Abstract
1. Extracellular recordings were made from directionally selective ganglion cell units in the isolated frog retina and decapitated Necturus preparation.
2. Intracellular recordings were made from individual photoreceptor cells in the frog and Necturus retinae while stimuli which had evoked directionally selective responses at the ganglion cell level were presented. No evidence for inhibition of photoreceptors for any direction of movement of the light stimulus was found. This appeared to rule out a mechanism for directional selectivity involving inhibition of photoreceptor potentials.
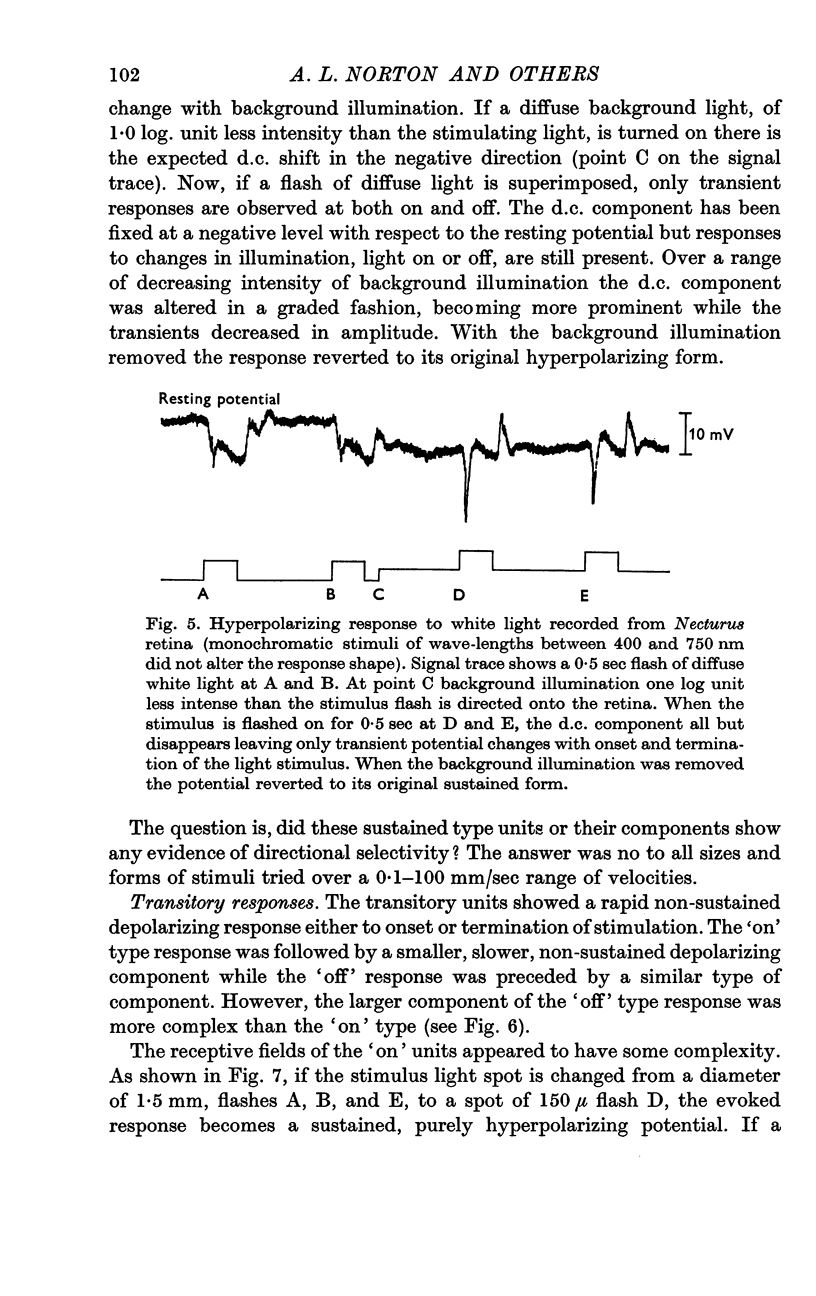
3. Intracellular recordings were made from the nuclear layer between photoreceptors and ganglion cells in Necturus. The responses were of two types: either transitory or sustained.
