Abstract
1. The effect of various cardioactive steroids on the activity of a microsomal (Na+ + K+)-activated ATPase from rat intestinal mucosa has been studied and compared with their effects on L-phenylalanine and D-galactose transport by rings of rat intestine in vitro. A similar comparison between the sensitivities to ouabain of microsomal (Na+ + K+)-ATPase and of phenylalanine transport in the intestines of the mouse, guinea-pig and toad has been made.
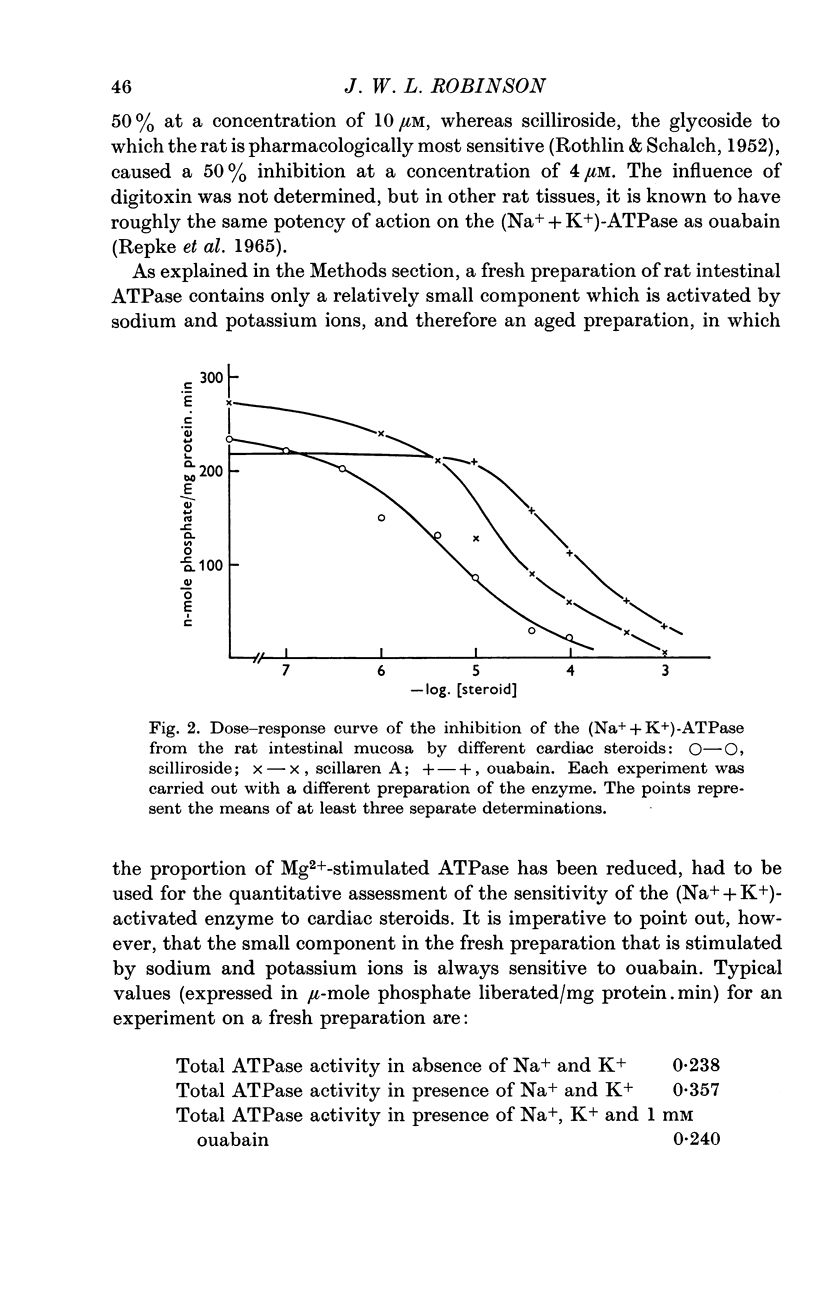
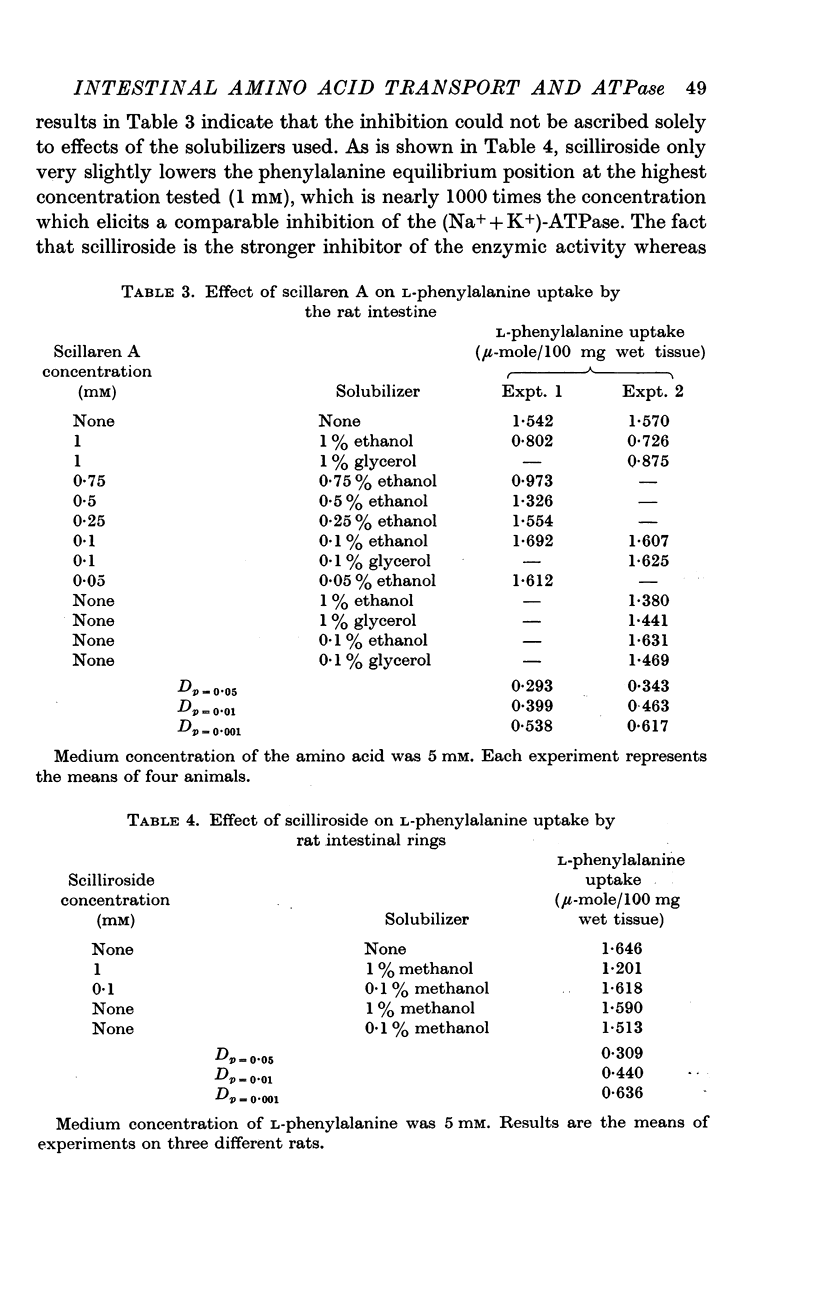
2. The rat intestinal enzyme is 50% inhibited by a concentration of 1 × 10-4M ouabain, 1 × 10-5M scillaren A and 4 × 10-6M scilliroside. At concentrations which almost completely inhibit the (Na+ + K+)-ATPase activity, these steroids have no effect on the transport of phenylalanine or galactose by the rat intestine. Only at concentrations of 1 × 10-3M are scillaren A and scilliroside able to reduce phenylalanine accumulation significantly, the same concentration of ouabain being effective only in the absence of external potassium ions. Digitoxin, 1 × 10-4M, a comparatively apolar glycoside, had no action on phenylalanine transport in the rat intestine.
