Abstract
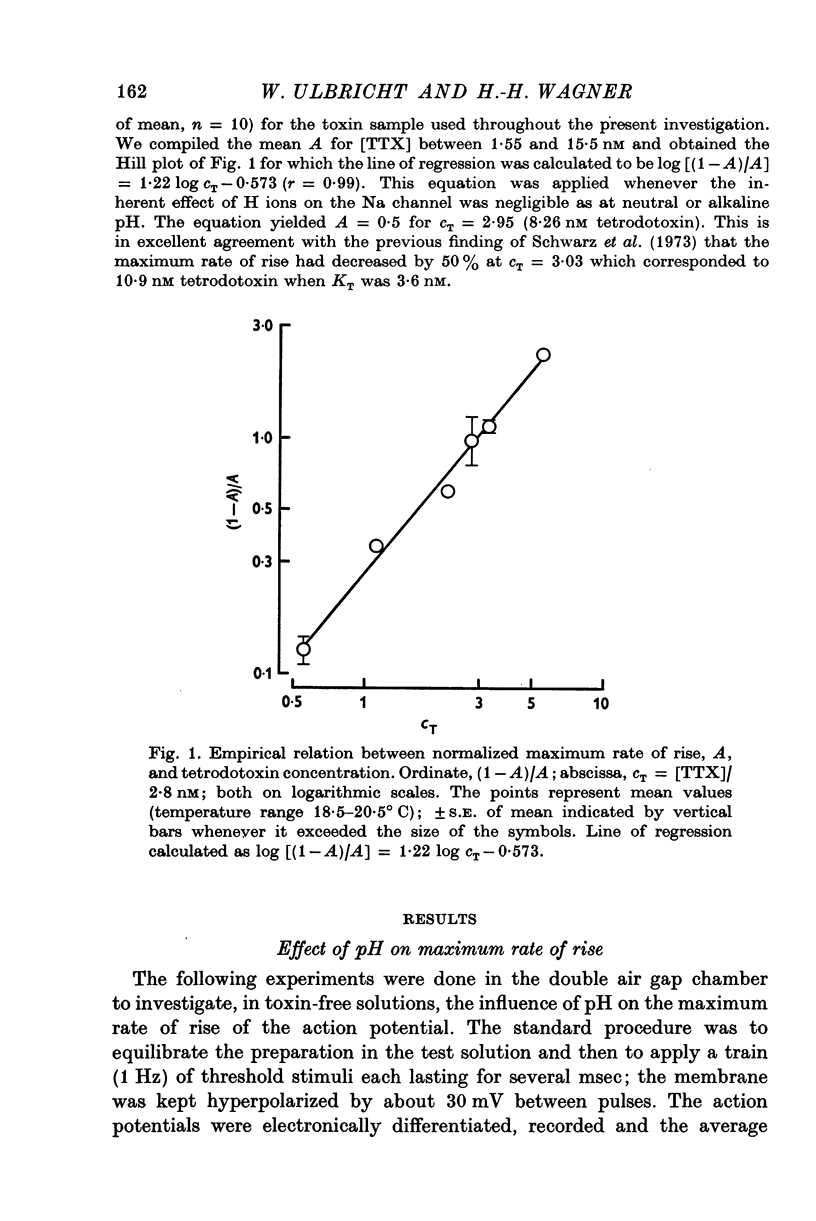
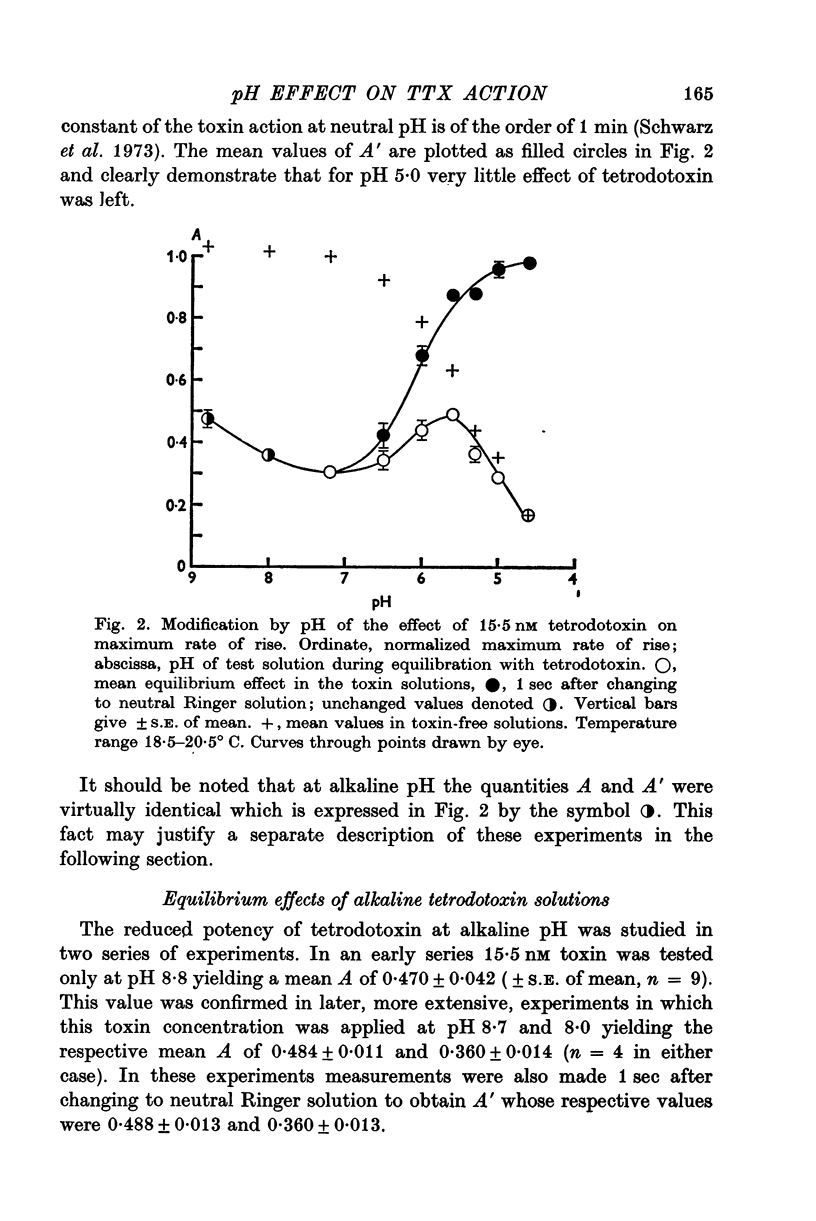
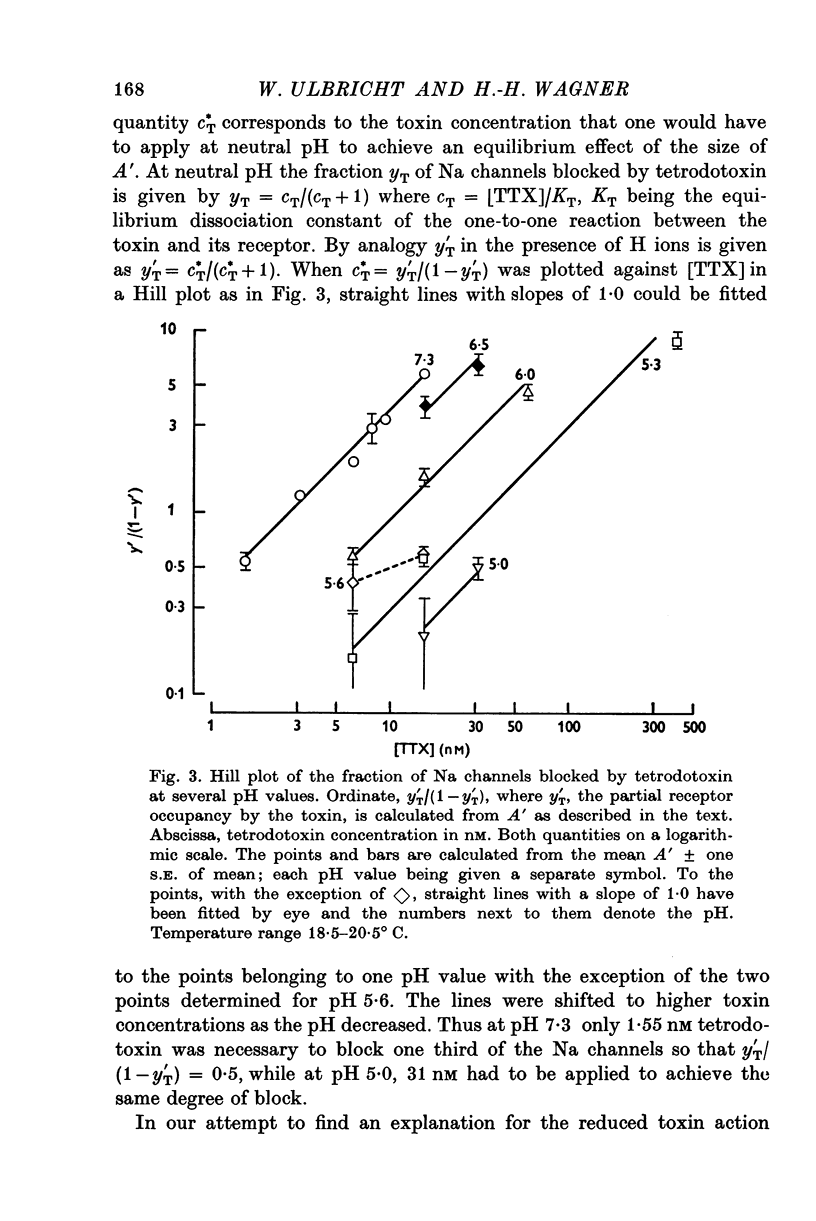
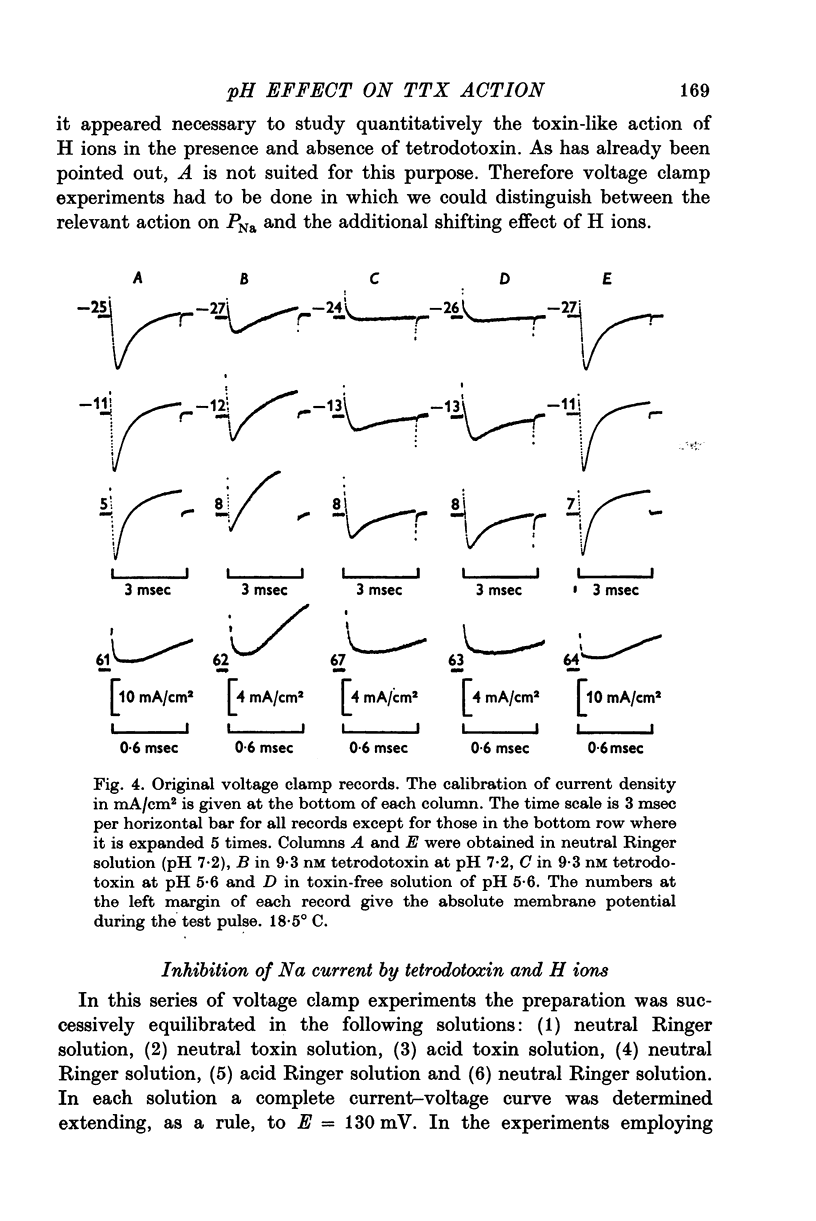
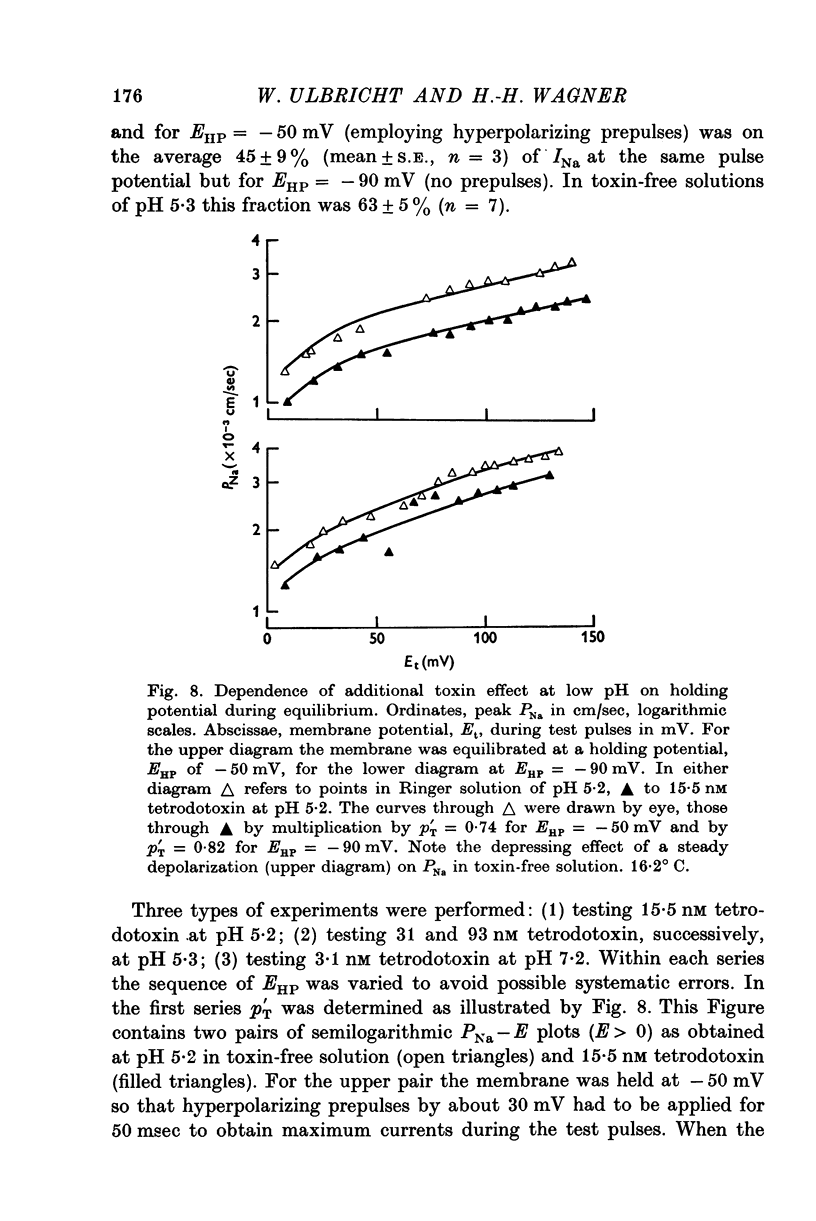
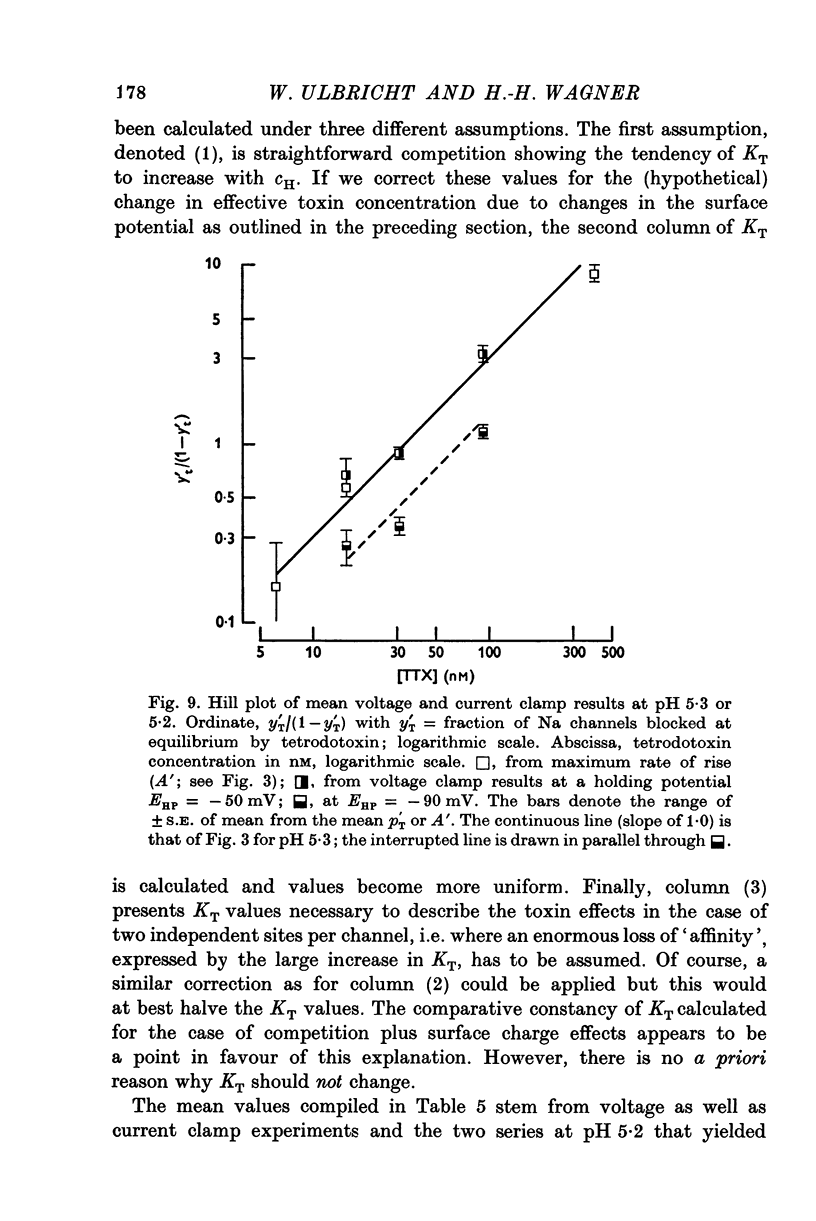
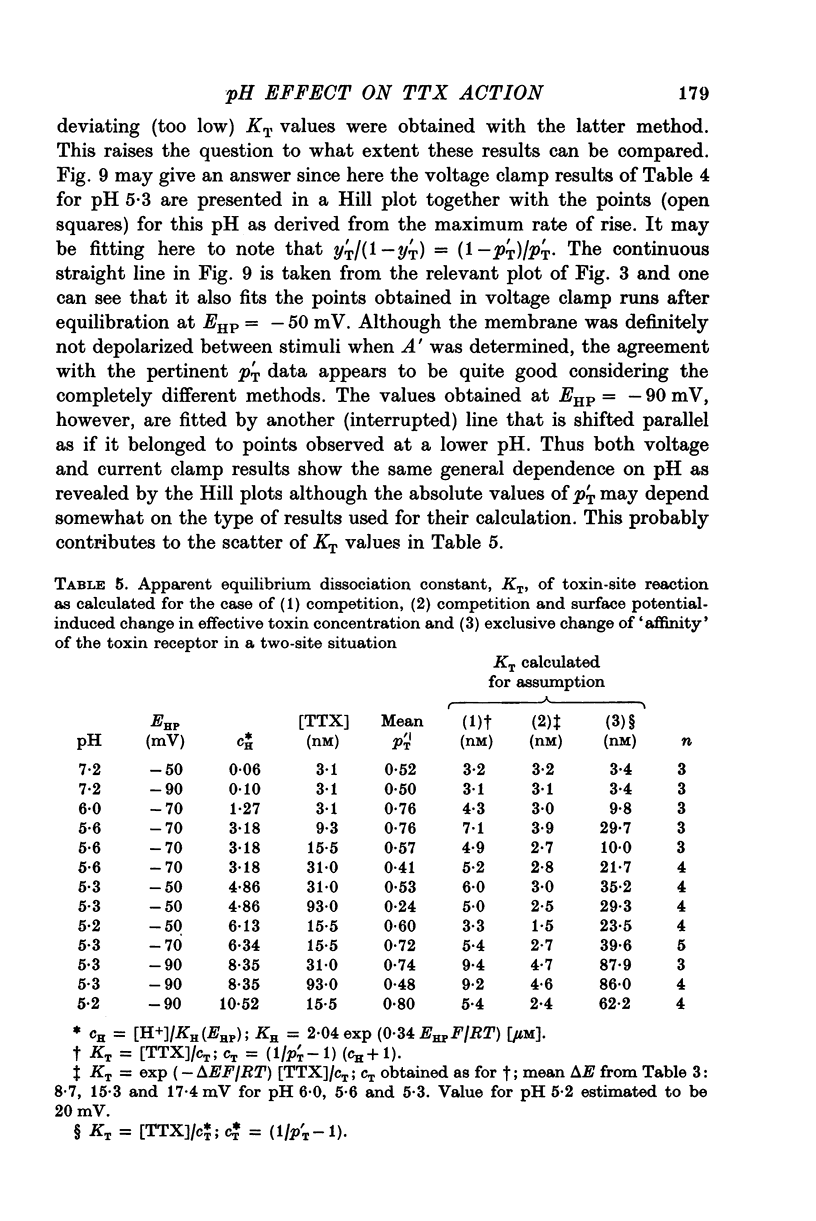
1. The experiments were done on single nodes of Ranvier of Rana esculenta. The effects of tetrodotoxin and H ions were determined either by the reduction of the maximum rate of rise, VA, of action potentials evoked with threshold stimuli or in the voltage clamp by the decrease of the peak Na permeability, PNa. 2. With the tetrodotoxin sample used throughout the investigation the equilibrium dissociation constant, KT, of the toxin-receptor reaction at neutral pH was determined to be 2-8 nM. Between 1-55 and 15-5 nM tetrodotoxin the normalized value, A, of VA, was found to be related to the normalized toxin concentration cT = [TTX]/2-8 nM by the empirical equation log [(1-A)/A] = 1-22 log cT-0-573. 