Abstract
1. The experiments were designed to investigate the effects of longitudinal muscle displacements on neurones of the motor cortex of anaesthetized Cebus monkeys and thus test the hypothesis that signals from muscle spindles may modify motor cortical output. The effects of sinusoidal stretching of the extensor digitorum communis (EDC) at frequencies varying from 6 to 300 Hz and of step and rhomboidal stretches were studied in neurones of the motor cortex. For comparison, neurones of the primary receiving area for low-threshold muscle afferents, cortical area 3a, were also included in this study. Neurones of the motor cortex were subdivided into corticospinal (PT) neurones and non-corticospinal (non-PT) neurones. 2. Threshold stretch amplitudes were clearly higher for neurones of area 4 (PT and non-PT) than for 3a neurones. However, a conspicuous fall in threshold stretch amplitude was observed for all three neurone populations when the frequency of sinusoidal stretching was increased (highest frequency: 300 Hz). A small number of non-PT and PT neurones responded to vibration amplitudes of less than 100 mum and some of these low-threshold cells of area 4 also responded to rhomboidal stretches of 8 mm/sec ramp velocity and 80 mum plateau amplitude. Increasing the stretch amplitude to twice threshold nearly doubled the output magnitude in all three cell types. Neurones of area 3a and non-PT neurones of area 4 had similar latencies, and these were significantly shorter than the latencies of PT neurones tested with trains of high frequency vibration. Dynamic response patterns were observed in all three cell types, but most frequently in 3a neurones. 