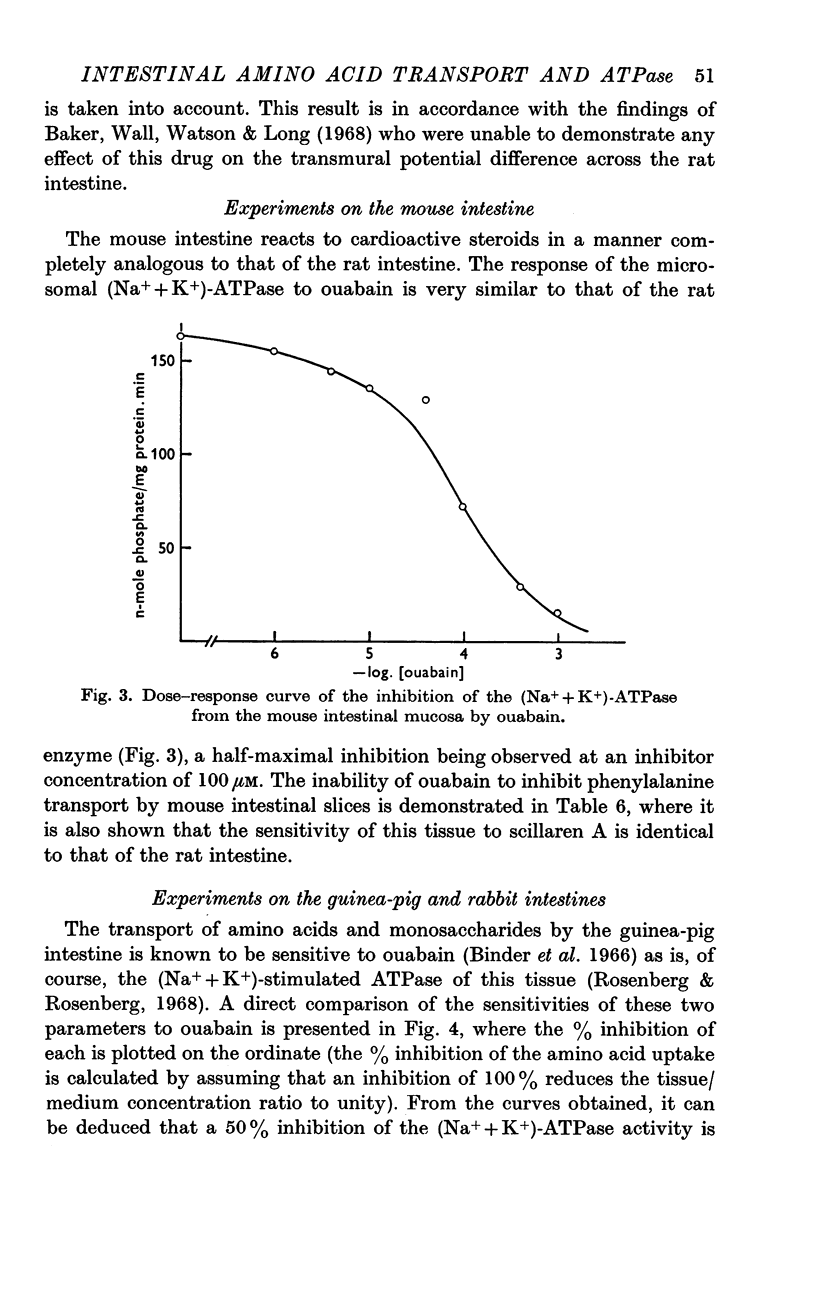
3. The effect of ouabain on the (Na+ + K+)-ATPase and phenylalanine transport system in the mouse intestine is completely analogous to its effect on these parameters in the rat.
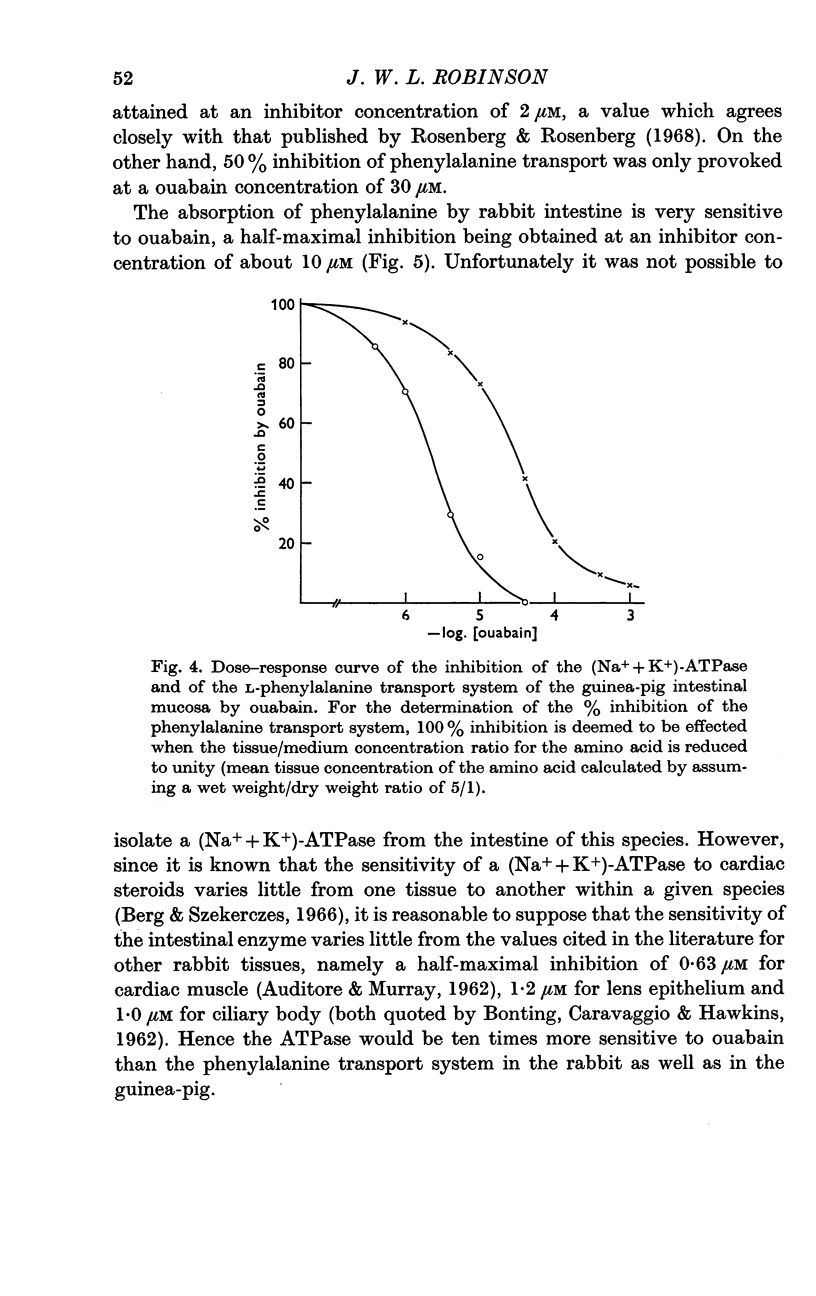
4. A half-maximal inhibition of guinea-pig intestinal (Na+ + K+)-ATPase by ouabain occurs at an inhibitor concentration of 2 × 10-6M, but phenylalanine transport by this tissue is only