3. On increasing the pH (up to 8-8) the effect of tetrodotoxin diminished as revealed by an increase in A. The apparent reduction of cT (as calculated from A) suggests that the toxin is active only in its cationic forms. 4. Weakly acid tetrodotoxin solutions (7-3 less than pH less than or equal to 5-5) reduced A to a lesser degree than did neutral toxin solutions in spite of the inherent depressing effect of acid pH on A (A = 0-5 at about pH 5-5). In more acid toxin solutions A decreased again and at pH 4-6 it was about equal to the value in toxin-free solution. 5. When, after equilibrium in an acid toxin solution, the perfusate was suddenly changed to neutral Ringer solution A jumped to a higher value A' as measured 1 sec after the switch. Since the blocking effect of hydrogen ions subsided within a fraction of a second while the time constant of the toxin washout is of the order of 1 min, A' reflects the number of Na channels blocked by tetrodotoxin at acid pH. 6. In acid toxin-free solution the peak PNa as obtained in voltage clamp experiments was reduced by a voltage-dependent factor (cH + 1)-1 with CH = [H+]/KH(E) and KH(E) = 2-04 muM exp (0-34 EF/RT). Adding tetrodotoxin resulted in another reduction by a constant factor p'T. 7. Experiments employing various combinations of toxin concentration (3-1-93 nM) and pH values (7-3-5-2) confirm the decreased toxin effect at low pH. Moreover, p'T was smaller (the additional toxin effect larger) when the membrane had been kept depolarized and thus cH reduced during equilibration. This suggests that tetrodotoxin cations and H ions compete for the same blocking site. A quantitative fit, however, requires additional assumptions.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Jr, Palti Y. The effects of external potassium and long duration voltage conditioning on the amplitude of sodium currents in the giant axon of the squid, Loligo pealei. J Gen Physiol. 1969 Nov;54(5):589–606. doi: 10.1085/jgp.54.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer T. I., Raftery M. A. Partial characterization of a tetrodotoxin-binding component from nerve membrane. