Abstract
1. The influx and the efflux of 204Tl and 42K were measured in intact squid giant axons. 2. The resting efflux of 204Tl was found to be about one half of 42K and to have a temperature coefficient (Q10) of 1-3 as compared to 1-1 for K. 3. The extra efflux of 204Tl associated with nerve impulses was 30% greater than 42K. 4. From either Cl or NO3 sea water, the resting influx of 204Tl was about three times that of 42K. Ouabain reduced the influx of either isotope by about two thirds without changing the Tl/K ratio of the fluxes. This indicates that the Na pump can transport Tl. 5. From NO3 sea water the extra influx of 204Tl assoicated with nerve impulses was about the same as 42K. From Cl sea water there was no detectable extra influx of 204Tl. 6. The flux ratio, ouabain-insensitive influx/efflux, was different for the two ions. The resting flux ratio for Tl was consistent with a passive non-interacting flux, whereas K movements were consistent with 'single file' passage through the membrane. 7. The extra flux associated with nerve impulses is different from the resting flux both in Tl/K selectivity and in the effect of anion in the sea water. There is also a much higher flux per unit time during the nerve impulse. These differences suggest differences in the mechanisms underlying ion permeability at rest and during nervous activity.
Full text
PDF

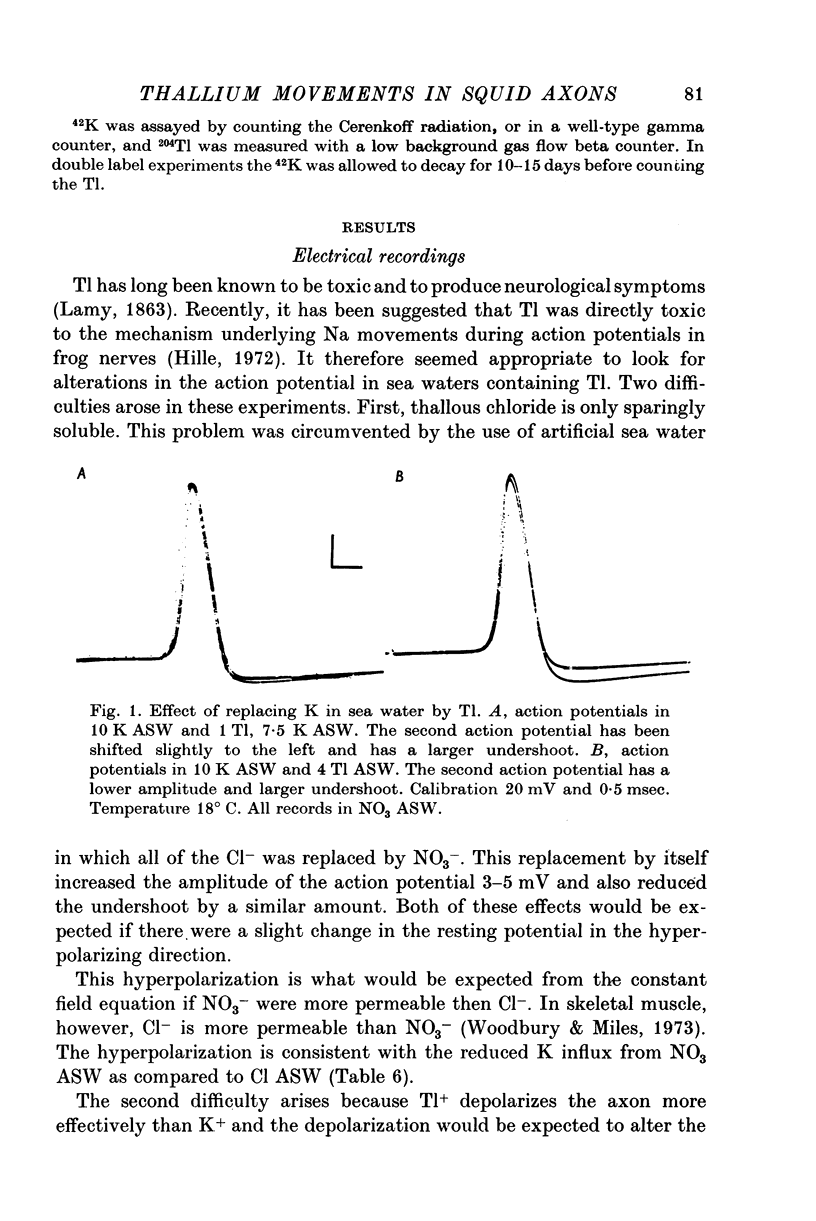

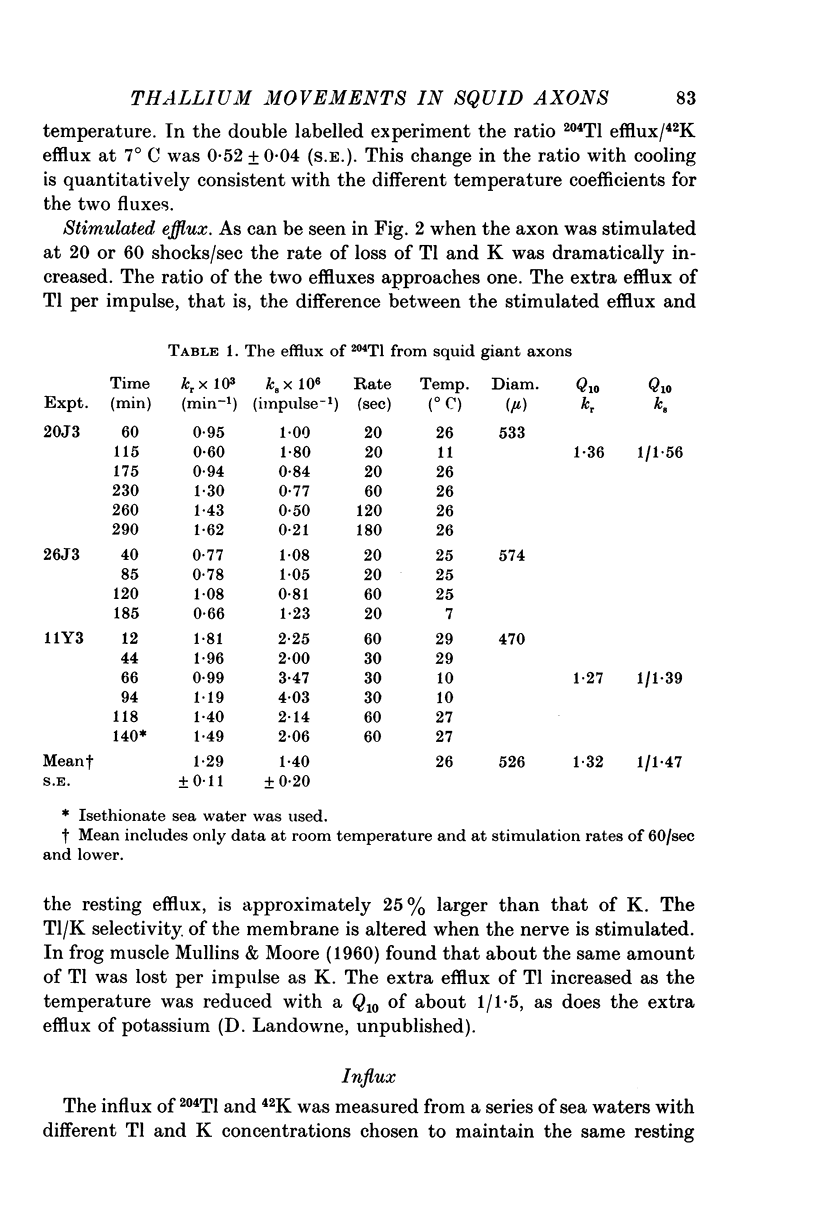
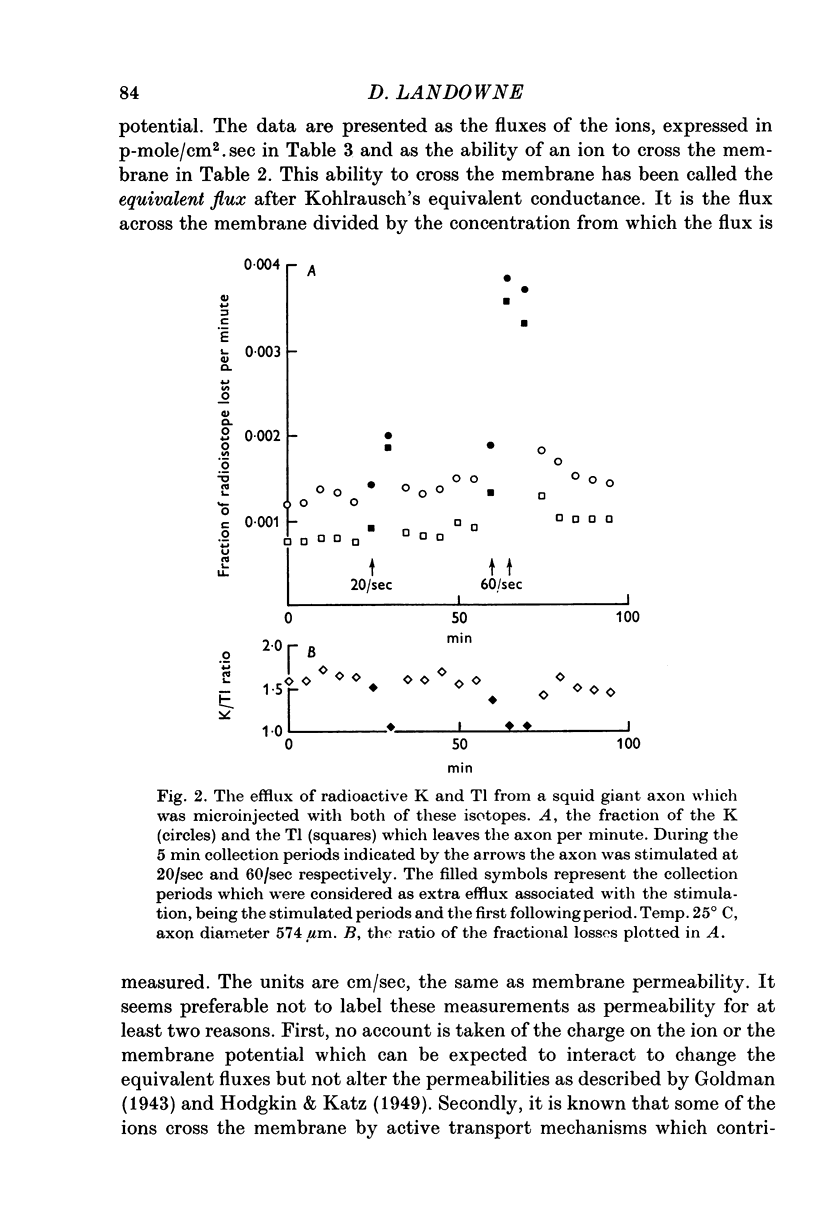


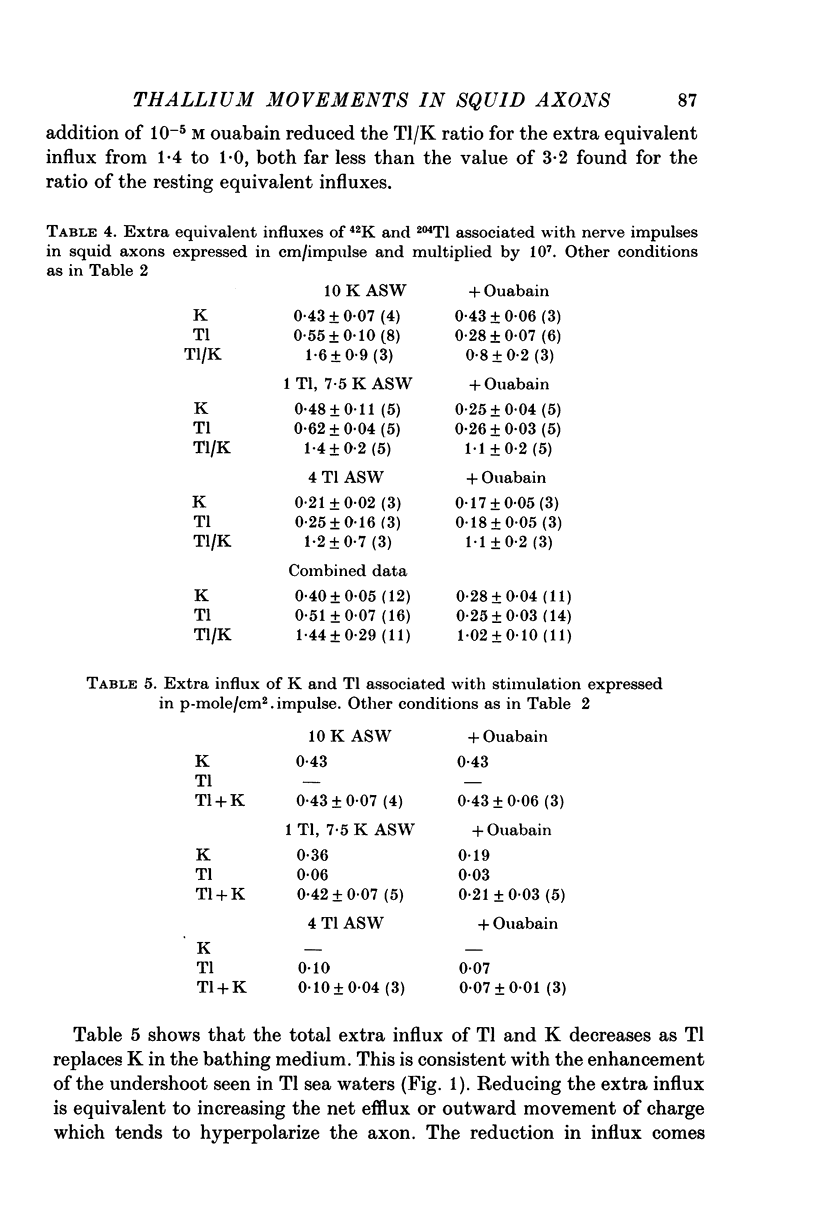








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Connelly C. M. Some properties of the external activation site of the sodium pump in crab nerve. J Physiol. 1966 Jul;185(2):270–297. doi: 10.1113/jphysiol.1966.sp007987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten J. S., Blank M. Thallium activation of the (Na+--K+)-activated ATPase of rabbit kidney. Biochim Biophys Acta. 1968 Apr 24;159(1):160–166. doi: 10.1016/0005-2744(68)90254-4. [DOI] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. L. The effects of injecting 'energy-rich' phosphate compounds on the active transport of ions in the giant axons of Loligo. J Physiol. 1960 Jul;152:561–590. doi: 10.1113/jphysiol.1960.sp006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., KEYNES R. D. The permeability of the squid giant axon to radioactive potassium and chloride ions. J Physiol. 1960 Nov;154:177–189. doi: 10.1113/jphysiol.1960.sp006572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Landowne D. The temperature dependence of the movement of sodium ions associated with nerve impulses. J Physiol. 