half-maximally reduced at a concentration of 3 × 10-5M. Similarly, in the rabbit intestine, there appears to be a difference of an order of magnitude between the sensitivities of the two parameters.
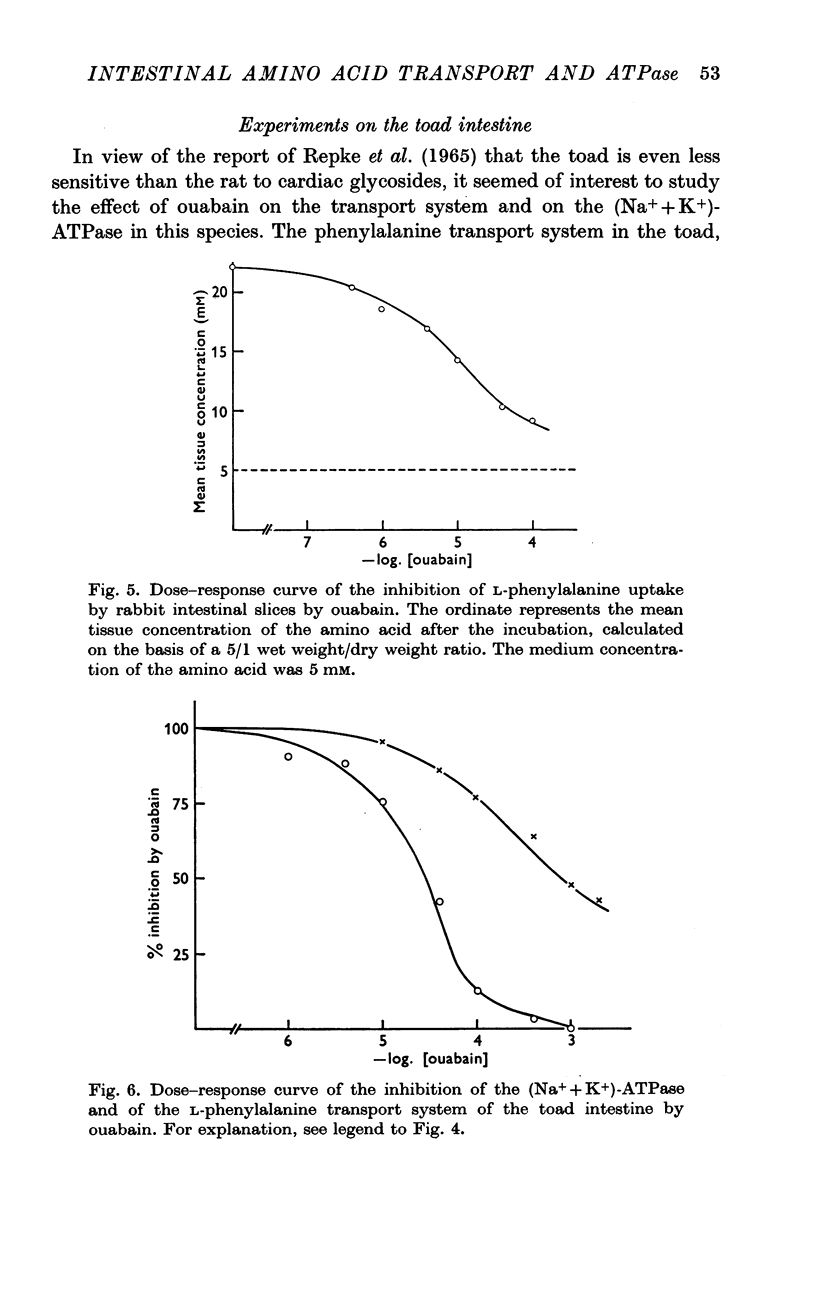
5. In the toad, 50% inhibition of the enzymic activity is observed at a concentration of 3 × 10-5M ouabain, whereas a concentration of 8 × 10-4M is required to reduce phenylalanine accumulation by one half.
