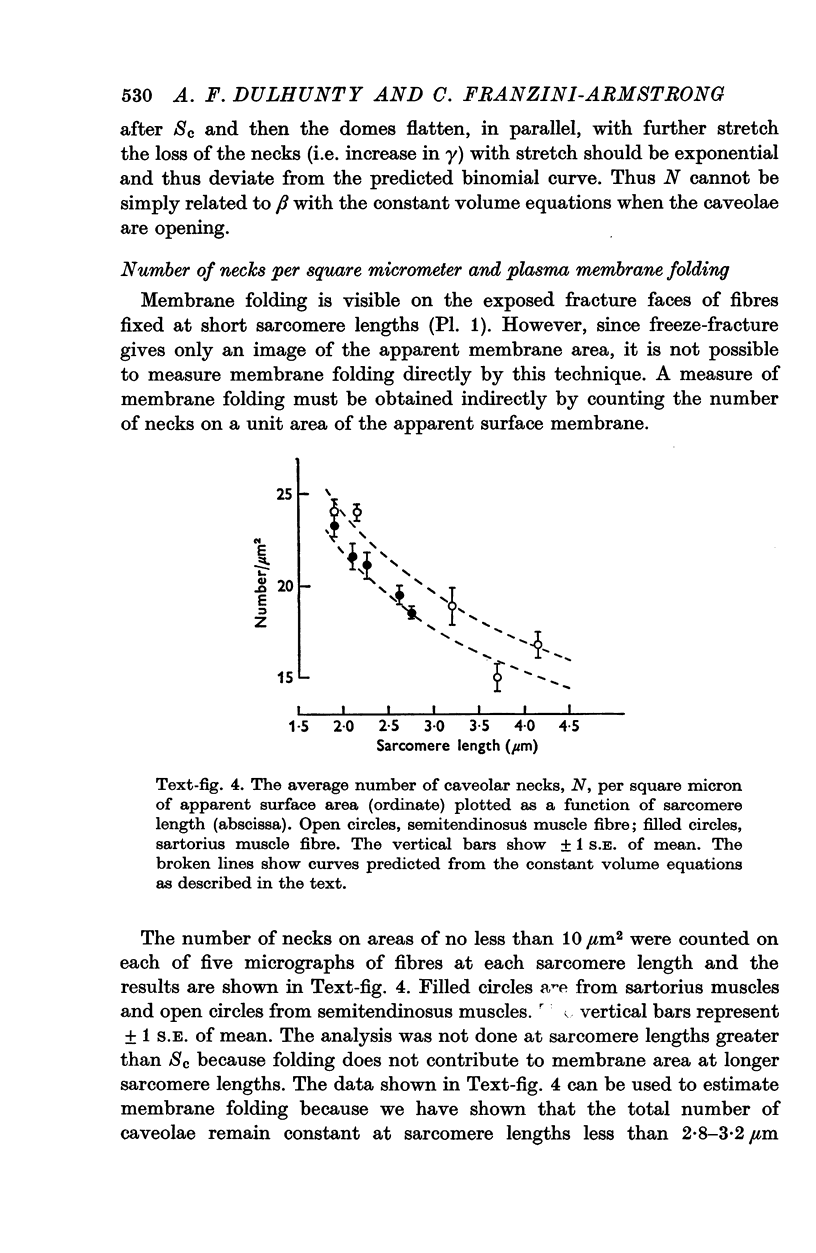
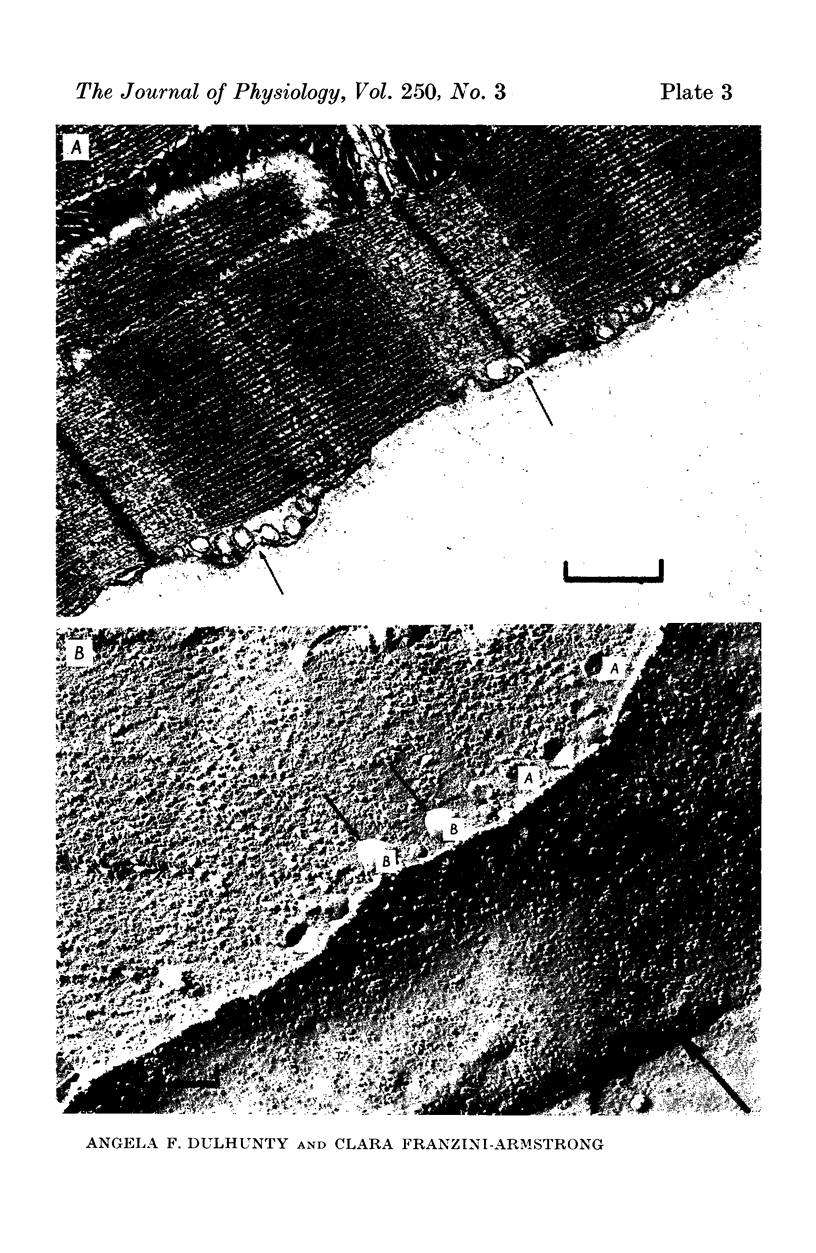
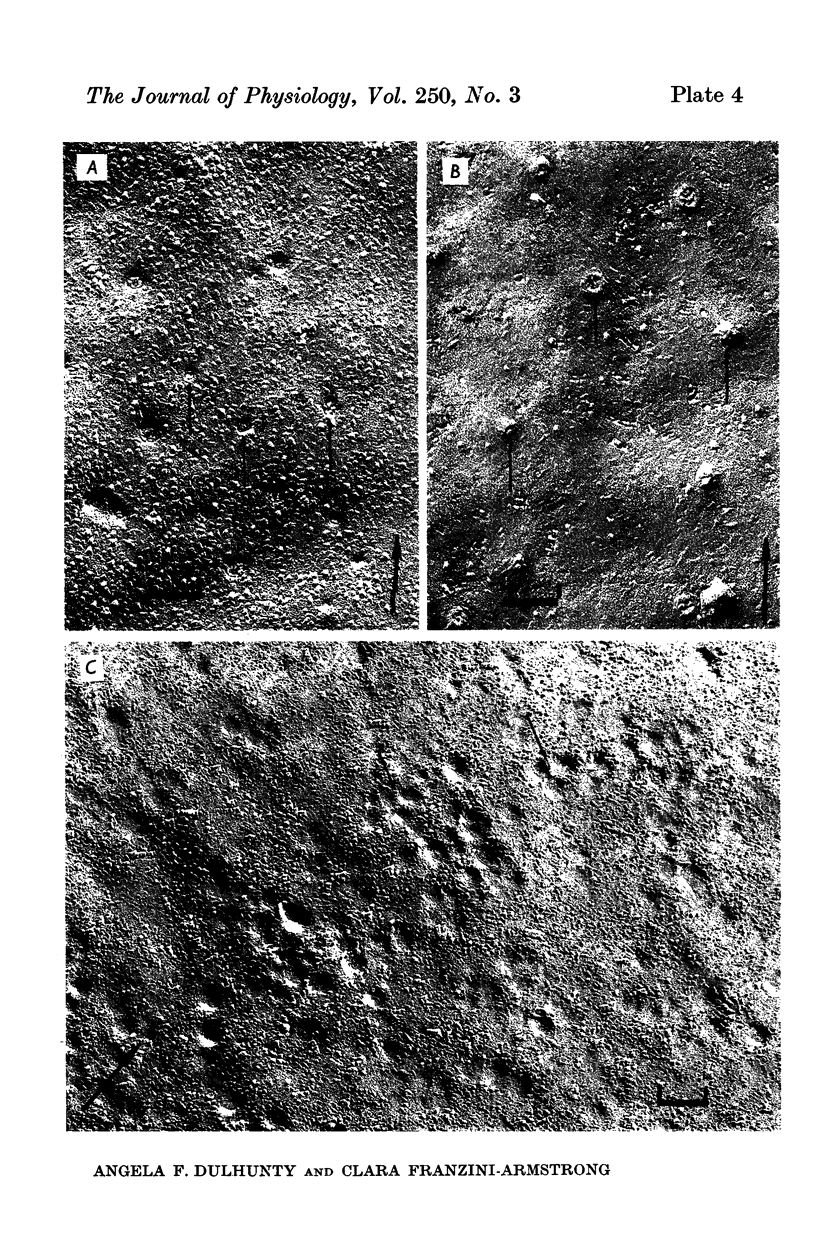
The plasmalemmal area of striated muscle fibres is greater than the apparent surface area (A = circumference x length) because of variable folds and the invaginations of the caveolae and T-tubules. Freeze-fracture replicas of the surface membrane of sartorius and semitendinosus muscles from Rana pipiens have been used to determine the numbers and distribution of folds and caveolae at different sarcomere lengths. (1) The plasmalemma folds are variable in size and shape, but are always oriented perpendicular to the long axis of the fibre. The folds vary with stretch, being more prominent at short sarcomere lengths. The caveolae are elliptical invaginations of the plasmalemma which open to the outside by a narrow "neck" of