Abstract
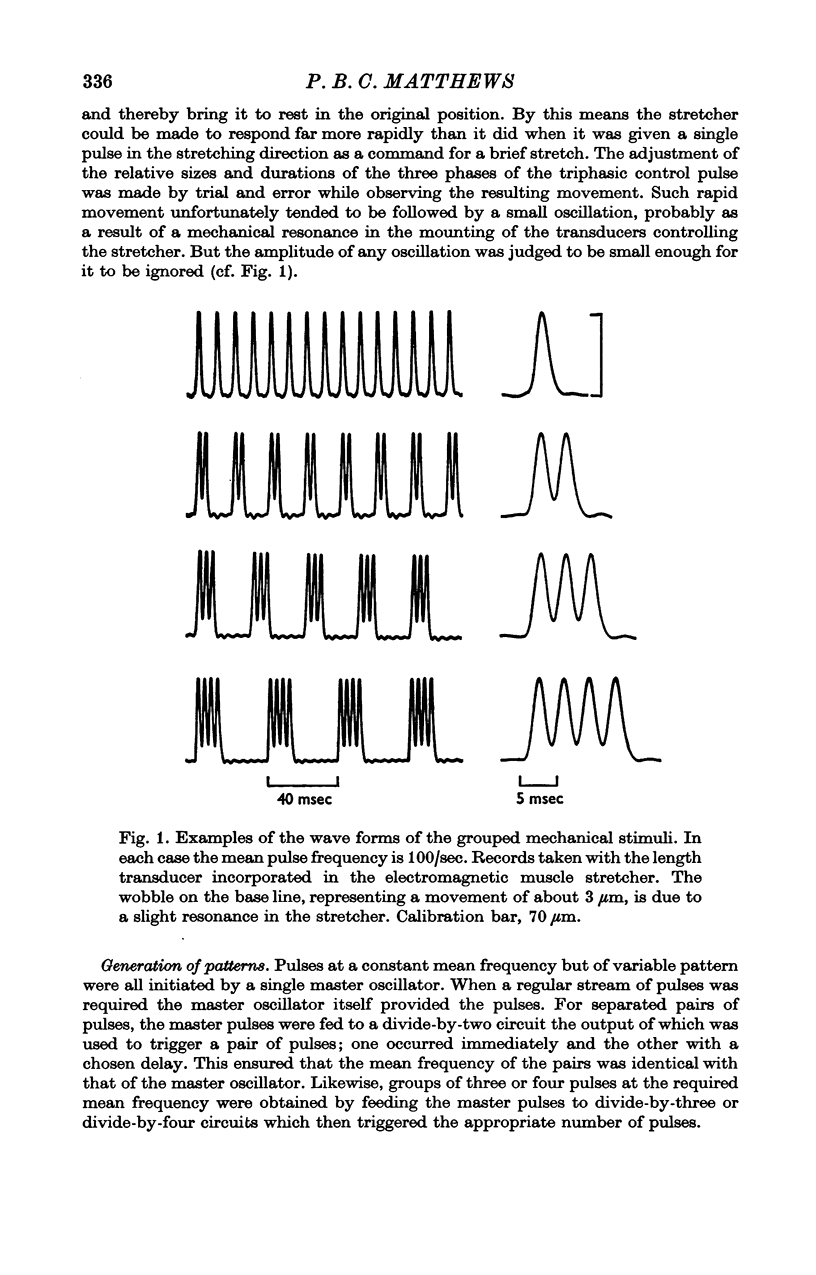
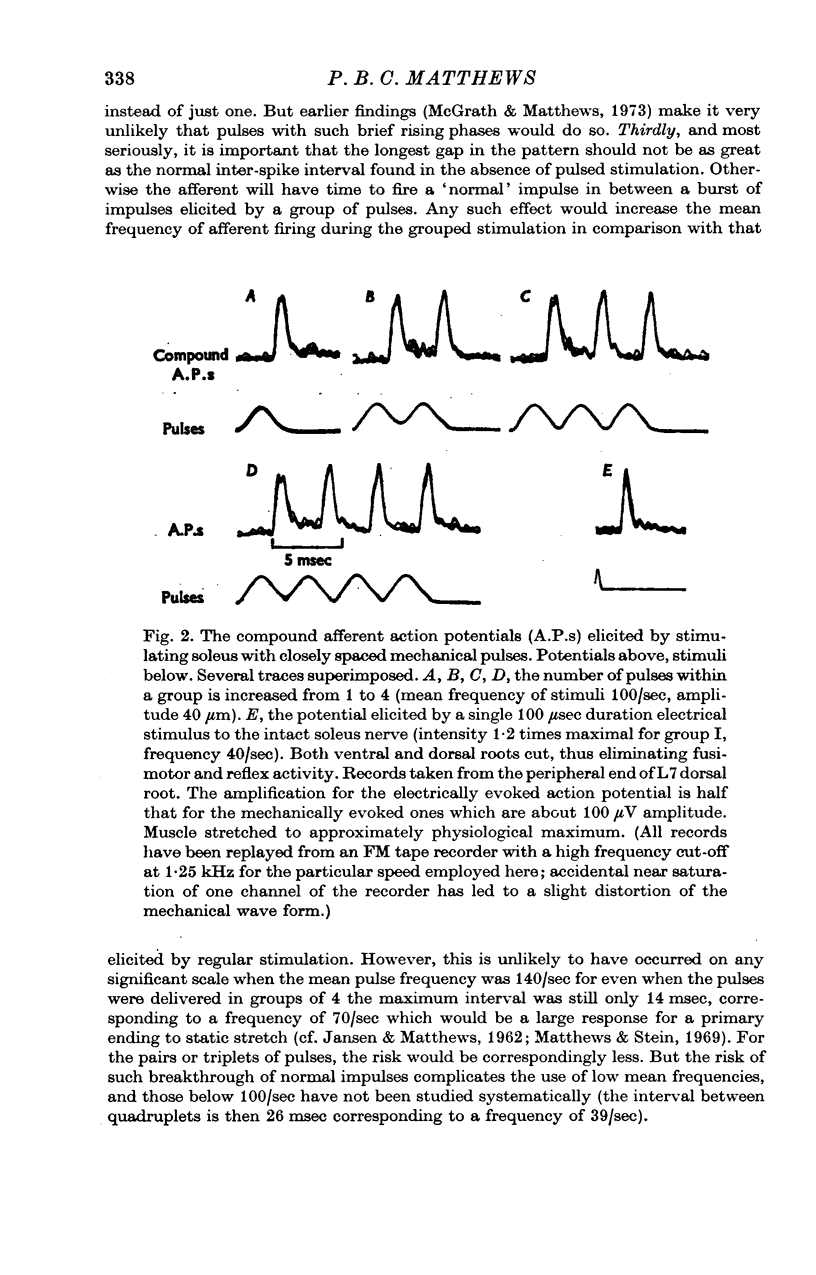
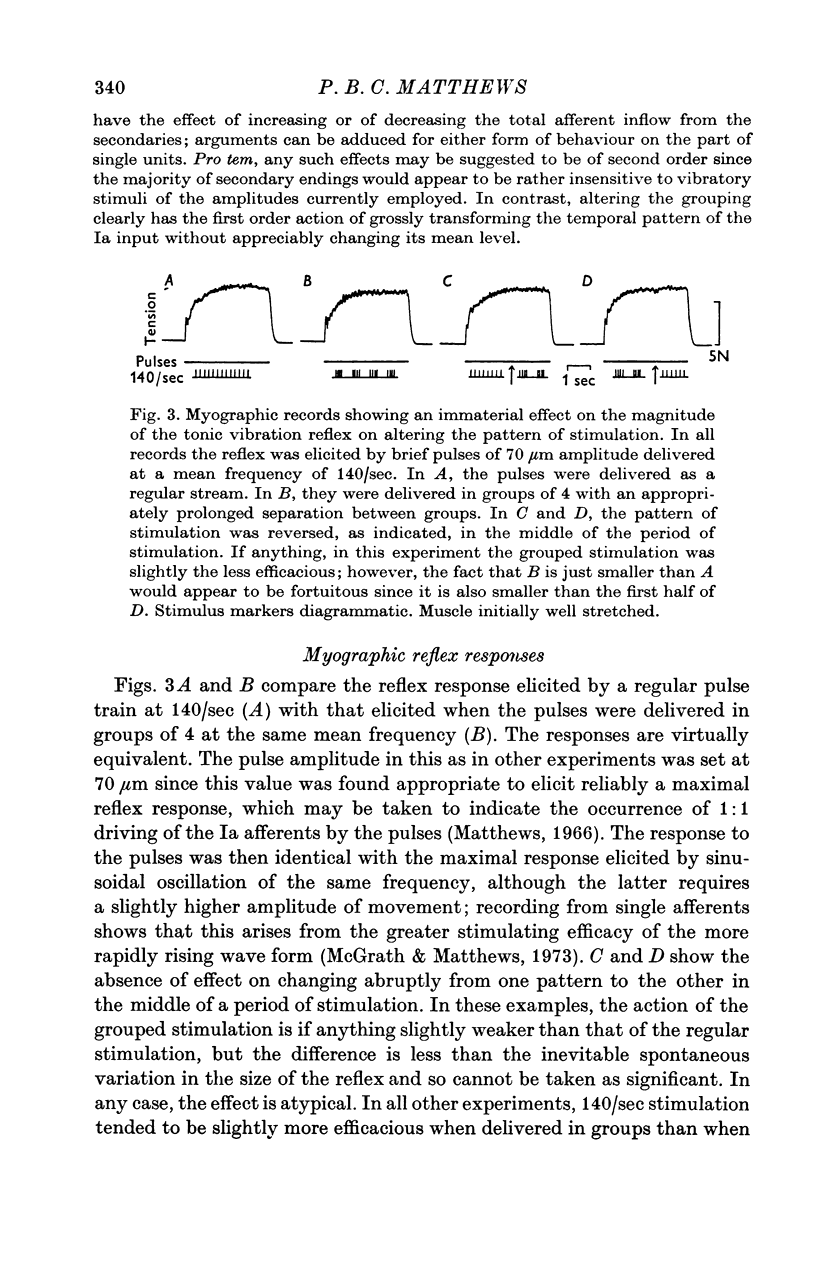
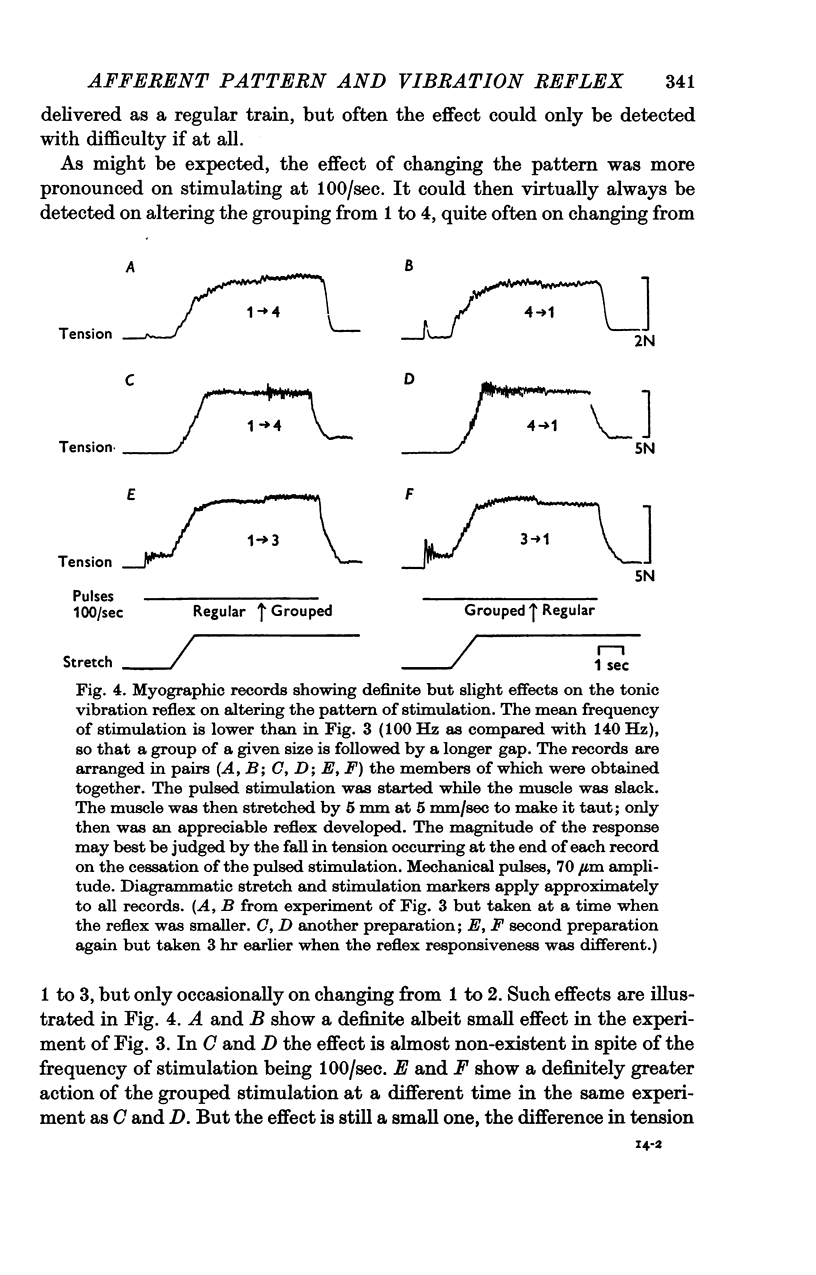
1. A study has been made of the effect of varying the temporal arrangement of the mechanical stimuli used to elicit the tonic vibration reflex in the soleus muscle of the decerebrate cat. The reflex was elicited by brief mechanical pulses, applied repetitively, either as a regular series or, at the same mean frequency, in groups of 2, 3 or 4 pulses with a separation between the pulses of 3-5 msec. Mean frequencies of 140/sec and 100/sec were used. The amplitude of the pulses was such that it could be presumed that each pulse excited every Ia fibre from soleus to discharge a spike, irrespective of the patterning employed. 2. Alterations in the stimulus pattern produced only minimal alterations in the size of the resultant reflex recorded myographically. The grouped stimulation regularly tended to produce the larger effect, but even with groups of 4 at 100/sec the modal effect was only 10% of the pre-existing response; expressed another way this was equivalent to an increase of 11 Hz in the mean frequency of stimulation. Thus under these conditions grouping the stimuli cannot have had an appreciable effect either in increasing the firing frequency of those motoneurones which were already active, or in recruiting those which were initially quiescent. 3. Recording from individual motor units with fine electrodes placed on the surface of the muscle showed that they were not significantly changing their frequency of firing on altering the pattern of stimulation. 