6. These findings are consistent with the suggestion that an (Na+ + K+)-stimulated ATPase is not the only enzyme in the epithelial cells of the intestinal mucosa that is responsible for sodium extrusion (the mechanism for sodium extrusion being intimately coupled with the mechanism for active amino acid transport); therefore, a second, as yet unidentified, enzyme system must be postulated to account for bulk sodium flow through the intestine.
Full text
PDF



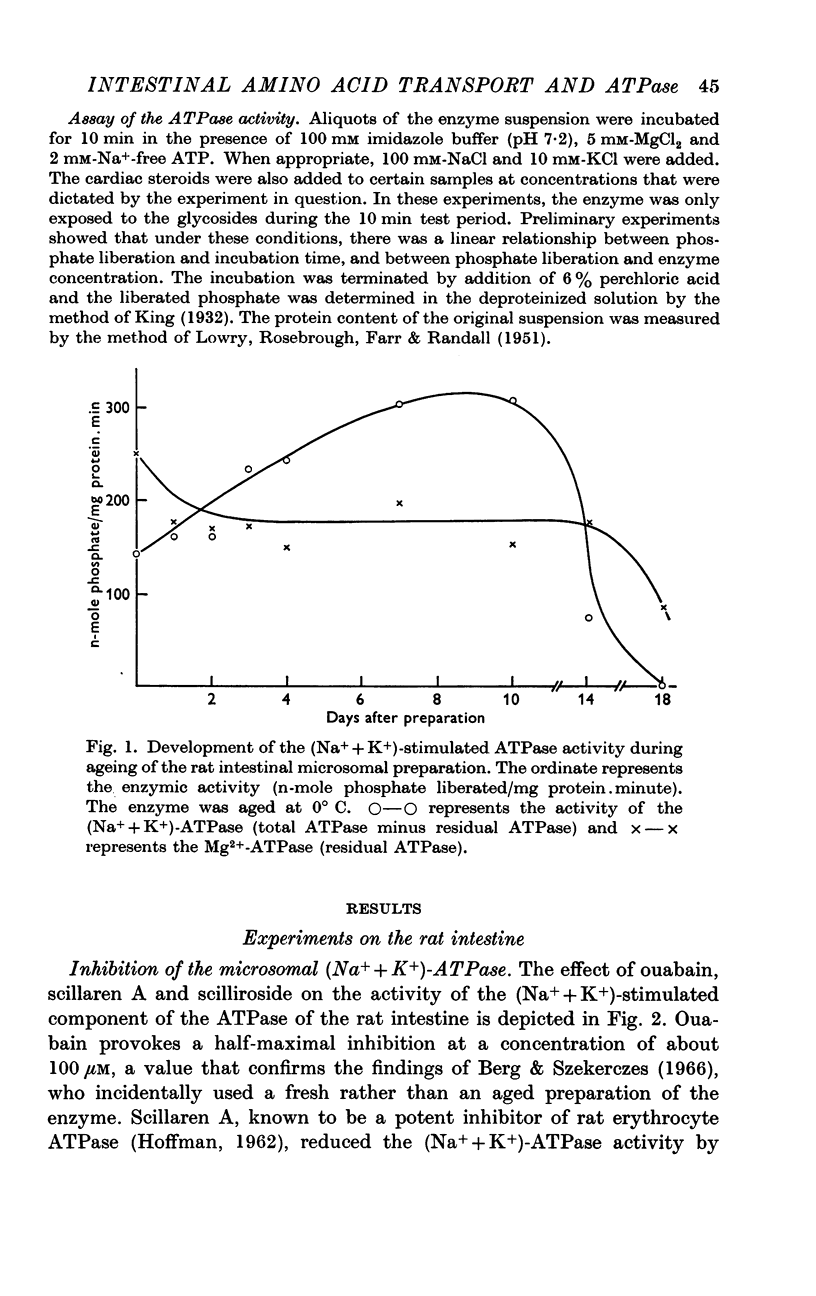










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUDITORE J. V., MURRAY L. Cardiac (microsomal) Na plus K adenosinetriphosphatase and its possible relationship to the active Na plus K transport system. Arch Biochem Biophys. 1962 Dec;99:372–382. doi: 10.1016/0003-9861(62)90282-5. [DOI] [PubMed] [Google Scholar]
- BONTING S. L., CARAVAGGIO L. L., HAWKINS N. M. Studies on sodium-potassium-activated adenosinetriphosphatase. IV. Correlation with cation transport sensitive to cardiac glycosides. Arch Biochem Biophys. 1962 Sep;98:413–419. doi: 10.1016/0003-9861(62)90206-0. [DOI] [PubMed] [Google Scholar]
- Baker R. D., Wall M. J., Watson S., Long J. L. Transmural electrical potential across rat jejunum. Effects of eversion and rapid temperature changes. Biochim Biophys Acta. 1968 Jun 11;150(4):649–654. doi: 10.1016/0005-2736(68)90054-0. [DOI] [PubMed] [Google Scholar]
- Berg G. G., Chapman B. The sodium and potassium activated ATPase of intestinal epithelium. I. Location of enzymatic activity in the cell. J Cell Physiol. 1965 Jun;65(3):361–372. doi: 10.1002/jcp.1030650309. [DOI] [PubMed] [Google Scholar]
- Berg G. G., Szekerczes J. The sodium and potassium activated ATPase. II. Comparative study of intestinal epithelium and red cells. J Cell Physiol. 1966 Jun;67(3):487–500. doi: 10.1002/jcp.1040670313. [DOI] [PubMed] [Google Scholar]
- Binder H. J., Boyer M., Spiro H. M., Spencer R. P. Species differences in the response of amino acid transport to ouabain and a sodium-free medium. Comp Biochem Physiol. 1966 May;18(1):83–89. doi: 10.1016/0010-406x(66)90333-1. [DOI] [PubMed] [Google Scholar]
- Clausen T. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. II. Effects of K+-free medium, ouabain and insulin upon the fate of glucose in rat diaphragm. Biochim Biophys Acta. 1966 Jul 13;120(3):361–368. doi: 10.1016/0926-6585(66)90303-7. [DOI] [PubMed] [Google Scholar]
- Crane R. K. Na+ -dependent transport in the intestine and other animal tissues. Fed Proc. 1965 Sep-Oct;24(5):1000–1006. [PubMed] [Google Scholar]
- Curran P. F., Schultz S. G., Chez R. A., Fuisz R. E. Kinetic relations of the Na-amino acid interaction at the mucosal border of intestine. J Gen Physiol. 1967 May;50(5):1261–1286. doi: 10.1085/jgp.50.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer D., Müller F., Kuhfahl E. Zum Problem der Kopplung zwischen aktivem Natrium-und Monosaccharidtransport in der Dünndarmschleimhaut. Acta Biol Med Ger. 1967;18(5):555–561. [PubMed] [Google Scholar]
