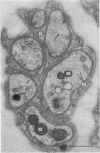
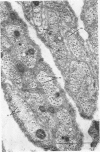
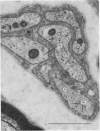
Abstract
1. Podophyllotoxin, colchicine and griseofulvin inhibit the intra-axonal movement of noradrenaline storage vesicles in cat hypogastric nerve/inferior mesenteric ganglion preparations maintained in vitro, cause the disappearance of axonal microtubules and inhibit the assembly of microtubules from tubulin in vitro. The order of potency of the three effects is podophyllotoxin greater than colchicine greater than griseofulvin. 2. Lumicolchicine is without effect on the three parameters and does not interfere with the binding of tritiated colchicine to tubulin. 3. Podophyllotoxin causes a more rapid loss of microtubules from axons than the same concentration of colchicine. 4. The experiments provide strong evidence that microtubules are components of the system responsible for the intra-axonal migration of noradrenaline storage vesicles.
Full text
PDF

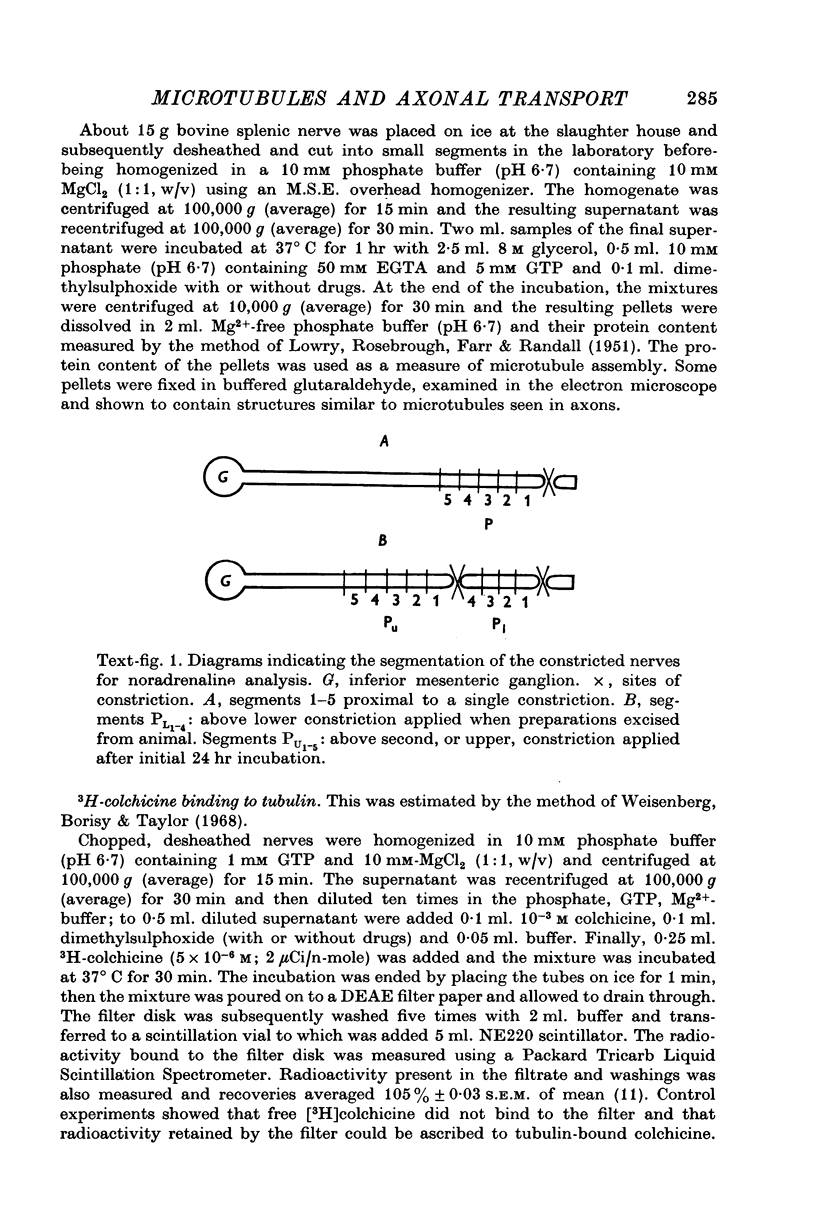

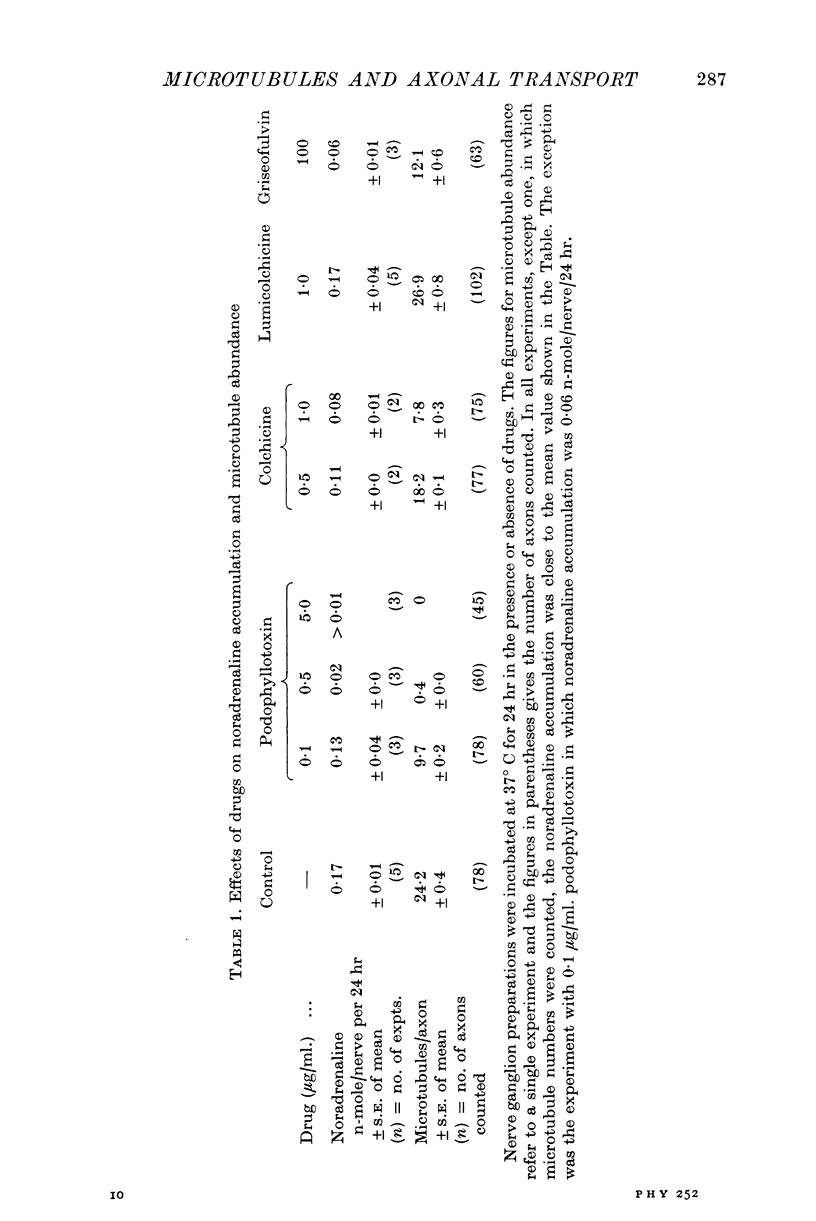
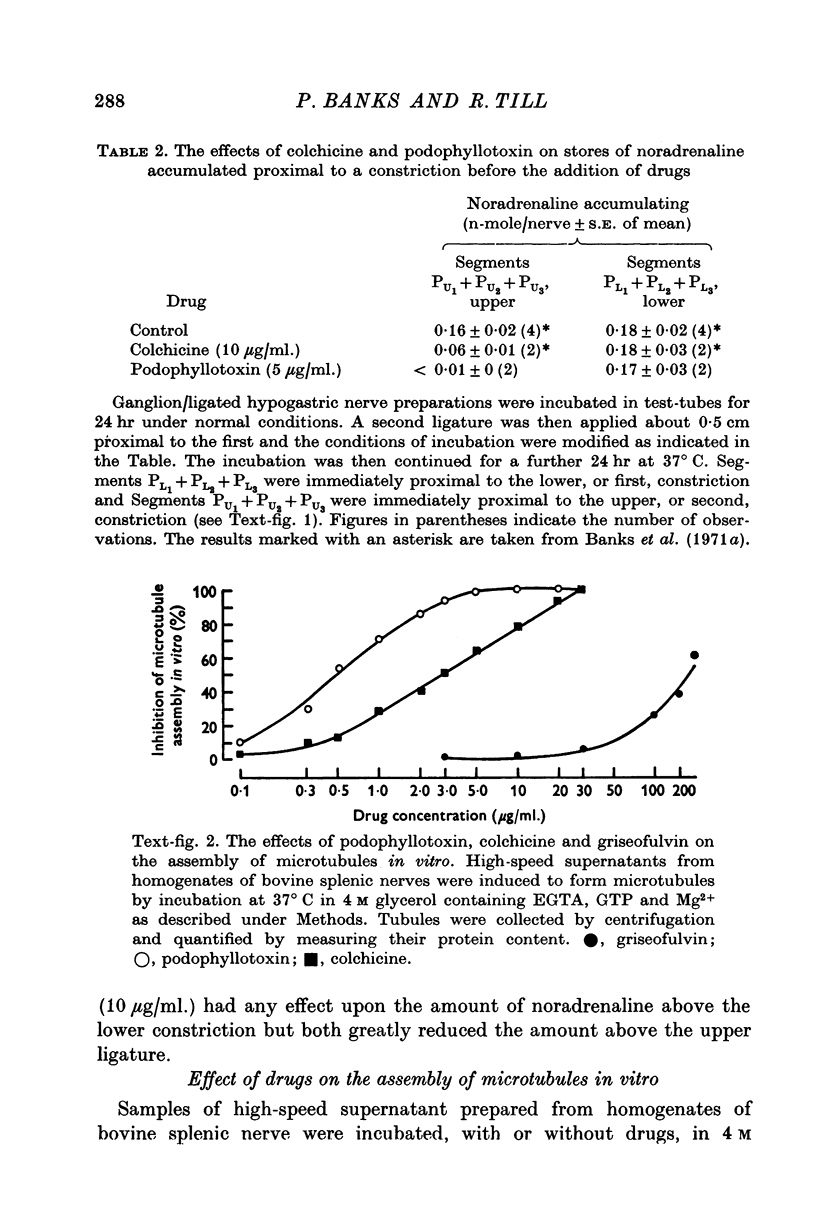

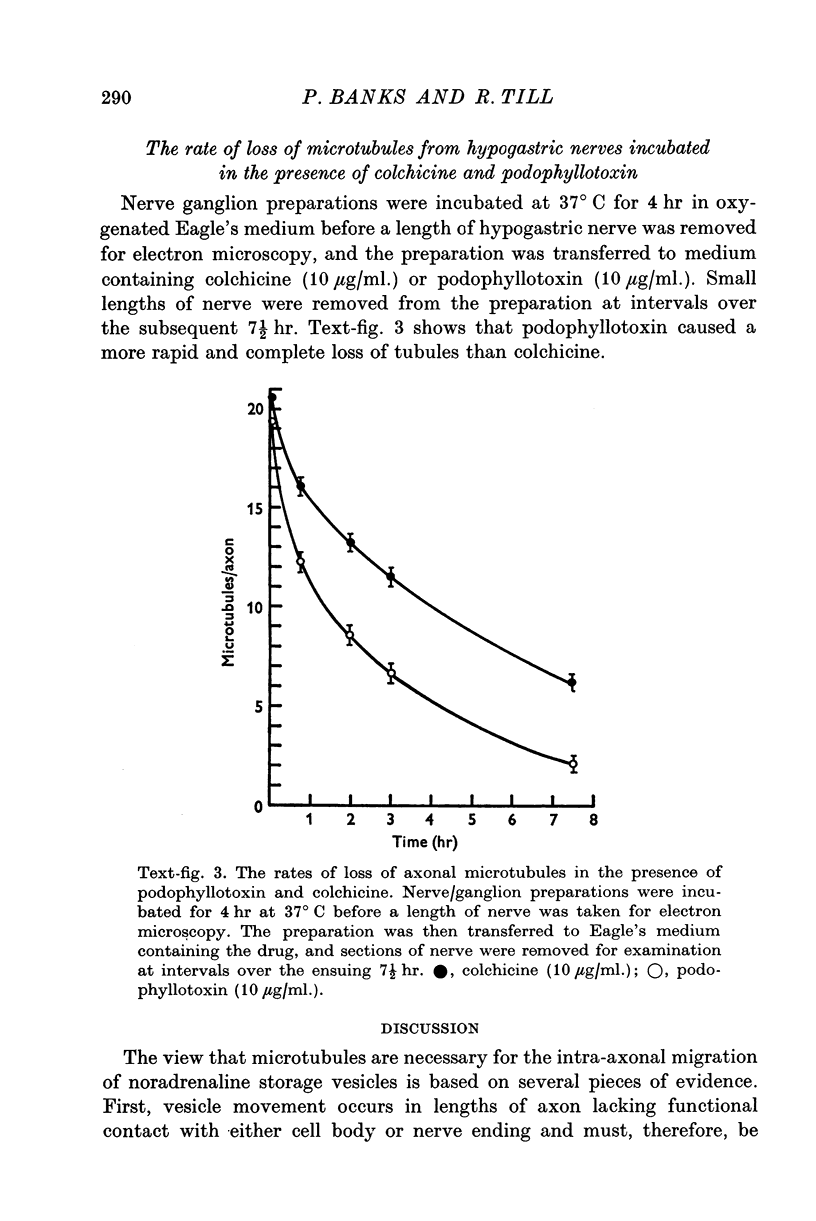




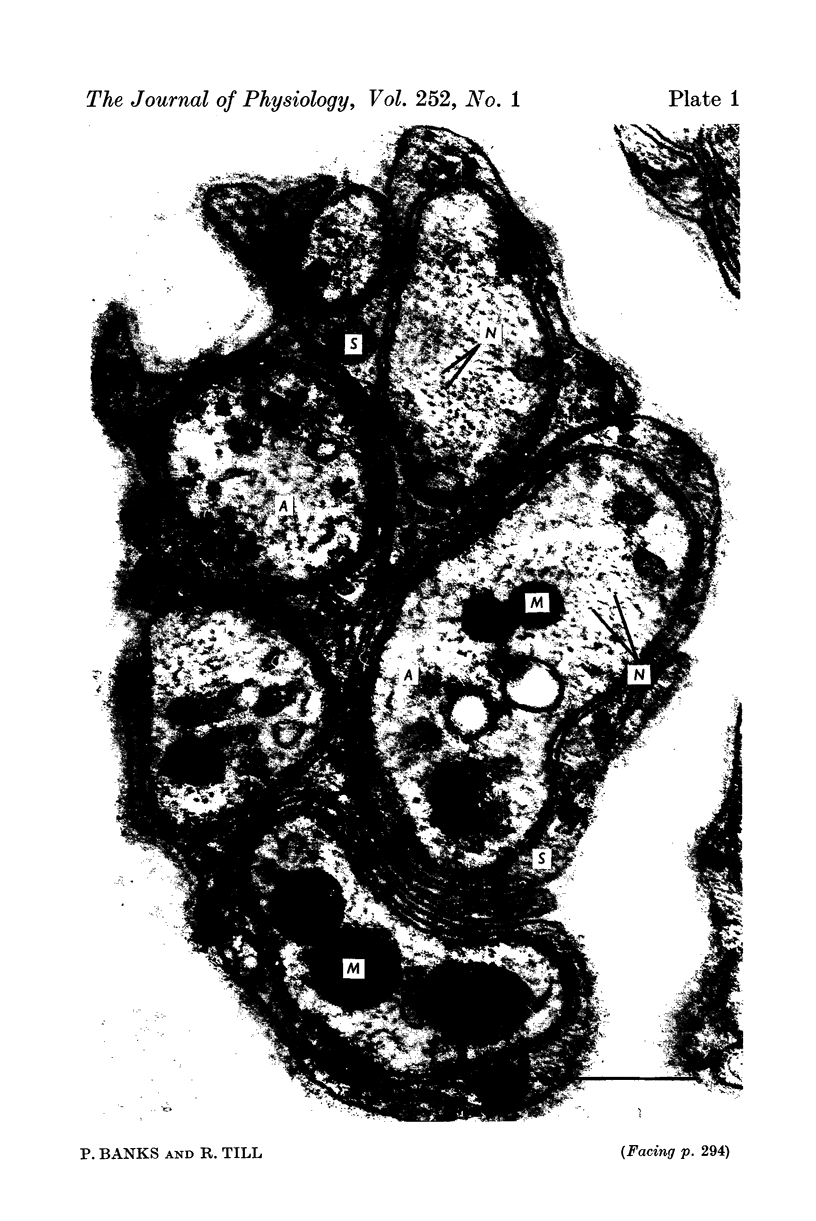
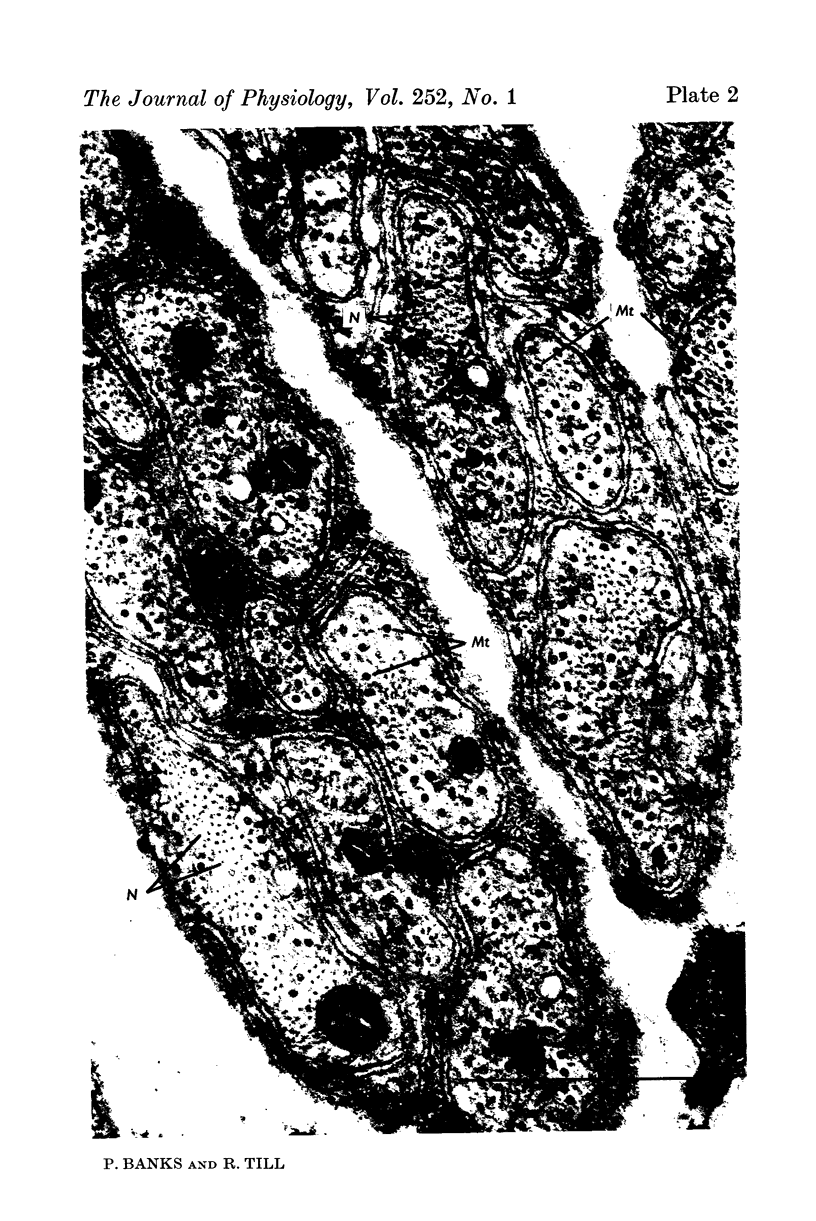
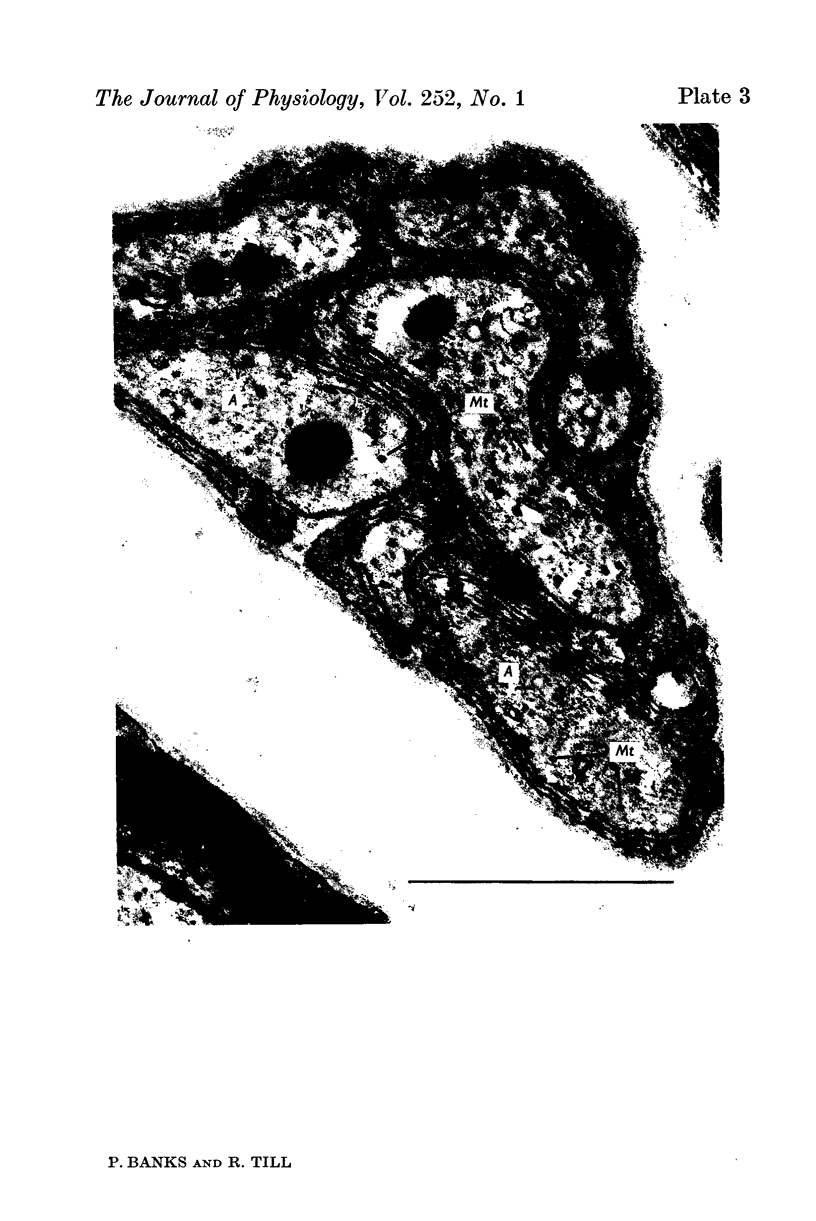
