Abstract
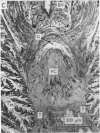
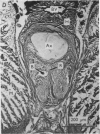
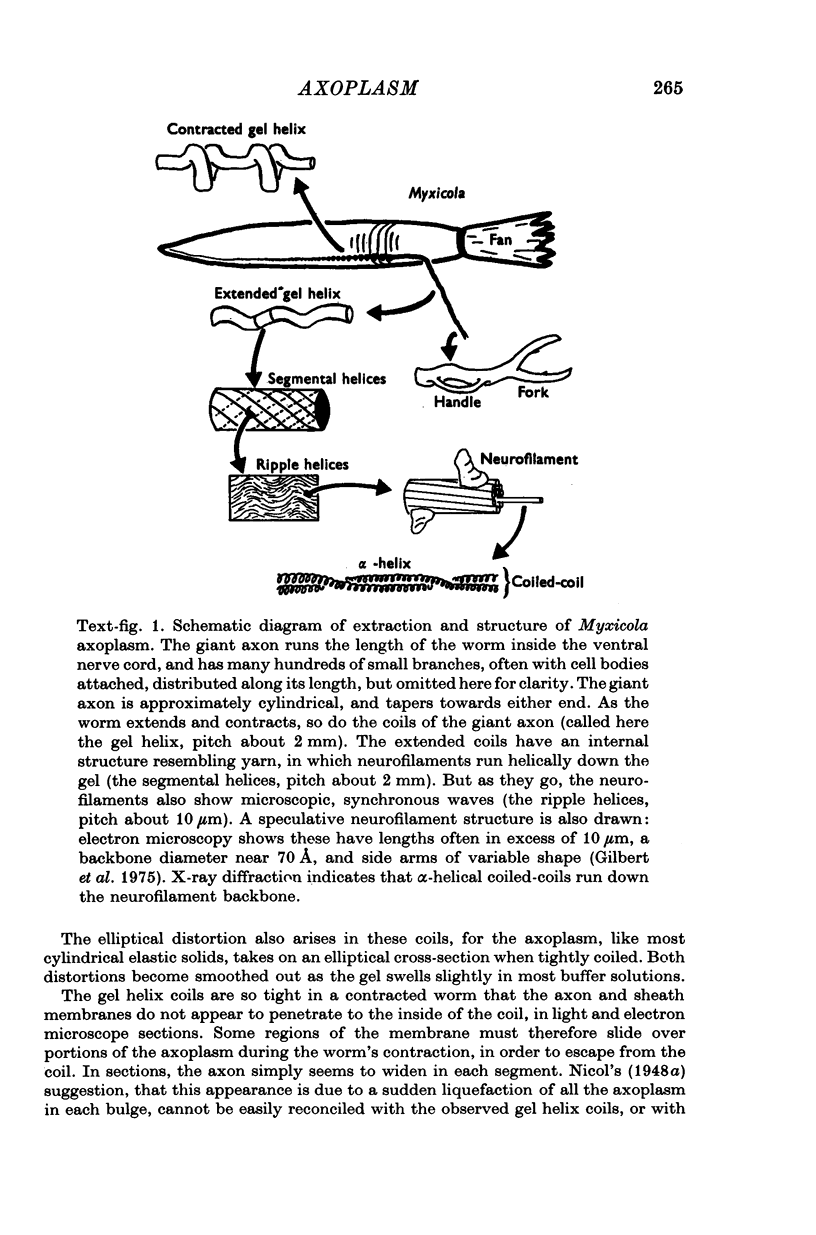
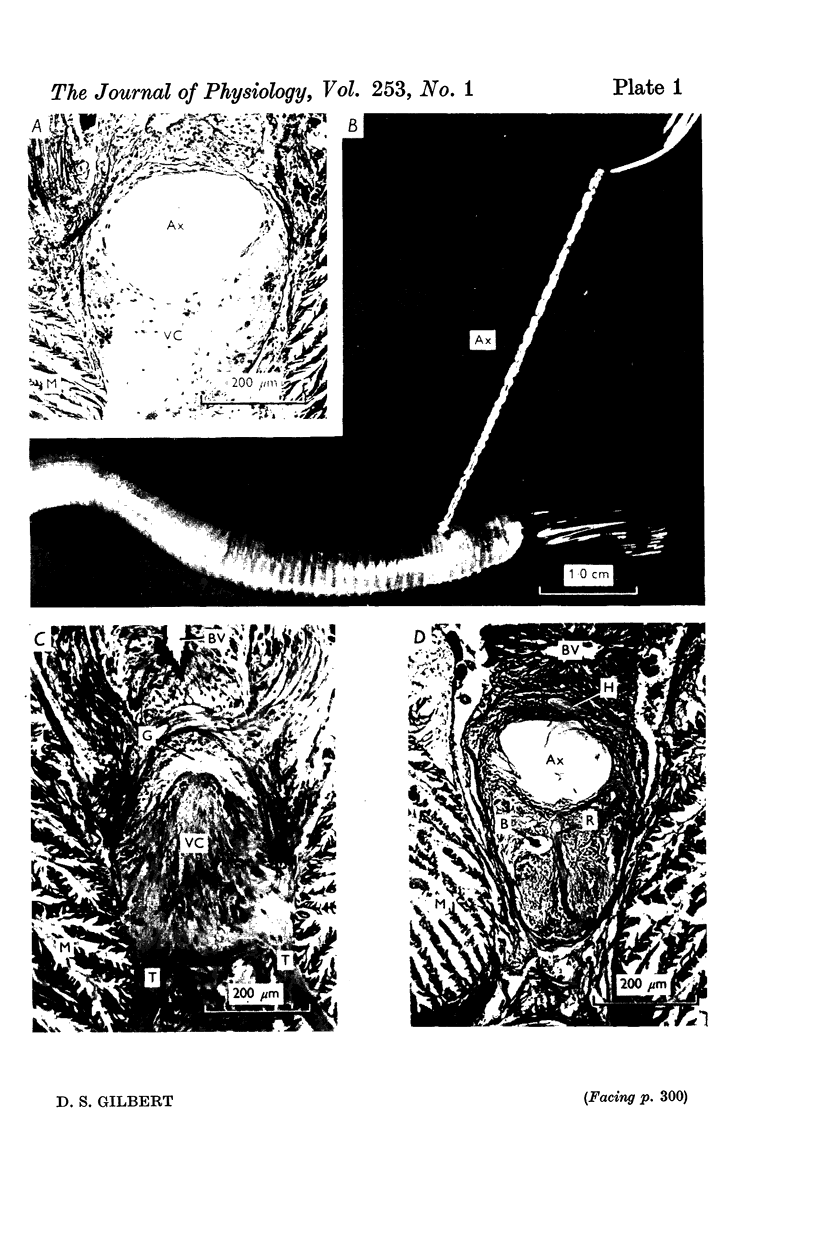
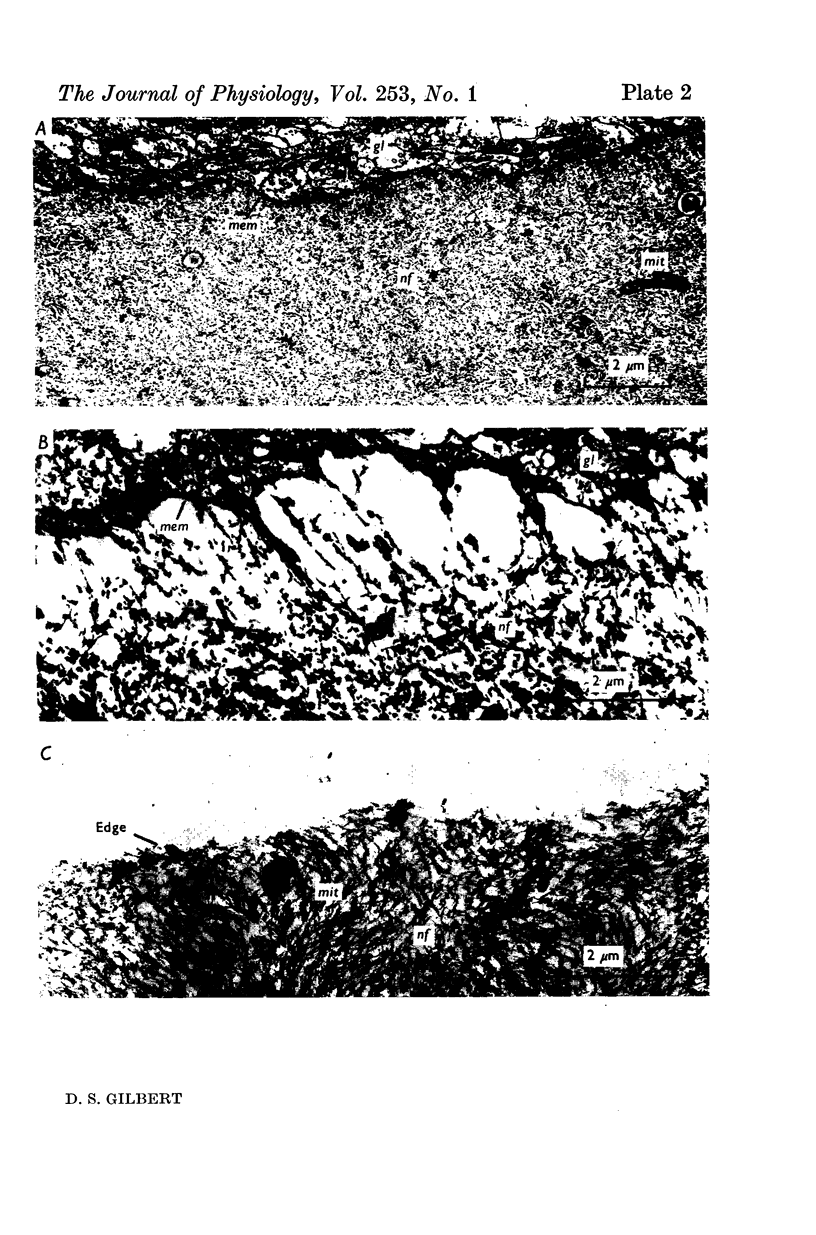
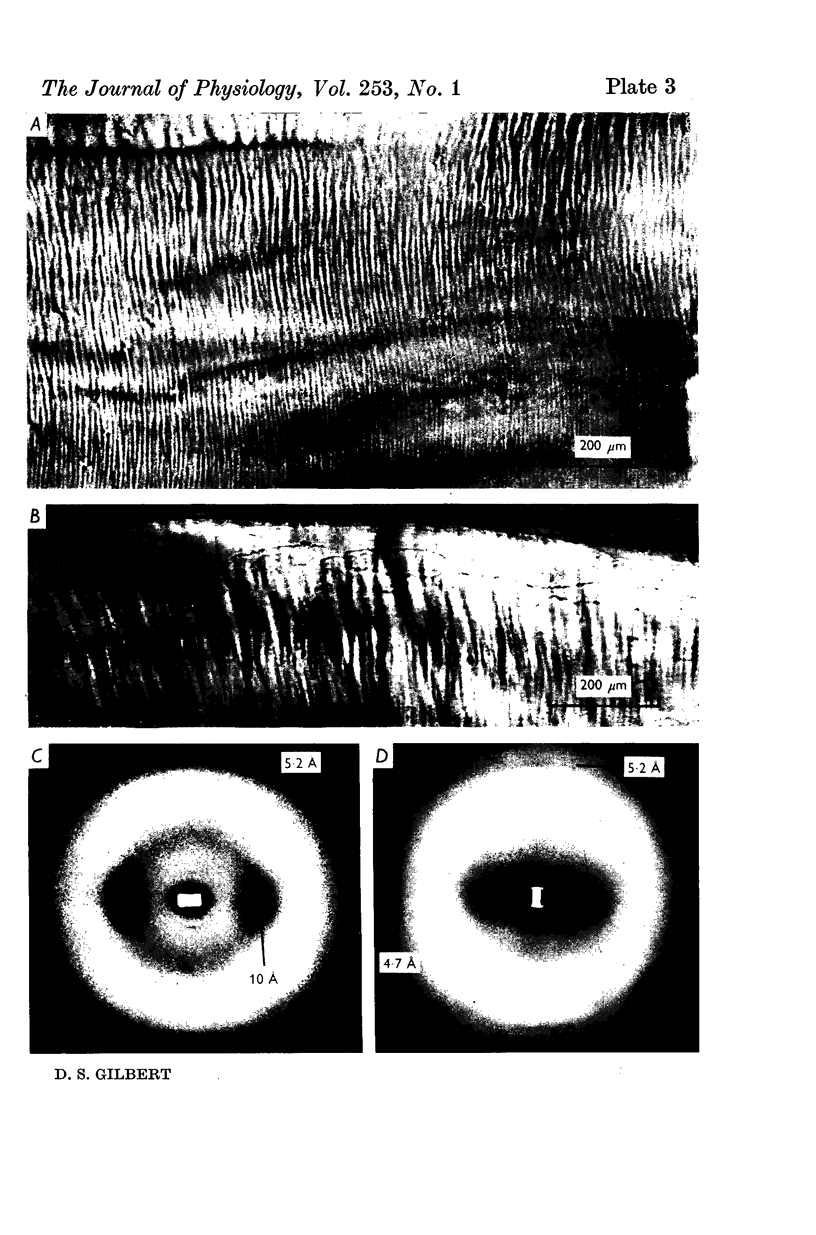
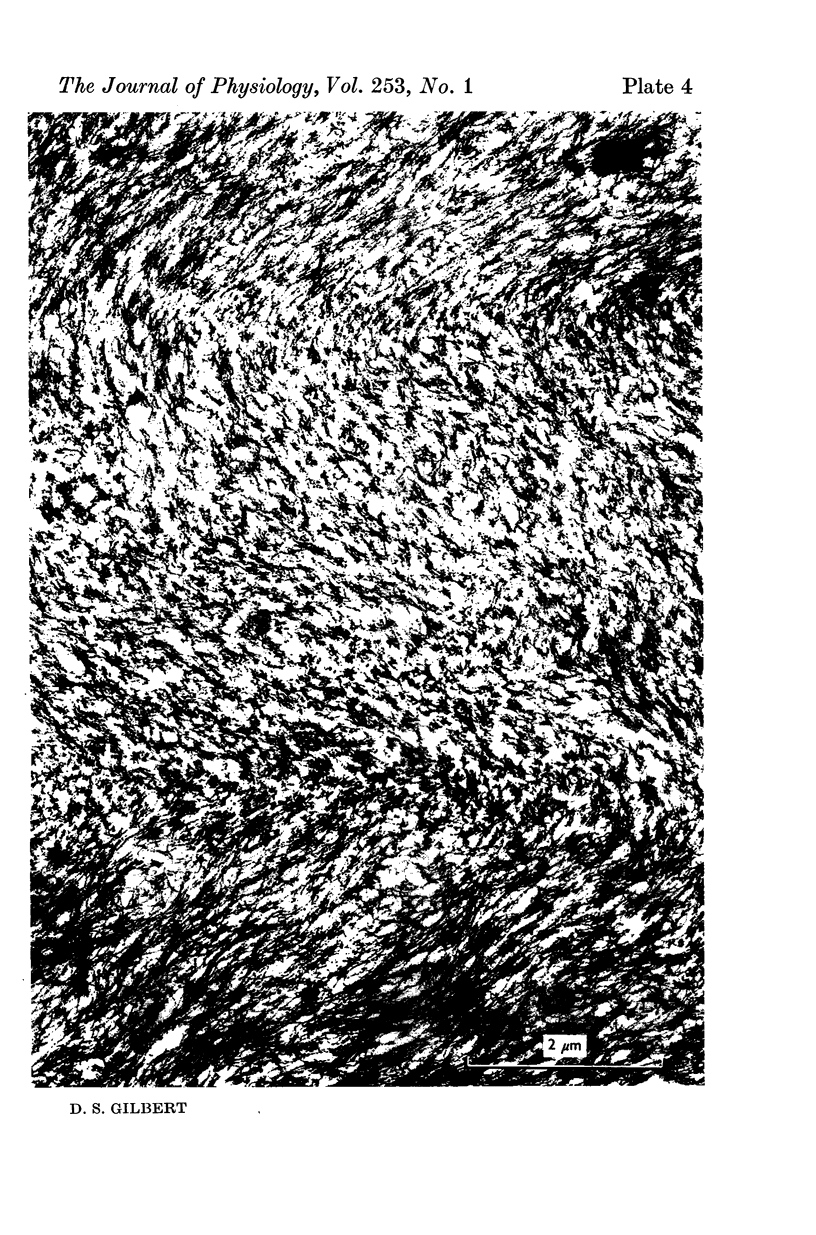
A technique is described for extracting axoplasm from the giant axon of a marine worm, Myxicola infundibulum. The operation can be completed in 10 sec. 2. Axoplasm is pulled from the