- FAUST R. G. EFFECTS OF BILE SALTS, SODIUM DEOXYCHOLATE, STROPHANTHIN-G AND METABOLIC INHIBITORS ON THE ABSORPTION OF D-GLUCOSE BY THE RAT JEJUNUM, IN VITRO. J Cell Physiol. 1964 Feb;63:55–64. doi: 10.1002/jcp.1030630106. [DOI] [PubMed] [Google Scholar]
- FOX M., THIER S., ROSENBERG L., SEGAL S. IONIC REQUIREMENTS FOR AMINO ACID TRANSPORT IN THE RAT KIDNEY CORTEX SLICE. I. INFLUENCE OF EXTRACELLULAR IONS. Biochim Biophys Acta. 1964 Jan 27;79:167–176. doi: 10.1016/0926-6577(64)90049-x. [DOI] [PubMed] [Google Scholar]
- Forth W., Rummel W. Wirkung von Herzglykosiden auf Calcium-, Natrium-, Wasser- und Glukosetransport am isolierten Dünndarm. Helv Physiol Pharmacol Acta. 1967;25(1):8–23. [PubMed] [Google Scholar]
- GROBECKER H., PIECHOWSKI U., GREEFF K. DIE WIRKUNG DES K-STROPHANTHINS UND DIGITONINS AUF DEN IONENTRANSPORT UND DIE MEMBRAN-ATPASE DER ERYTHROCYTEN VON MENSCHEN, MEERSCHWEINCHEN UND RATTEN. Med Exp Int J Exp Med. 1963;9:273–282. [PubMed] [Google Scholar]
- Gajdos A. La pompe à sodium. Presse Med. 1967 Dec 2;75(51):2615–2620. [PubMed] [Google Scholar]
- Gordon E. E. Influence of ouabain on metabolism of rat kidney. Biochim Biophys Acta. 1965 Jul 8;104(2):606–608. doi: 10.1016/0304-4165(65)90370-3. [DOI] [PubMed] [Google Scholar]
- HOLLANDS B. C., SMITH M. W. PHOSPHATASES OF THE GOLDFISH INTESTINE. J Physiol. 1964 Dec;175:31–37. doi: 10.1113/jphysiol.1964.sp007501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. F., Kregenow F. M. The characterization of new energy dependent cation transport processes in red blood cells. Ann N Y Acad Sci. 1966 Jul 14;137(2):566–576. doi: 10.1111/j.1749-6632.1966.tb50182.x. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Epstein F. H. The physiological role of sodium-potassium activated adenosine triphosphatase in the active transport of cations across biological membranes. Isr J Med Sci. 1967 Jan-Feb;3(1):155–166. [PubMed] [Google Scholar]
- Kessler R. H., Landwehr D., Quintanilla A., Weseley S. A., Kaufmann W., Arcila H., Urbaitis B. K. Effects of certain inhibitors on renal sodium reabsorption and ATP specific activity. Nephron. 1968;5(6):474–488. doi: 10.1159/000179657. [DOI] [PubMed] [Google Scholar]
- Kessler R. H. The effects of metabolic inhibitors and diuretics on sodium chloride reabsorption and oxidative metabolism in the mammalian kidney. Ann N Y Acad Sci. 1966 Nov 22;139(2):356–361. doi: 10.1111/j.1749-6632.1966.tb41209.x. [DOI] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLUCH M., PONZ F. OUABAIN-NA+ RELATION IN THE INHIBITION OF THE ACTIVE TRANSPORT OF SUGARS BY THE INTESTINE IN VIVO. Rev Esp Fisiol. 1964 Dec;20:185–191. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lauterbach F. Comparison of intestinal penetration of cortisol and convallatoxin: demonstration of a transport mechanism for cardiotonic steroids. Biochim Biophys Acta. 1968 Jan 3;150(1):146–155. doi: 10.1016/0005-2736(68)90018-7. [DOI] [PubMed] [Google Scholar]