4. Gross electromyographic recording showed that the motor discharge was locked in time to the mechanical stimuli and of appropriate latency for it to be presumed that the actual discharge of impulses was triggered by Ia monosynaptic action. 5. Similar insensitivity to the temporal pattern of the afferent input was found when the motoneurones were excited via two separate channels, one being the pulsed mechanical stimulation of soleus, the other being the weak electrical stimulation of the nerve to the medial head of gastrocnemius; altering the relative timing of the two sets of stimuli had little effect on the myographic result. 6. Thus, during tonic firing the timing of the motor output reflects the timing of the afferent input while the mean motor output reflects the mean value of the afferent input, as seems physiologically appropriate. The latter finding is, however, paradoxical; as detailed in an appendix the amount of motor firing produced by synchronous Ia monosynaptic action might be expected to increase on grouping the stimuli and so apparently favouring e.p.s.p. summation.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVORD E. C., Jr, FUORTES M. G. Reflex activity of extensor motor units following muscular afferent excitation. J Physiol. 1953 Nov 28;122(2):302–321. doi: 10.1113/jphysiol.1953.sp005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasijević R., Todorović A. B., Vuco J. The differential reflex excitability of alpha motoneurons of decerebrate cats caused by vibration applied to the tendon of the gastrocnemius medialis muscle. Brain Res. 1968 Nov;11(2):336–346. doi: 10.1016/0006-8993(68)90029-2. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Engberg I., Matthews P. B. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967 Oct;192(3):773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E. Composite nature of the monosynaptic excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1114–1137. doi: 10.1152/jn.1967.30.5.1114. [DOI] [PubMed] [Google Scholar]