approximately 20 nm. The caveolar lumen has an average long dimension of 81.6 +/- 11.7 nm and an average short dimension of 66.9 +/- 7.9 nm. The caveolar "necks" only can be seen in freeze-fracture replicas and these are distributed in two circumferential bands on either side of the Z-line, and in longitudinal bands separated by distances of 1-5 mum. In the sartorius muscle, at a sarcomere length of 2.8 mum, there is an average number of thirty-seven caveolae per square micrometer of fibre surface. (2) During passive stretch the opening of folds provides membrane for the necessary increase in surface area up to a sarcomere length of about 3.0 mum. This length is defined as the critical sarcomere length (Sc). The number of caveolae remains constant at all sarcomere lengths less than Sc and thus their "necks" have been used as membrane markers to determine the amount of folding at different sarcomere lengths. The membrane area contained in folds and caveolae is expressed as a fraction of the apparent surface area (A). For example, in the sartorius muscle, at a sarcomere length of 2.4 mum, the membrane area, excluding the T-tubules, is: A + 0.1A (folding) + 0.7A (caveolae) = 1.8A. (3) For stretch beyond Sc membrane is provided by the opening of caveolae. At a sarcomere length of about 8 mum all the caveolae are open and the fibres rupture with further stretch. (4) The relative contributions of folds and caveolae vary with sarcomere length in a way that is consistent with assumptions of constant volume and plasmalemma area. The maintenance of constant plasmalemma area, even after excessive stretch, suggests that the plasmalemma is relatively inelastic in this situation.