- Lauterbach F. Die Wirkung Cardiotoner Steroide auf die Enterale Resorption aktiv transportierter und Diffundierender Substanzen und auf deren Beziehung zu Na+-Konzentration und Na+-Transport. Biochim Biophys Acta. 1967 May 2;135(2):273–285. doi: 10.1016/0005-2736(67)90121-6. [DOI] [PubMed] [Google Scholar]
- Lauterbach F. Uber Unterschiede im Mechanismus der enteralen Resorption kardiotoner Steroide und anderer Pharmaka. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1967;257(4):432–457. [PubMed] [Google Scholar]
- Nadal J., Prous J., Ponz F. Pso de ouabaina a través de sacos de yeyuno de rata in vitro. Rev Esp Fisiol. 1967 Dec;23(4):241–242. [PubMed] [Google Scholar]
- Newey H., Sanford P. A., Smyth D. H. Some effects of ouabain and potassium on transport and metabolism in rat small intestine. J Physiol. 1968 Jan;194(1):237–248. doi: 10.1113/jphysiol.1968.sp008404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHLIN E., SCHALCH W. R. Zur Pharmakologie und Toxikologie des Scillirosids und des Scillirosidins. Helv Physiol Pharmacol Acta. 1952;10(4):427–437. [PubMed] [Google Scholar]
- RUMMEL W., STUPP H. F. [The effect of potassium and calcium on salt, glucose and water absorption by the isolated small intestine]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1960;240:79–92. [PubMed] [Google Scholar]
- Repke K., Est M., Portius H. J. Uber die Ursache der Speciesunterschiede in der Digitalisempfindlichkeit. Biochem Pharmacol. 1965 Dec;14(12):1785–1802. doi: 10.1016/0006-2952(65)90269-8. [DOI] [PubMed] [Google Scholar]
- Robinson J. W. Beziehung zwischen der Herzglykosid-Empfindlichkeit des Aminosäuretransportes und der Na+ -K+ -stimulierten ATPase im Rattendunndarm. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;260(2):192–192. [PubMed] [Google Scholar]
- Robinson J. W., Förster H. Effect of ethacrynic acid and furosemide on the transport systems of the rat intestine. Naturwissenschaften. 1968 May;55(5):232–233. doi: 10.1007/BF00606224. [DOI] [PubMed] [Google Scholar]
- Robinson J. W. Les relations entre les ions de sodium et l'absorption intestinale d'acides aminés. Biochim Biophys Acta. 1966 Sep 5;126(1):61–72. [PubMed] [Google Scholar]
- Robinson J. W. The loss of intestinal transport capacity following preincubation in sodium-free media in vitro. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;294(2):182–200. doi: 10.1007/BF00363605. [DOI] [PubMed] [Google Scholar]