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3634–3637. doi: 10.1073/pnas.69.12.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camougis G., Takman B. H., Tasse J. R. Potency difference between the zwitterion form and the cation forms of tetrodotoxin. Science. 1967 Jun 23;156(3782):1625–1627. doi: 10.1126/science.156.3782.1625. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Henderson R., Ritchie J. M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J Physiol. 1972 Dec;227(1):95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo L. A., Adelman W. J., Jr Equilibrium and kinetic properties of the interaction between tetrodotoxin and the excitable membrane of the squid giant axon. J Gen Physiol. 1970 Mar;55(3):309–335. doi: 10.1085/jgp.55.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Membrane currents in isolated frog nerve fibre under voltage clamp conditions. J Physiol. 1958 Aug 29;143(1):76–90. doi: 10.1113/jphysiol.1958.sp006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Sodium currents in the myelinated nerve fibre of Xenopus laevis investigated with the voltage clamp technique. J Physiol. 1959 Oct;148:188–200. doi: 10.1113/jphysiol.1959.sp006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin H., Neumcke B. Specific and unspecific charges at the sodium channels of the nerve membrane. Pflugers Arch. 1974;351(3):207–229. doi: 10.1007/BF00586919. [DOI] [PubMed] [Google Scholar]
- Drouin H., The R. The effect of reducing extracellular pH on the membrane currents of the ranvier node. Pflugers Arch. 1969;313(1):80–88. doi: 10.1007/BF00586331. [DOI] [PubMed] [Google Scholar]
- Goto T., Kishi Y., Takahashi S., Hirata Y. Tetrodotoxin. Tetrahedron. 1965 Aug;21(8):2059–2088. doi: 10.1016/s0040-4020(01)98344-9. [DOI] [PubMed] [Google Scholar]
- Henderson R., Ritchie J. M., Strichartz G. R. The binding of labelled saxitoxin to the sodium channels in nerve membranes. J Physiol. 1973 Dec;235(3):783–804. doi: 10.1113/jphysiol.1973.sp010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Wang J. H. Solubilization of a specific tetrodotoxin-binding component from garfish olfactory nerve membrane. Biochemistry. 