Abstract
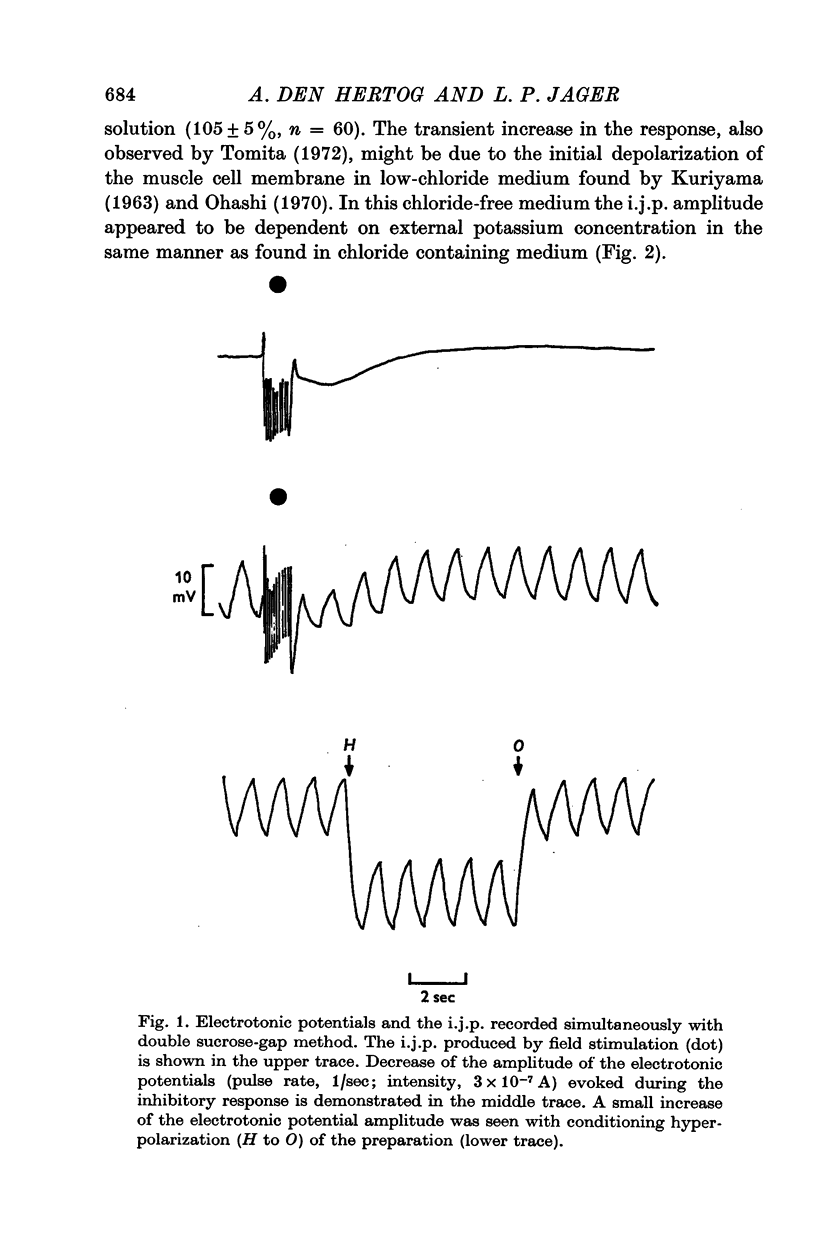
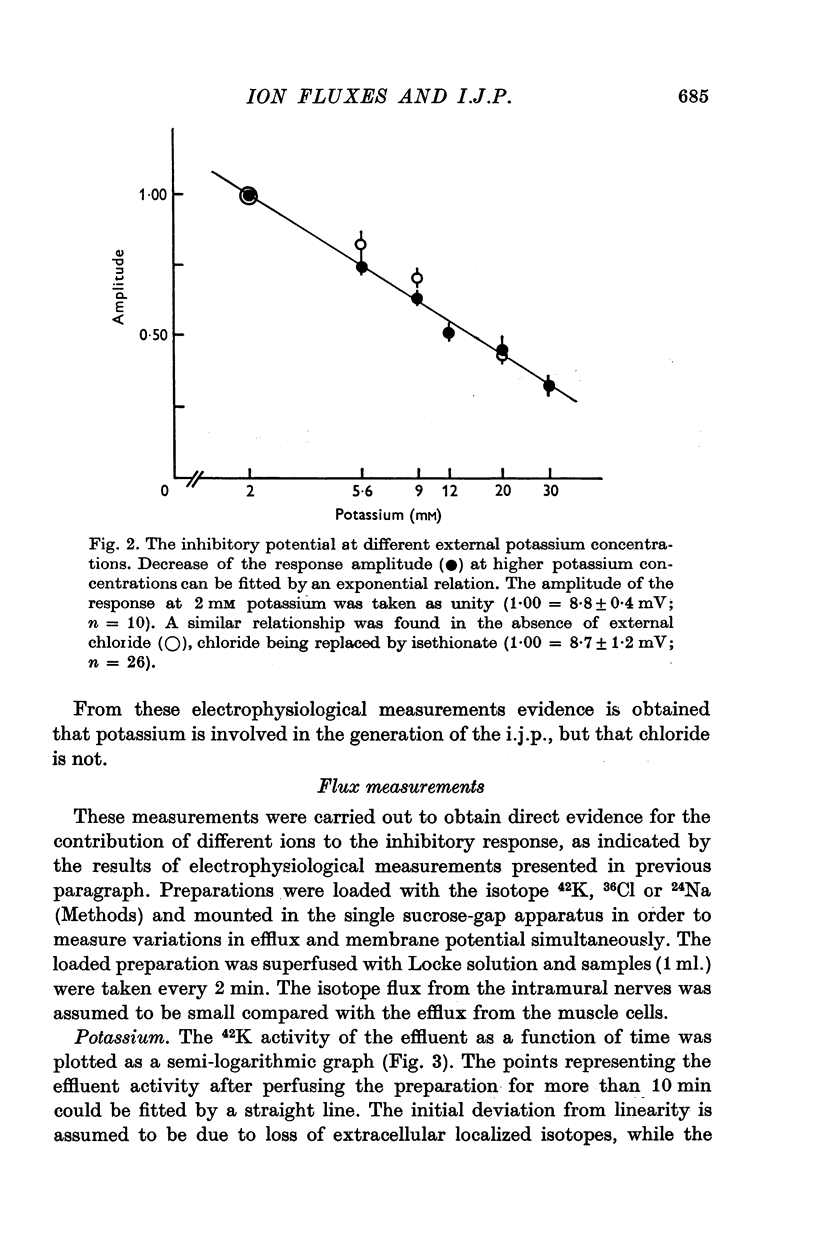
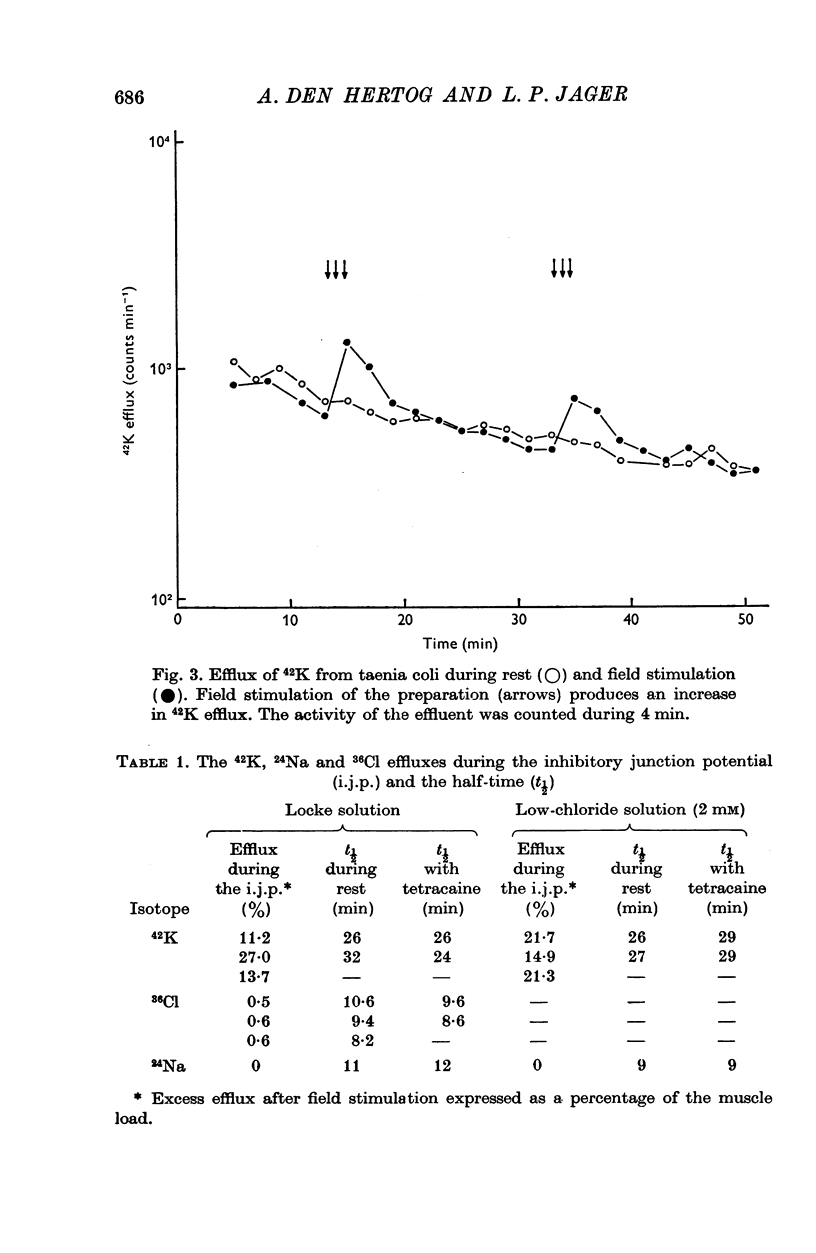
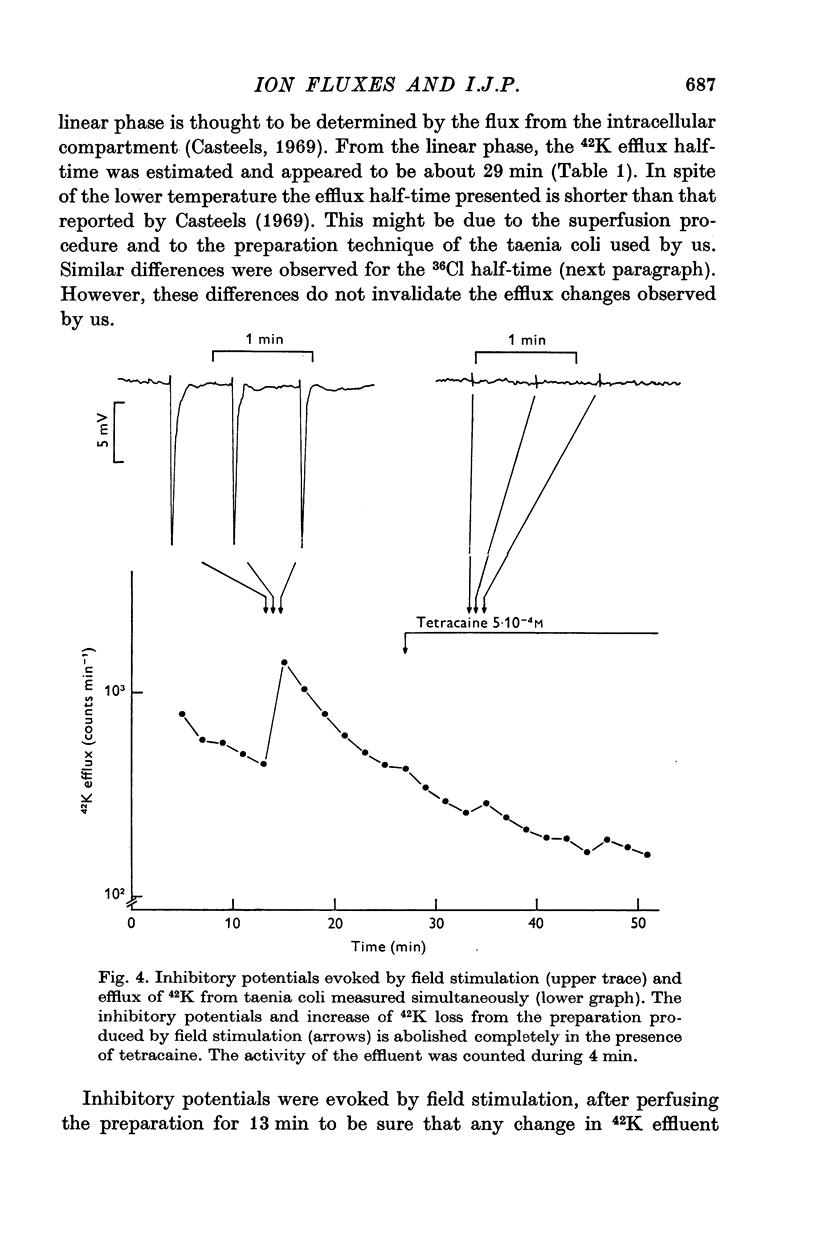
1. Contribution of different ions to the inhibitory junction potential (i.j.p.) in the guinea-pig taenia coli was studied by measuring the 42K, 24Na and 36Cl fluxes, the membrane resistance and the influence of various external ion concentrations. 2. The membrane resistance, as measured by the electrotonic potential, decreased transiently during the i.j.p. A maximal reduction of the electrotonic potential of about 50% was found at the top of the i.j.p. 3. The i.j.p. amplitude could be reduced by raising the external potassium concentration. Extrapolation of the relationship observed shows that the inhibitory response would be abolished at 115 mM potassium. Similar experiments were made in chloride-free medium, chloride being replaced by isethionate. Amplitude and time course of the response were not different in chloride containing Locke solution and chloride-free medium. 