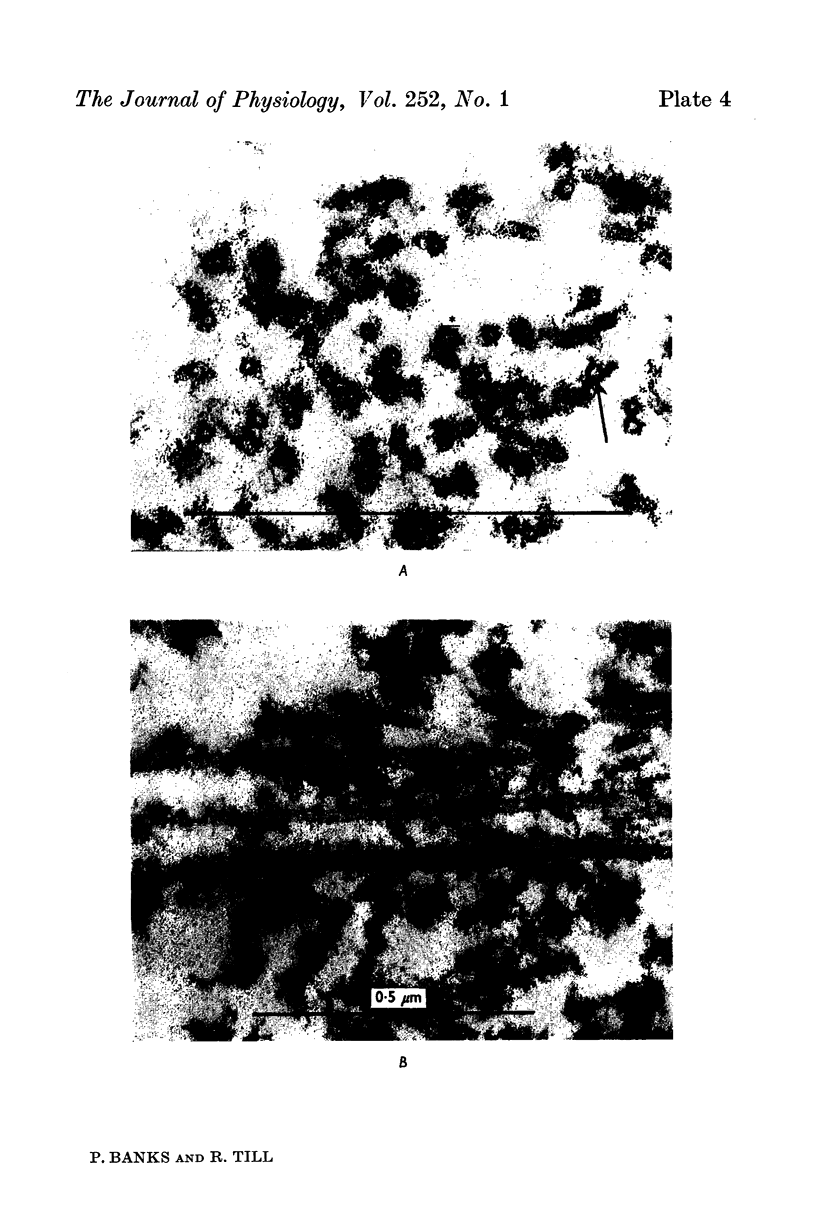
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks P., Mangnall D., Mayor D. The re-distribution of cytochrome oxidase, noradrenaline and adenosine triphosphate in adrenergic nerves constricted at two points. J Physiol. 1969 Feb;200(3):745–762. doi: 10.1113/jphysiol.1969.sp008720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks P., Mayor D. Intra-axonal transport in noradrenergic neurons in the sympathetic nervous system. Biochem Soc Symp. 1972;(36):133–149. [PubMed] [Google Scholar]