- Burke R. E. Firing patterns of gastrocnemius motor units in the decerebrate cat. J Physiol. 1968 Jun;196(3):631–654. doi: 10.1113/jphysiol.1968.sp008527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E. Group Ia synaptic input to fast and slow twitch motor units of cat triceps surae. J Physiol. 1968 Jun;196(3):605–630. doi: 10.1113/jphysiol.1968.sp008526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. The isometric responses of mammalian muscles. J Physiol. 1930 Jun 27;69(4):377–385. doi: 10.1113/jphysiol.1930.sp002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RITCHIE J. M., SCHAUMANN W. A study of the effect of the pattern of electrical stimulation of the aortic nerve on the reflex depressor responses. J Physiol. 1956 Jul 27;133(1):232–242. doi: 10.1113/jphysiol.1956.sp005581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R. Neuromuscular interaction in postural tone of the cat's isometric soleus muscle. J Physiol. 1958 Oct 31;143(3):387–402. doi: 10.1113/jphysiol.1958.sp006067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillary H. L., Kennedy D. Neuromuscular effects of impulse pattern in a crustacean motoneuron. J Neurophysiol. 1969 Jul;32(4):607–612. doi: 10.1152/jn.1969.32.4.607. [DOI] [PubMed] [Google Scholar]
- Grillner S., Udo M. Motor unit activity and stiffness of the contracting muscle fibres in the tonic stretch reflex. Acta Physiol Scand. 1971 Mar;81(3):422–424. doi: 10.1111/j.1748-1716.1971.tb04916.x. [DOI] [PubMed] [Google Scholar]