4. The half-times of 42K, 24Na and 36Cl effluxes during rest were 29, 10 and 9 min respectively. The 42K-efflux from the preparation was markedly increased to about three times the resting efflux during field stimulation. In low-chloride solution a similar effect on 42K-efflux was observed during field stimulation. Only a slight increase in the chloride efflux was observed but the sodium efflux was not affected during field stimulation. 5. From the results presented it is concluded that the inhibitory junction potential is caused by a selective increase in potassium permeability of the smooth-muscle cell membrane.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Burnstock G., Holman M. Transmission from intramural inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Feb;182(3):541–558. doi: 10.1113/jphysiol.1966.sp007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Properties of the inhibitory potential of smooth muscle as observed in the response to field stimulation of the guinea-pig taenia coli. J Physiol. 1967 Apr;189(2):299–315. doi: 10.1113/jphysiol.1967.sp008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTEELS R., KURIYAMA H. MEMBRANE POTENTIAL AND IONIC CONTENT IN PREGNANT AND NON-PREGNANT RAT MYOMETRIUM. J Physiol. 1965 Mar;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Calculation of the membrane potential in smooth muscle cells of the guinea-pig's taenia coli by the Goldman equation. J Physiol. 1969 Nov;205(1):193–208. doi: 10.1113/jphysiol.1969.sp008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. The distribution of chloride ions in the smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Apr;214(2):225–243. doi: 10.1113/jphysiol.1971.sp009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager L. P., Den Hertog A. D. Effect of temperature on the transmitter release from the "purinergic" nerves in the guinea-pig taenia coli. Eur J Pharmacol. 1974 Dec;29(2):201–205. doi: 10.1016/0014-2999(74)90018-1. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H. The influence of potassium, sodium and chloride on the membrane potential of the smooth muscle of taenia coli. J Physiol. 1963 Apr;166:15–28. doi: 10.1113/jphysiol.1963.sp007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oashi H. An estimate of the proportion of the resting membrane conductance of the smooth muscle of guinea-pog taenia coli attributable to chloride. J Physiol. 1970 Sep;210(2):405–419. doi: 10.1113/jphysiol.1970.sp009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Conductance change during the inhibitory potential in the guinea-pig taenia coli. J Physiol. 1972 Sep;225(3):693–703. doi: 10.1113/jphysiol.1972.sp009964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Membrane capacity and resistance of mammalian smooth muscle. J Theor Biol. 1966 Nov;12(2):216–227. doi: 10.1016/0022-5193(66)90114-7. [DOI] [PubMed] [Google Scholar]
- den Hertog A. Some further observations on the electrogenic sodium pump in non-myelinated nerve fibres. J Physiol. 1973 Jun;231(3):493–509. doi: 10.1113/jphysiol.1973.sp010245. [DOI] [PMC free article] [PubMed] [Google Scholar]