1974 Jan;236(1):95–111. doi: 10.1113/jphysiol.1974.sp010424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEHRING P. J., HAMMOND P. B. THE UPTAKE OF THALLIUM BY RABBIT ERYTHROCYTES. J Pharmacol Exp Ther. 1964 Aug;145:215–221. [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Movements of Na and K in single muscle fibres. J Physiol. 1959 Mar 3;145(2):405–432. doi: 10.1113/jphysiol.1959.sp006150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The potassium permeability of a giant nerve fibre. J Physiol. 1955 Apr 28;128(1):61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Eaton D. C., Stuart A. E., Rosenthal N. P. Cation selectivity of the resting membrane of squid axon. J Membr Biol. 1972;9(4):373–384. [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to metal cations in myelinated nerve. J Gen Physiol. 1972 Jun;59(6):637–658. doi: 10.1085/jgp.59.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic movements during nervous activity. J Physiol. 1951 Jun;114(1-2):119–150. doi: 10.1113/jphysiol.1951.sp004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko V. K., Kolchinskaia L. I., Parkhomenko N. T. Talii i natriievyi nasos erytrotsytiv. Ukr Biokhim Zh. 1973;45(1):42–46. [PubMed] [Google Scholar]
- MULLINS L. J., MOORE R. D. The movement of thallium ions in muscle. J Gen Physiol. 1960 Mar;43:759–773. doi: 10.1085/jgp.43.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Potassium fluxes in dialyzed squid axons. J Gen Physiol. 1969 Jun;53(6):704–740. doi: 10.1085/jgp.53.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben J., Kayne F. J. Thallium-205 nuclear magnetic resonance study of pyruvate kinase and its substrates. Evidence for a substrate-induced conformational change. J Biol Chem. 1971 Oct 25;246(20):6227–6234. [PubMed] [Google Scholar]
- Spencer P. S., Peterson E. R., Madrid R., Raine C. S. Effects of thallium salts on neuronal mitochondria in organotypic cord-ganglia-muscle combination cultures. J Cell Biol. 1973 Jul;58(1):79–95. doi: 10.1083/jcb.58.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen G., Sommermeyer K. Untersuchungen über die Fluoreszenz des Tl+ in wässrigen Lösungen bei Anregung durch ultraviolettes Licht. Biophysik. 1968;5(3):192–206. doi: 10.1007/BF01189033. [DOI] [PubMed] [Google Scholar]
- TASAKI I., TEORELL T., SPYROPOULOS C. S. Movement of radioactive tracers across squid axon membrane. Am J Physiol. 1961 Jan;200:11–22. doi: 10.1152/ajplegacy.1961.200.1.11. [DOI] [PubMed] [Google Scholar]
- Woodbury J. W., Miles P. R. Anion conductance of frog muscle membranes: one channel, two kinds of pH dependence. J Gen Physiol. 1973 Sep;62(3):324–353. doi: 10.1085/jgp.62.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]