1972 Nov 21;11(24):4565–4569. doi: 10.1021/bi00774a022. [DOI] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Pharmacological modifications of the sodium channels of frog nerve. J Gen Physiol. 1968 Feb;51(2):199–219. doi: 10.1085/jgp.51.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to organic cations in myelinated nerve. J Gen Physiol. 1971 Dec;58(6):599–619. doi: 10.1085/jgp.58.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y., Nishiyama A. Actions of saxitoxin on peripheral neuromuscular systems. J Physiol. 1965 Sep;180(1):50–66. [PMC free article] [PubMed] [Google Scholar]
- Koppenhöfer E., Vogel W. Wirkung von Tetrodotoxin und Tetraäthylammoniumchlorid an der Innenseite der Schnürringsmembran von Xenopus laevis. Pflugers Arch. 1969;313(4):361–380. doi: 10.1007/BF00593959. [DOI] [PubMed] [Google Scholar]
- Mozhayeva G. N., Naumov A. P. Tetraethylammonium ion inhibition of potassium conductance of the nodal membrane. Biochim Biophys Acta. 1972 Dec 1;290(1):248–255. doi: 10.1016/0005-2736(72)90067-3. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Moore J. W., Frazier D. T. Dependence of tetrodotoxin blockage of nerve membrane conductance on external pH. J Pharmacol Exp Ther. 1969 Oct;169(2):224–228. [PubMed] [Google Scholar]
- Nonner W. A new voltage clamp method for Ranvier nodes. Pflugers Arch. 1969;309(2):176–192. doi: 10.1007/BF00586967. [DOI] [PubMed] [Google Scholar]
- Ogura Y., Mori Y. Mechanism of local anesthetic action of crystalline tetrodotoxin and its derivatives. Eur J Pharmacol. 1968 Apr;3(1):58–67. doi: 10.1016/0014-2999(68)90049-6. [DOI] [PubMed] [Google Scholar]
- SMITH M. E., SMITH L. B. Piperazine dihydrochloride and glycylglycine as non-toxic buffers in distilled water and in sea water. Biol Bull. 1949 Jun;96(3):233–237. [PubMed] [Google Scholar]
- STAMPFLI R. Die Strom-Spannungs-Charakteristik der erregbaren Membran eines einzelnen Schnürrings und ihre Abhängigkeit von der Ionenkonzentration. Helv Physiol Pharmacol Acta. 1958;16(2):127–145. [PubMed] [Google Scholar]
- Schwarz J. R., Ulbricht W., Wagner H. H. The rate of action of tetrodotoxin on myelinated nerve fibres of Xenopus laevis and Rana esculenta. J Physiol. 1973 Aug;233(1):167–194. doi: 10.1113/jphysiol.1973.sp010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K., Ikuma S., Kawamura M., Tachikawa R., Sakai K. Tetrodotoxin. VII. On the structure of tetrodotoxin and its derivatives. Chem Pharm Bull (Tokyo) 1964 Nov;12(11):1357–1374. doi: 10.1248/cpb.12.1357. [DOI] [PubMed] [Google Scholar]
- Ulbricht W., Wagner H. H. The influence of pH on the rate of tetrodotoxin action on myelinated nerve fibres. J Physiol. 1975 Oct;252(1):185–202. doi: 10.1113/jphysiol.1975.sp011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierhaus J., Ulbricht W. Effect of a sudden change in sodium concentration on repetitively evoked action potentials of single nodes of Ranvier. Pflugers Arch. 1971;326(1):76–87. doi: 10.1007/BF00586795. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]