3. It is concluded that, in Cebus monkeys, signals from both primary and secondary muscle spindle endings from forelimb muscles reach the motor cortex. Under the present experimental conditions, the input from the primaries to the motor cortex was effective only if these spindle receptors were driven maximally by vibratory stimuli. The particularly low probability of stretch-evoked discharges of cortico-spinal neurones in the anaesthetized preparation may be explained by a low gain in transmission from input to output cells of the motor cortex.
Full text
PDF



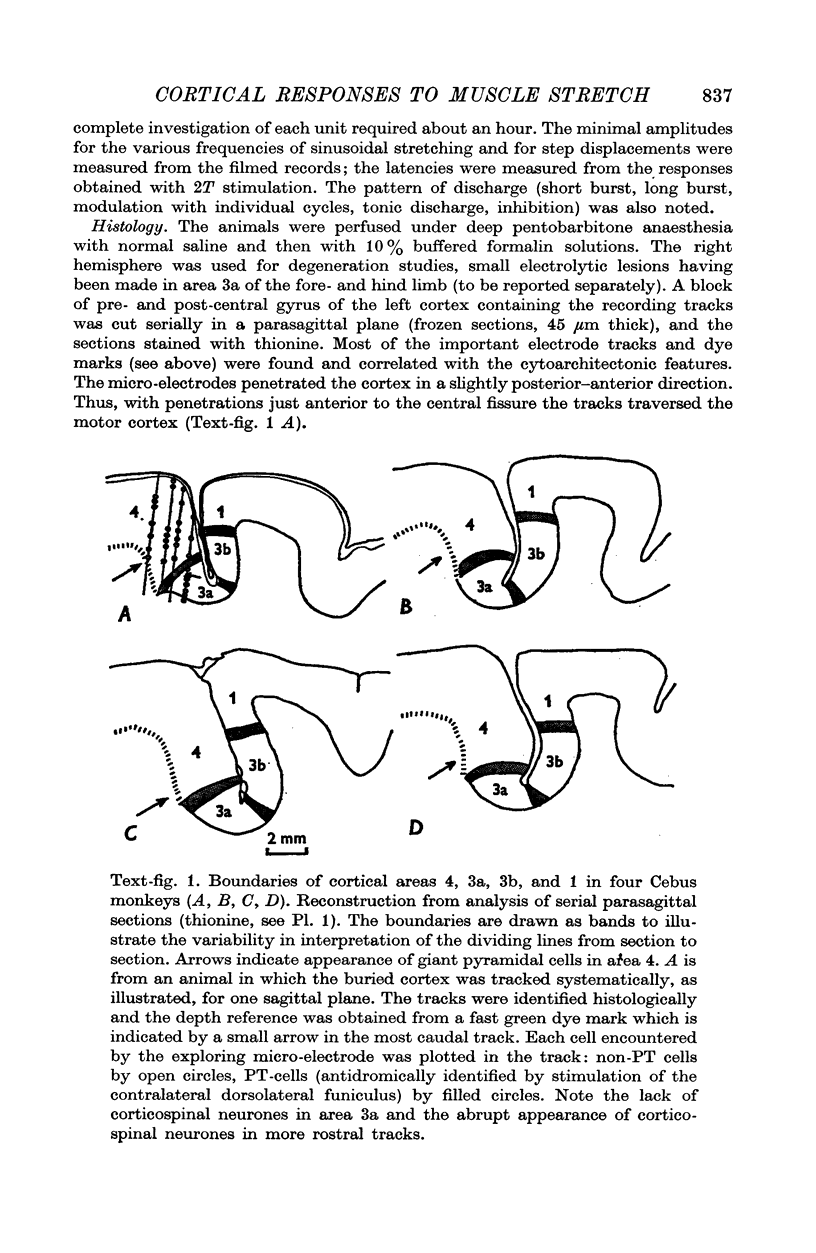

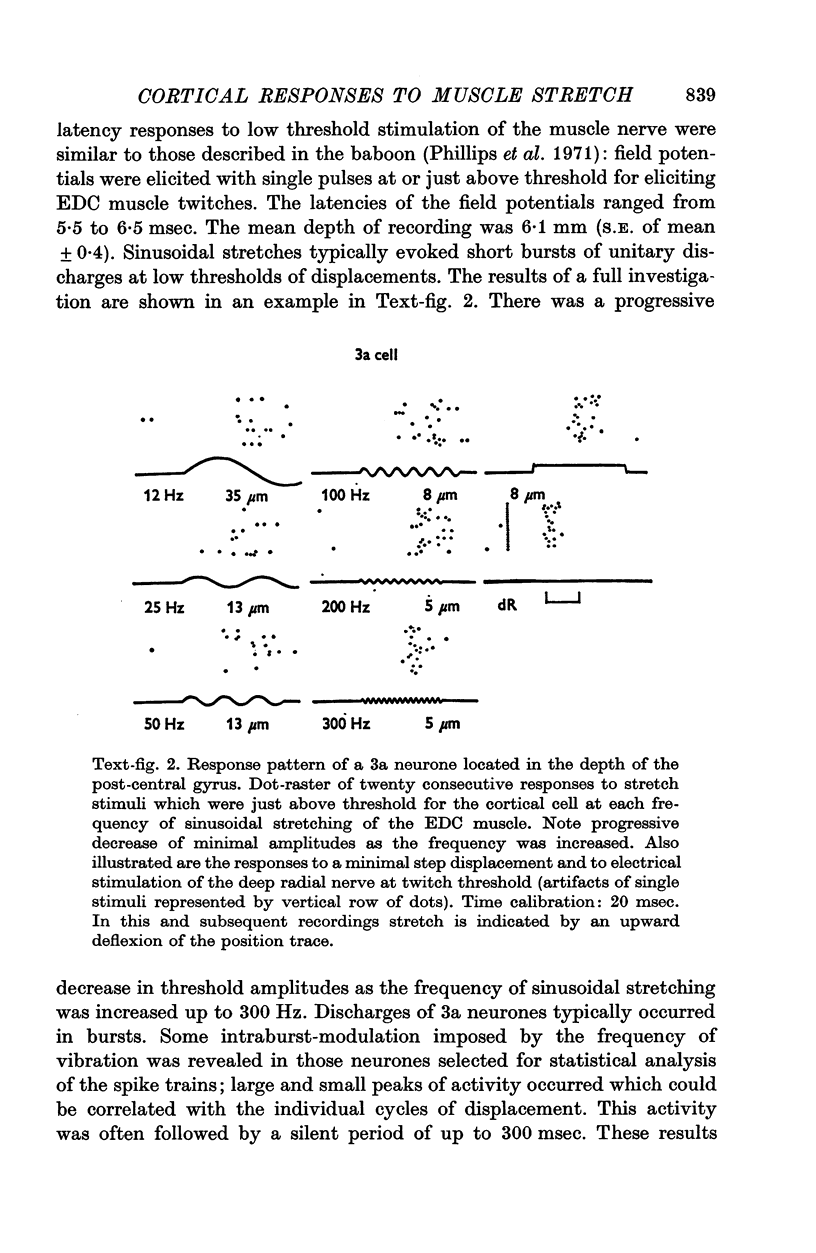















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS B. D., ROBSON J. G. 'Weightless' micro-electrodes for recording extracellular unit action potentials from the central nervous system. Nature. 1960 Apr 16;186:246–247. doi: 10.1038/186246a0. [DOI] [PubMed] [Google Scholar]
- Conrad B., Matsunami K., Meyer-Lohmann J., Wiesendanger M., Brooks V. B. Cortical load compensation during voluntary elbow movements. Brain Res. 1974 May 17;71(2-3):507–514. doi: 10.1016/0006-8993(74)90994-9. [DOI] [PubMed] [Google Scholar]
- Evarts E. V. Motor cortex reflexes associated with learned movement. Science. 1973 Feb 2;179(4072):501–503. doi: 10.1126/science.179.4072.501. [DOI] [PubMed] [Google Scholar]
- Evarts E. V., Tanji J. Gating of motor cortex reflexes by prior instruction. Brain Res. 1974 May 17;71(2-3):479–494. doi: 10.1016/0006-8993(74)90992-5. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Latency measurements compatible with a cortical pathway for the stretch reflex in man. J Physiol. 1973 Apr;230(1):58P–59P. [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in human voluntary movement. Nature. 