axon of a living worm as a long, clear cylinder, up to 35 cm long and 70 mg wet weight. The worm regenerates a new giant axon in about 4 months. 3. Myxicola axoplasm is a gel, 87% water, held together by protein neurofilaments. It contains small amounts of mitochondria and vesicles, but no detectable microtubules. 4. The internal structure of the gel is superficially similar to that of yarn. Closer inspection with light and electron microscopy, and X-ray diffraction, show it to be organized in a hierarchy of helical forms. Squid giant axons have a similar structure. 5. Initial estimates of the bulk physical properties of extracted Myxicola axoplasm give: breaking strength, 1400 g/cm2; specific gravity, 1-05 g/cm3; birefringence, 1-6 X 10(-4); index of refraction, 1-351; resistivity, 57 omega cm. These average values are shown to be compatible with the observed structure and composition. 6. Despite its mechanical strength, the axoplasm gel is so hydrated that Na+, K+ and homarine diffuse through it at rates approaching those in free solution. Fewer than about 5% of each of these ions are tightly bound to the gel. 7. It is argued that (a) the structure and physical properties of Myxicola axoplasm are representative of those in other axons, (b) the compound helix architecture results from twist of parallel, cross-linked fibrous proteins, and (c) this sturcture serves as a flexible internal skeleton for nerve cell processes.