- Banks P., Mayor D., Mitchell M., Tomlinson D. Studies on the translocation of noradrenaline-containing vesicles in post-ganglionic sympathetic neurones in vitro. Inhibition of movement by colchicine and vinblastine and evidence for the involvement of axonal microtubules. J Physiol. 1971 Aug;216(3):625–639. doi: 10.1113/jphysiol.1971.sp009544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks P., Mayor D., Tomlinson D. R. Further evidence for the involvement of microtubules in the intra-axonal movement of noradrenaline storage granules. J Physiol. 1971 Dec;219(3):755–761. doi: 10.1113/jphysiol.1971.sp009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlström A. Axoplasmic transport (with particular respect to adrenergic neurons). Philos Trans R Soc Lond B Biol Sci. 1971 Jun 17;261(839):325–358. doi: 10.1098/rstb.1971.0064. [DOI] [PubMed] [Google Scholar]
- Dahlström A. Effect of colchicine on transport of amine storage granules in sympathetic nerves of rat. Eur J Pharmacol. 1968 Dec;5(1):111–113. doi: 10.1016/0014-2999(68)90165-9. [DOI] [PubMed] [Google Scholar]
- Dahlström A. The transport of noradrenaline between two simultaneously performed ligations of the sciatic nerves of rat and cat. Acta Physiol Scand. 1967 Mar;69(3):158–166. doi: 10.1111/j.1748-1716.1967.tb03507.x. [DOI] [PubMed] [Google Scholar]