- Iansek R., Redman S. J. An analysis of the cable properties of spinal motoneurones using a brief intracellular current pulse. J Physiol. 1973 Nov;234(3):613–636. doi: 10.1113/jphysiol.1973.sp010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSEN J. K., MATTHEWS P. B. The effects of fusimotor activity on the static responsiveness of primary and secondary endings of muscle spindles in the decerebrate cat. Acta Physiol Scand. 1962 Aug;55:376–386. doi: 10.1111/j.1748-1716.1962.tb02451.x. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Miller S., Porter R., Redman S. J. The time course of minimal excitory post-synaptic potentials evoked in spinal motoneurones by group Ia afferent fibres. J Physiol. 1971 Jun;215(2):353–380. doi: 10.1113/jphysiol.1971.sp009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. Evidence that the secondary as well as the primary endings of the muscle spindles may be responsible for the tonic stretch reflex of the decerebrate cat. J Physiol. 1969 Oct;204(2):365–393. doi: 10.1113/jphysiol.1969.sp008918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Stein R. B. The regularity of primary and secondary muscle spindle afferent discharges. J Physiol. 1969 May;202(1):59–82. doi: 10.1113/jphysiol.1969.sp008795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. The dependence of tension upon extension in the stretch reflex of the soleus muscle of the decerebrate cat. J Physiol. 1959 Oct;147(3):521–546. doi: 10.1113/jphysiol.1959.sp006260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. The reflex excitation of the soleus muscle of the decerebrate cat caused by vibbration applied to its tendon. J Physiol. 