1972 Jul 21;238(5360):140–143. doi: 10.1038/238140a0. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Wong Y. C., Kwan H. C. Distributed feedback systems for muscle control. Brain Res. 1974 May 17;71(2-3):495–505. doi: 10.1016/0006-8993(74)90993-7. [DOI] [PubMed] [Google Scholar]
- Phillips C. G., Powell T. P., Wiesendanger M. Projection from low-threshold muscle afferents of hand and forearm to area 3a of baboon's cortex. J Physiol. 1971 Sep;217(2):419–446. doi: 10.1113/jphysiol.1971.sp009579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosén I., Asanuma H. Peripheral afferent inputs to the forelimb area of the monkey motor cortex: input-output relations. Exp Brain Res. 1972;14(3):257–273. doi: 10.1007/BF00816162. [DOI] [PubMed] [Google Scholar]
- Tardieu C., Tabary J. C., Tardieu G. Etude mécanique et électromyographique des réponses à différentes perturbations du maintein postural. J Physiol (Paris) 1968 Jul-Aug;60(4):243–259. [PubMed] [Google Scholar]
- Thomas R. C., Wilson V. J. Precise localization of Renshaw cells with a new marking technique. Nature. 1965 Apr 10;206(980):211–213. doi: 10.1038/206211b0. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M. Input from muscle and cutaneous nerves of the hand and forearm to neurones of the precentral gyrus of baboons and monkeys. J Physiol. 1973 Jan;228(1):203–219. doi: 10.1113/jphysiol.1973.sp010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. C., Kwan H. C., Murphy J. T. Projection of primary muscle spindle afferents to motorsensory cortex. Can J Physiol Pharmacol. 1974 Apr;52(2):349–351. doi: 10.1139/y74-046. [DOI] [PubMed] [Google Scholar]