Full text
PDF







































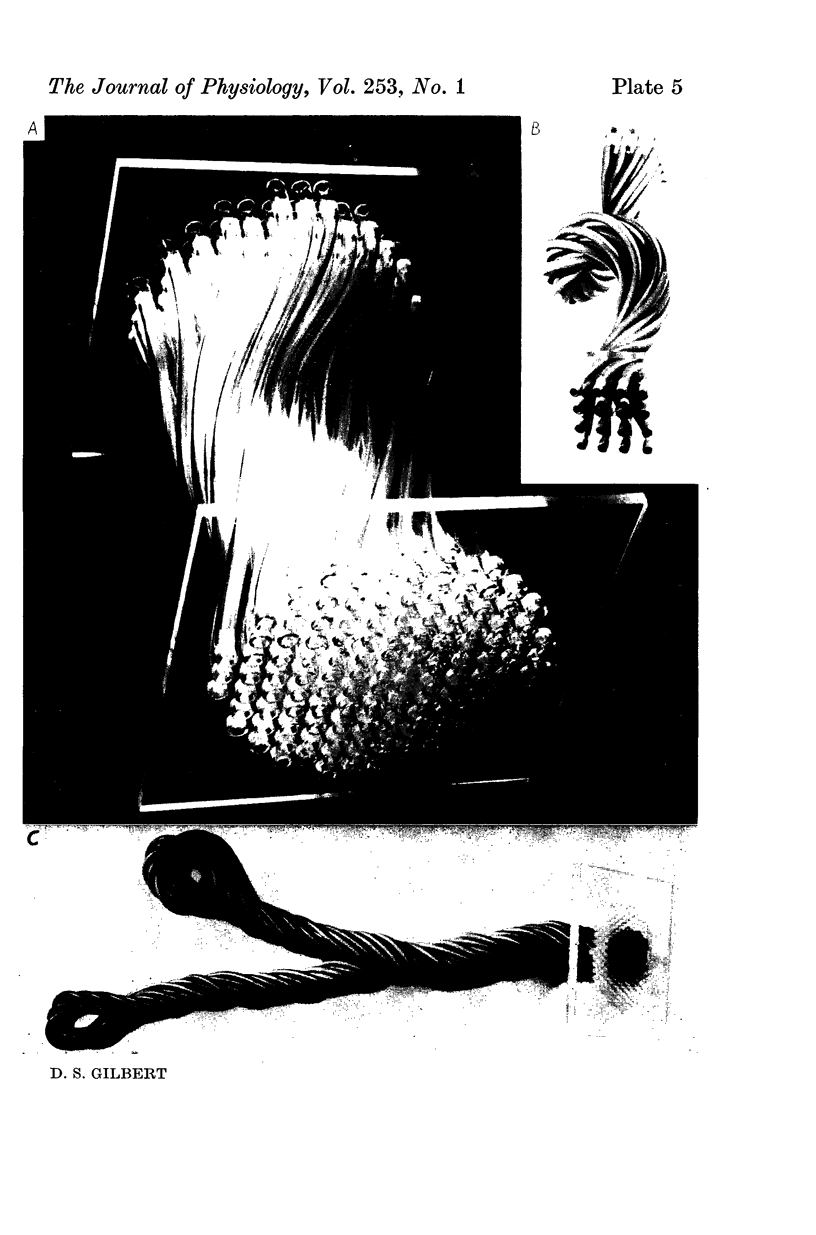

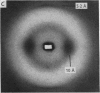
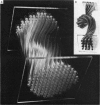
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemà S., Calissano P., Rusca G., Giuditta A. Identification of a calcium-binding, brain specific protein in the axoplasm of squid giant axons. J Neurochem. 1973 Mar;20(3):681–689. doi: 10.1111/j.1471-4159.1973.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Allen R. D., Francis D., Zeh R. Direct test of the positive pressure gradient theory of pseudopod extension and retraction in amoebae. Science. 1971 Dec 17;174(4015):1237–1240. doi: 10.1126/science.174.4015.1237. [DOI] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEN GEREN B. The formation from the Schwann cell surface of myelin in the peripheral nerves of chick embryos. Exp Cell Res. 1954 Nov;7(2):558–562. doi: 10.1016/s0014-4827(54)80098-x. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Schlaepfer W. Proceedings: Calcium uptake by axoplasm extruded from giant axons of Loligo. J Physiol. 1975 Jul;249(1):37P–38P. [PubMed] [Google Scholar]
- Binstock L., Goldman L. Giant axon of Myxicola: some membrane properties as observed under voltage clamp. Science. 1967 Dec 15;158(3807):1467–1469. doi: 10.1126/science.158.3807.1467. [DOI] [PubMed] [Google Scholar]
- Biondi R. J., Levy M. J., Weiss P. A. An engineering study of the peristaltic drive of axonal flow. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1732–1736. doi: 10.1073/pnas.69.7.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaurock A. E., Wilkins M. H. Structure of frog photoreceptor membranes. Nature. 1969 Aug 30;223(5209):906–909. doi: 10.1038/223906a0. [DOI] [PubMed] [Google Scholar]
- Bouteille M., Pease D. C. The tridimensional structure of native collagenous fibrils, their proteinaceous filaments. J Ultrastruct Res. 1971 May;35(3):314–338. doi: 10.1016/s0022-5320(71)80161-2. [DOI] [PubMed] [Google Scholar]
- Bray D. Model for membrane movements in the neural growth cone. Nature. 1973 Jul 13;244(5411):93–96. doi: 10.1038/244093a0. [DOI] [PubMed] [Google Scholar]
- Bunge M. B. Fine structure of nerve fibers and growth cones of isolated sympathetic neurons in culture. J Cell Biol. 1973 Mar;56(3):713–735. doi: 10.1083/jcb.56.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge R. P. Glial cells and the central myelin sheath. Physiol Rev. 1968 Jan;48(1):197–251. doi: 10.1152/physrev.1968.48.1.197. [DOI] [PubMed] [Google Scholar]
- CRAGG B. G. A physical theory of the growth of axons. J Cell Physiol. 1955 Feb;45(1):33–60. doi: 10.1002/jcp.1030450105. [DOI] [PubMed] [Google Scholar]
- Carpenter D. O., Hovey M. M., Bak A. F. Measurements of intracellular conductivity in Aplysia neurons: evidence for organization of water and ions. Ann N Y Acad Sci. 1973 Mar 30;204:502–533. doi: 10.1111/j.1749-6632.1973.tb30801.x. [DOI] [PubMed] [Google Scholar]
- Clarke E., Bearn J. G. The spiral nerve bands of Fontana. Brain. 1972;95(1):1–20. doi: 10.1093/brain/95.1.1. [DOI] [PubMed] [Google Scholar]