- Grisham L. M., Wilson L., Bensch K. G. Antimitotic action of griseofulvin does not involve disruption of microtubules. Nature. 1973 Aug 3;244(5414):294–296. doi: 10.1038/244294a0. [DOI] [PubMed] [Google Scholar]
- Karlsson J. O., Hansson H. A., Sjöstrand J. Effect of colchicine on axonal transport and morphology of retinal ganglion cells. Z Zellforsch Mikrosk Anat. 1971;115(2):265–283. doi: 10.1007/BF00391128. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mayor D., Kapeller K. Fluorescence microscopy and electron microscopy of adrenergic nerves after constriction at two points. J R Microsc Soc. 1967;87(2):277–294. [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Nucleoside transport in mammalian cells. Inhibition by colchicine. Biochemistry. 1972 Jul 4;11(14):2573–2578. doi: 10.1021/bi00764a003. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- PAGET G. E., WALPOLE A. L. Some cytological effects of griseofulvin. Nature. 1958 Nov 8;182(4645):1320–1321. doi: 10.1038/1821320a0. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., McClure W. O. Microtubules and axoplasmic transport. Brain Res. 1974 Jun 20;73(2):333–337. doi: 10.1016/0006-8993(74)91053-1. [DOI] [PubMed] [Google Scholar]
- Price M. T. The effects of colchicine and lumicolchicine on the rapid phase of axonal transport in the rabbit visual system. Brain Res. 1974 Sep 13;77(3):497–501. doi: 10.1016/0006-8993(74)90638-6. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Wilson L., Bamburg J. R., Mizel S. B., Grisham L. M., Creswell K. M. Interaction of drugs with microtubule proteins. Fed Proc. 1974 Feb;33(2):158–166. [PubMed] [Google Scholar]
- Zweig M. H., Chignell C. F. Interaction of some colchicine analogs, vinblastine and podophyllotoxin with rat brain microtubule protein. Biochem Pharmacol. 1973 Sep 1;22(17):2141–2150. doi: 10.1016/0006-2952(73)90113-5. [DOI] [PubMed] [Google Scholar]