Full text
PDF





















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASELLA C. Tensile force in total striated muscle, isolated fibre and sarcolemma. Acta Physiol Scand. 1950 Dec;21(4):380–401. doi: 10.1111/j.1748-1716.1950.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Dulhunty A. F., Gage P. W. Electrical properties of toad sartorius muscle fibres in summer and winter. J Physiol. 1973 May;230(3):619–641. doi: 10.1113/jphysiol.1973.sp010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT G. F. X-RAY DIFFRACTION STUDIES ON STRIATED AND SMOOTH MUSCLES. Proc R Soc Lond B Biol Sci. 1964 Oct 27;160:467–472. doi: 10.1098/rspb.1964.0057. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., Gage P. W. Ionic conductances of the surface and transverse tubular membranes of frog sartorius fibers. J Gen Physiol. 1969 Mar;53(3):279–297. doi: 10.1085/jgp.53.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezerman E. B., Ishikawa H. Differentiation of the sarcoplasmic reticulum and T system in developing chick skeletal muscle in vitro. J Cell Biol. 1967 Nov 1;35(2):405–420. doi: 10.1083/jcb.35.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALK G., FATT P. LINEAR ELECTRICAL PROPERTIES OF STRIATED MUSCLE FIBRES OBSERVED WITH INTRACELLULAR ELECTRODES. Proc R Soc Lond B Biol Sci. 1964 Apr 14;160:69–123. doi: 10.1098/rspb.1964.0030. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Capacitance of the surface and transverse tubular membrane of frog sartorius muscle fibers. J Gen Physiol. 1969 Mar;53(3):265–278. doi: 10.1085/jgp.53.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L. A note on conduction velocity. J Physiol. 1954 Jul 28;125(1):221–224. doi: 10.1113/jphysiol.1954.sp005152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H. E. X-ray analysis and the problem of muscle. Proc R Soc Lond B Biol Sci. 1953 Mar 11;141(902):59–62. doi: 10.1098/rspb.1953.0017. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. Analysis of the membrane capacity in frog muscle. J Physiol. 1972 Feb;221(1):121–136. doi: 10.1113/jphysiol.1972.sp009743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. The effect of change in length on conduction velocity in muscle. J Physiol. 1954 Jul 28;125(1):215–220. doi: 10.1113/jphysiol.1954.sp005151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Bastian J. Double sucrose-gap method applied to single muscle fiber of Xenopus laevis. J Gen Physiol. 1974 Feb;63(2):235–256. doi: 10.1085/jgp.63.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PODOLSKY R. J. THE MAXIMUM SARCOMERE LENGTH FOR CONTRACTION OF ISOLATED MYOFIBRILS. J Physiol. 1964 Jan;170:110–123. doi: 10.1113/jphysiol.1964.sp007317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D., Schild R. F. The distribution of the T-system along the sarcomeres of frog and toad sartorius muscles. J Physiol. 1968 Jan;194(1):249–258. doi: 10.1113/jphysiol.1968.sp008405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane splitting in freeze-ethching. Covalently bound ferritin as a membrane marker. J Cell Biol. 1970 Jun;45(3):598–605. doi: 10.1083/jcb.45.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayns D. G., Simpson F. O., Bertaud W. S. Surface features of striated muscle. II. Guinea-pig skeletal muscle. J Cell Sci. 1968 Dec;3(4):475–482. doi: 10.1242/jcs.3.4.475. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H. The sarcolemma of skeletal muscle fibres as demonstrated by a replica technique. Cell Tissue Res. 1974;150(3):377–387. doi: 10.1007/BF00220144. [DOI] [PubMed] [Google Scholar]
- Schneider M. F. Linear electrical properties of the transverse tubules and surface membrane of skeletal muscle fibers. J Gen Physiol. 1970 Nov;56(5):640–671. doi: 10.1085/jgp.56.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillack T. W., Marchesi V. T. Demonstration of the outer surface of freeze-etched red blood cell membranes. J Cell Biol. 1970 Jun;45(3):649–653. doi: 10.1083/jcb.45.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdiosera R., Clausen C., Eisenberg R. S. Impedance of frog skeletal muscle fibers in various solutions. J Gen Physiol. 1974 Apr;63(4):460–491. doi: 10.1085/jgp.63.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi G., Vergara J., Ramón F. On the connection between the transverse tubules and the plasma membrane in frog semitendinosus skeletal muscle. Are caveolae the mouths of the transverse tubule system? J Cell Biol. 1975 Mar;64(3):734–740. doi: 10.1083/jcb.64.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]