- Cole K. S., Hodgkin A. L. MEMBRANE AND PROTOPLASM RESISTANCE IN THE SQUID GIANT AXON. J Gen Physiol. 1939 May 20;22(5):671–687. doi: 10.1085/jgp.22.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crewther W. G., Fraser R. D., Lennox F. G., Lindley H. The chemistry of keratins. Adv Protein Chem. 1965;20:191–346. doi: 10.1016/s0065-3233(08)60390-3. [DOI] [PubMed] [Google Scholar]
- DAVISON P. F., TAYLOR E. W. Physical-chemical studies of proteins of squid nerve axoplasm, with special reference to the axon fibrous protein. J Gen Physiol. 1960 Mar;43:801–823. doi: 10.1085/jgp.43.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE ROBERTIS E., THORNBURG W. Polarization and electron microscope study of frog nerve axoplasm. J Biophys Biochem Cytol. 1956 Jul 25;2(4):475–482. doi: 10.1083/jcb.2.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. P. Colchicine inhibition of nerve fiber formation in vitro. J Cell Biol. 1972 Apr;53(1):164–176. doi: 10.1083/jcb.53.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison P. F., Winslow B. The protein subunit of calf brain neurofilament. J Neurobiol. 1974;5(2):119–133. doi: 10.1002/neu.480050204. [DOI] [PubMed] [Google Scholar]
- Day W. A., Gilbert D. S. X-ray diffraction pattern of axoplasm. Biochim Biophys Acta. 1972 Dec 28;285(2):503–506. doi: 10.1016/0005-2795(72)90342-x. [DOI] [PubMed] [Google Scholar]
- Dipolo R. Calcium efflux from internally dialyzed squid giant axons. J Gen Physiol. 1973 Nov;62(5):575–589. doi: 10.1085/jgp.62.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M. Surface area per lipid molecule in the intact membrane of the human red cell. Nature. 1969 Sep 20;223(5212):1279–1280. doi: 10.1038/2231279a0. [DOI] [PubMed] [Google Scholar]
- Fuller F. B. The writhing number of a space curve. Proc Natl Acad Sci U S A. 1971 Apr;68(4):815–819. doi: 10.1073/pnas.68.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G., North R. A. Proceedings: Intracellular recording and electron microscopy of the same myenteric plexus neurone. J Physiol. 1974 Jul;240(2):28P–30P. [PubMed] [Google Scholar]
- Gilbert D. S. Axoplasm chemical composition in Myxicola and solubility properties of its structural proteins. J Physiol. 1975 Dec;253(1):303–319. doi: 10.1113/jphysiol.1975.sp011191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. S. Helical structure of Myxicola axoplasm. Nat New Biol. 1972 Jun 14;237(76):195–passim. doi: 10.1038/newbio237195a0. [DOI] [PubMed] [Google Scholar]
- Gilbert D. S. Helical structure of axoplasm. J Physiol. 1972 Apr;222(1):44P–45P. [PubMed] [Google Scholar]
- Gilbert D. S., Newby B. J. Neurofilament disguise, destruction and discipline. Nature. 1975 Aug 14;256(5518):586–589. doi: 10.1038/256586a0. [DOI] [PubMed] [Google Scholar]
- Gilbert D. S., Shaw T. I. Extrusion and perfusion of the giant nerve fibre of Myxicola infundibulum. J Physiol. 1969 Sep;204(1):28P–29P. [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Inactivation of the sodium current in Myxicola giant axons. Evidence for coupling to the activation process. J Gen Physiol. 1972 Jun;59(6):659–675. doi: 10.1085/jgp.59.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., MORITA H., NAKA K. TRANSMISSION THROUGH DISTRIBUTED SYNAPSES BETWEEN TWO GIANT AXONS OF A SABELLID WORM. Comp Biochem Physiol. 1964 Dec;13:453–460. doi: 10.1016/0010-406x(64)90037-4. [DOI] [PubMed] [Google Scholar]