1966 May;184(2):450–472. doi: 10.1113/jphysiol.1966.sp007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath G. J., Matthews P. B. Evidence from the use of vibration during procaine nerve block that the spindle group II fibres contribute excitation to the tonic stretch reflex of the decerebrate cat. J Physiol. 1973 Dec;235(2):371–408. doi: 10.1113/jphysiol.1973.sp010392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano O., Wand P., Sontag K. H. Excitation of Renshaw cells by orthodromic group Ia volleys following vibration of extensor muscles. Pflugers Arch. 1974 Jan 11;347(2):137–144. doi: 10.1007/BF00592395. [DOI] [PubMed] [Google Scholar]
- Porter R., Muir R. B. The meaning for motoneurones of the temporal pattern of natural activity in pyramidal tract neurones of conscious monkeys. Brain Res. 1971 Nov;34(1):127–142. doi: 10.1016/0006-8993(71)90355-6. [DOI] [PubMed] [Google Scholar]
- Rack P. M., Westbury D. R. The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol. 1969 Oct;204(2):443–460. doi: 10.1113/jphysiol.1969.sp008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W., Burke R. E., Smith T. G., Nelson P. G., Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967 Sep;30(5):1169–1193. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Ohye C. Polysynaptic activation of extensor motorneurones from group Ia fibres in the cat spinal cord. Experientia. 1964 Nov 15;20(11):628–629. doi: 10.1007/BF02144828. [DOI] [PubMed] [Google Scholar]
- Westbury D. R. A study of stretch and vibration reflexes of the cat by intracellular recording from motoneurones. J Physiol. 1972 Oct;226(1):37–56. doi: 10.1113/jphysiol.1972.sp009972. [DOI] [PMC free article] [PubMed] [Google Scholar]