- Robinson J. W., Vannotti A. Etude de l'absorption intestinale des acides aminés en physiopathologie. Schweiz Med Wochenschr. 1966 Aug 6;96(31):1002–1007. [PubMed] [Google Scholar]
- Rosenberg I. H., Coleman A. L., Rosenberg L. E. The role of sodium ion in the transport of amino acids by the intestine. Biochim Biophys Acta. 1965 May 25;102(1):161–171. doi: 10.1016/0926-6585(65)90210-4. [DOI] [PubMed] [Google Scholar]
- Rosenberg I. H., Rosenberg L. E. Localization and characterization of adenosine triphosphatase in guinea pig intestinal epithelium. Comp Biochem Physiol. 1968 Mar;24(3):975–985. doi: 10.1016/0010-406x(68)90809-8. [DOI] [PubMed] [Google Scholar]
- Ruedas G., Weiss C. Die Wirkung von Anderungen der Natriumkonzentration im Perfusionsmedium und von Strophanthin auf die Glucoseresorption der isolierten Rattenniere. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;298(1):12–22. [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- SMITH M. W. THE IN VITRO ABSORPTION OF WATER AND SOLUTES FROM THE INTESTINE OF GOLDFISH, CARASSIUS AURATUS. J Physiol. 1964 Dec;175:38–49. doi: 10.1113/jphysiol.1964.sp007502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J. Kationenpumpen. Bull Schweiz Akad Med Wiss. 1967 Dec;23(3):260–270. [PubMed] [Google Scholar]
- Schoner W., von Ilberg C., Kramer R., Seubert W. On the mechanism of Na+- and K+-stimulated hydrolysis of adenosine triphosphate. 1. Purification and properties of a Na+-and K+-activated ATPase from ox brain. Eur J Biochem. 1967 May;1(3):334–343. doi: 10.1007/978-3-662-25813-2_45. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F., Chez R. A., Fuisz R. E. Alanine and sodium fluxes across mucosal border of rabbit ileum. J Gen Physiol. 1967 May;50(5):1241–1260. doi: 10.1085/jgp.50.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz P. Electron transport systems in microsomes. Origin and functional nature of microsomes. Fed Proc. 1965 Sep-Oct;24(5):1153–1155. [PubMed] [Google Scholar]
- Smith M. W., Colombo V. E., Munn E. A. Influence of temperature acclimatization on the ionic activation of goldfish intestinal adenosine triphosphatase. Biochem J. 1968 May;107(5):691–698. doi: 10.1042/bj1070691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier S. O. Amino acid accumulation in the toad bladder. Relationship to transepithelial sodium transport. Biochim Biophys Acta. 1968 Mar 1;150(2):253–262. doi: 10.1016/0005-2736(68)90168-5. [DOI] [PubMed] [Google Scholar]
- WARDLAW A. C., VANBELLE G. STATISTICAL ASPECTS OF THE MOUSE DIAPHRAGM TEST FOR INSULIN. Diabetes. 1964 Nov-Dec;13:622–633. doi: 10.2337/diab.13.6.622. [DOI] [PubMed] [Google Scholar]
- Whittembury G. Sodium and water transport in kidney proximal tubular cells. J Gen Physiol. 1968 May;51(5 Suppl):303S+–303S+. [PubMed] [Google Scholar]