- HINKE J. A. The measurement of sodium and potassium activities in the squid axon by means of cation-selective glass micro-electrodes. J Physiol. 1961 Apr;156:314–335. doi: 10.1113/jphysiol.1961.sp006678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of calcium on the axoplasm of giant nerve fibers. J Exp Biol. 1949 Oct;26(3):292-4, pl. doi: 10.1242/jeb.26.3.292. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The mobility and diffusion coefficient of potassium in giant axons from Sepia. J Physiol. 1953 Mar;119(4):513–528. doi: 10.1113/jphysiol.1953.sp004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallpike J. F., Adams C. W. Proteolysis and myelin breakdown: a review of recent histochemical and biochemical studies. Histochem J. 1969 Nov;1(6):559–578. doi: 10.1007/BF01012862. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L. The membrane resistance of a non-medullated nerve fibre. J Physiol. 1947 Jul 31;106(3):305–318. doi: 10.1113/jphysiol.1947.sp004214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huneeus F. C., Davison P. F. Fibrillar proteins from squid axons. I. Neurofilament protein. J Mol Biol. 1970 Sep 28;52(3):415–428. doi: 10.1016/0022-2836(70)90410-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. Muscular contraction and cell motility. Nature. 1973 Jun 22;243(5408):445–449. doi: 10.1038/243445a0. [DOI] [PubMed] [Google Scholar]
- KAMIYA N., SEIFRIZ W. Torsion in a protoplasmic thread. Exp Cell Res. 1954 Feb;6(1):1–16. doi: 10.1016/0014-4827(54)90143-3. [DOI] [PubMed] [Google Scholar]
- KOECHLIN B. A. On the chemical composition of the axoplasm of squid giant nerve fibers with particular reference to its ion pattern. J Biophys Biochem Cytol. 1955 Nov 25;1(6):511–529. doi: 10.1083/jcb.1.6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling G. N., Miller C., Ochsenfeld M. M. The physical state of solutes and water in living cells according to the association-induction hypothesis. Ann N Y Acad Sci. 1973 Mar 30;204:6–50. doi: 10.1111/j.1749-6632.1973.tb30770.x. [DOI] [PubMed] [Google Scholar]
- Metuzals J. Configuration of a filamentous network in the axoplasm of the squid (Loligo pealii L.) giant nerve fiber. J Cell Biol. 1969 Dec;43(3):480–505. doi: 10.1083/jcb.43.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metuzals J., Izzard C. S. Spatial patterns of threadlike elements in the axoplasm of the giant nerve fiber of the squid (Loligo pealii L.) as disclosed by differential interference microscopy and by electron microscopy. J Cell Biol. 1969 Dec;43(3):456–479. doi: 10.1083/jcb.43.3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICOL J. A. C. The function of the giant axon of Myxicola infundibulum Montagu. Can J Res. 1948 Aug;26(4):212–222. doi: 10.1139/cjr48d-018. [DOI] [PubMed] [Google Scholar]
- NICOL J. A. C. The giant nerve-fibres in the central nervous system of Myxicola (Polychaeta, Sabellidae). Q J Microsc Sci. 1948 Mar;89(Pt 1):1–45. [PubMed] [Google Scholar]
- Ochs S. Fast transport of materials in mammalian nerve fibers. Science. 1972 Apr 21;176(4032):252–260. doi: 10.1126/science.176.4032.252. [DOI] [PubMed] [Google Scholar]
- Orrego F. Protein degradation in squid giant axons. J Neurochem. 1971 Dec;18(12):2249–2254. doi: 10.1111/j.1471-4159.1971.tb00181.x. [DOI] [PubMed] [Google Scholar]
- PAULING L., COREY R. B. Compound helical configurations of polypeptide chains: structure of proteins of the alpha-keratin type. Nature. 1953 Jan 10;171(4341):59–61. doi: 10.1038/171059a0. [DOI] [PubMed] [Google Scholar]
- Peracchia C. Excitable membrane ultrastructure. I. Freeze fracture of crayfish axons. J Cell Biol. 1974 Apr;61(1):107–122. doi: 10.1083/jcb.61.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbard D., Chrambach A. Unified theory for gel electrophoresis and gel filtration. Proc Natl Acad Sci U S A. 1970 Apr;65(4):970–977. doi: 10.1073/pnas.65.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer W. W. Calcium-induced degeneration of axoplasm in isolated segments of rat peripheral nerve. Brain Res. 1974 Apr 5;69(2):203–215. doi: 10.1016/0006-8993(74)90002-x. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Albert S., DeVries G. H., Norton W. T. Isolation of filaments from brain. Science. 1971 Dec 17;174(4015):1242–1245. doi: 10.1126/science.174.4015.1242. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Villegas G. M. Electron microscopic study of the giant nerve fiber of the giant squid Dosidicus gigas. J Ultrastruct Res. 1969 Mar;26(5):501–504. doi: 10.1016/s0022-5320(69)90054-9. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. Electrical characteristics of Sepia axons. J Physiol. 1951 Jul;114(3):372–381. doi: 10.1113/jphysiol.1951.sp004628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS P., HISCOE H. B. Experiments on the mechanism of nerve growth. J Exp Zool. 1948 Apr;107(3):315–395. doi: 10.1002/jez.1401070302. [DOI] [PubMed] [Google Scholar]
- WEISS P. THE DYNAMICS OF THE MEMBRANE-BOUND INCOMPRESSIBLE BODY: A MECHANISM OF CELLULAR AND SUBCELLULAR MOTILITY. Proc Natl Acad Sci U S A. 1964 Oct;52:1024–1029. doi: 10.1073/pnas.52.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P. A., Mayr R. Neuronal organelles in neuroplasmic ("axonal") flow. II. Neurotubules. Acta Neuropathol. 1971;5(Suppl):198–120. [PubMed] [Google Scholar]
- Weiss P. A., Mayr R. Organelles in neuroplasmic ("axonal") flow: neurofilaments. Proc Natl Acad Sci U S A. 1971 Apr;68(4):846–850. doi: 10.1073/pnas.68.4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J., Besso J. A., Jr, Boldosser W. G., Parsons R. L. The fine structure of the nerve cord of Myxicola infundibulum (Annelida, Polychaeta). Z Zellforsch Mikrosk Anat. 1972;131(2):141–148. doi: 10.1007/BF00306923. [DOI] [PubMed] [Google Scholar]
- Wuerker R. B., Kirkpatrick J. B. Neuronal microtubules, neurofilaments, and microfilaments. Int Rev Cytol. 1972;33:45–75. doi: 10.1016/s0074-7696(08)61448-5. [DOI] [PubMed] [Google Scholar]