4. The sustained type responses could be divided into two classes depending on their receptive field organization. One type of sustained potential had a large receptive field without any evidence for a centre-surround antagonism and corresponded to the luminosity type S-potential recorded in fish. The other type had a smaller receptive field and showed a difference in sign of response between centre and surround if the centre was flooded with a steady light. This is very similar to what has been described for a type of on-centre, off-surround ganglion cell.
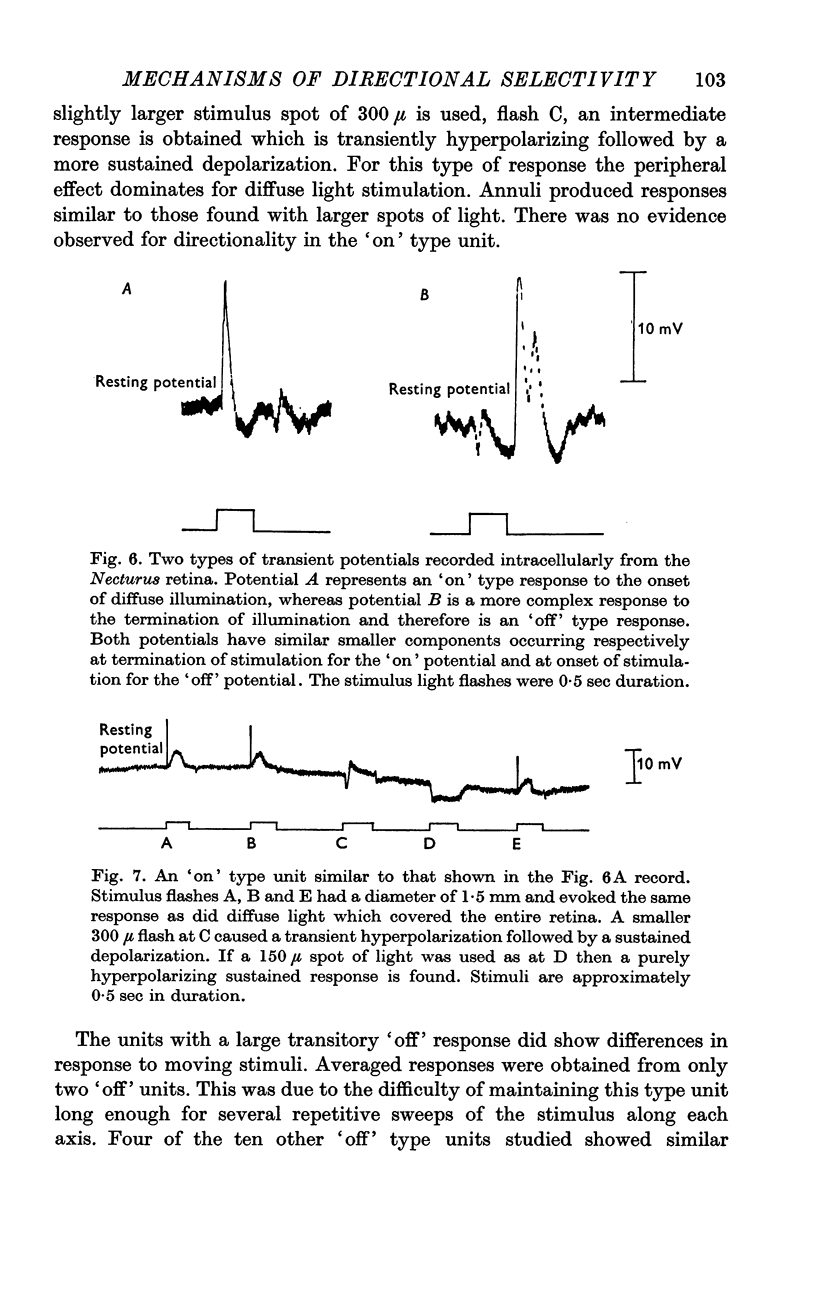
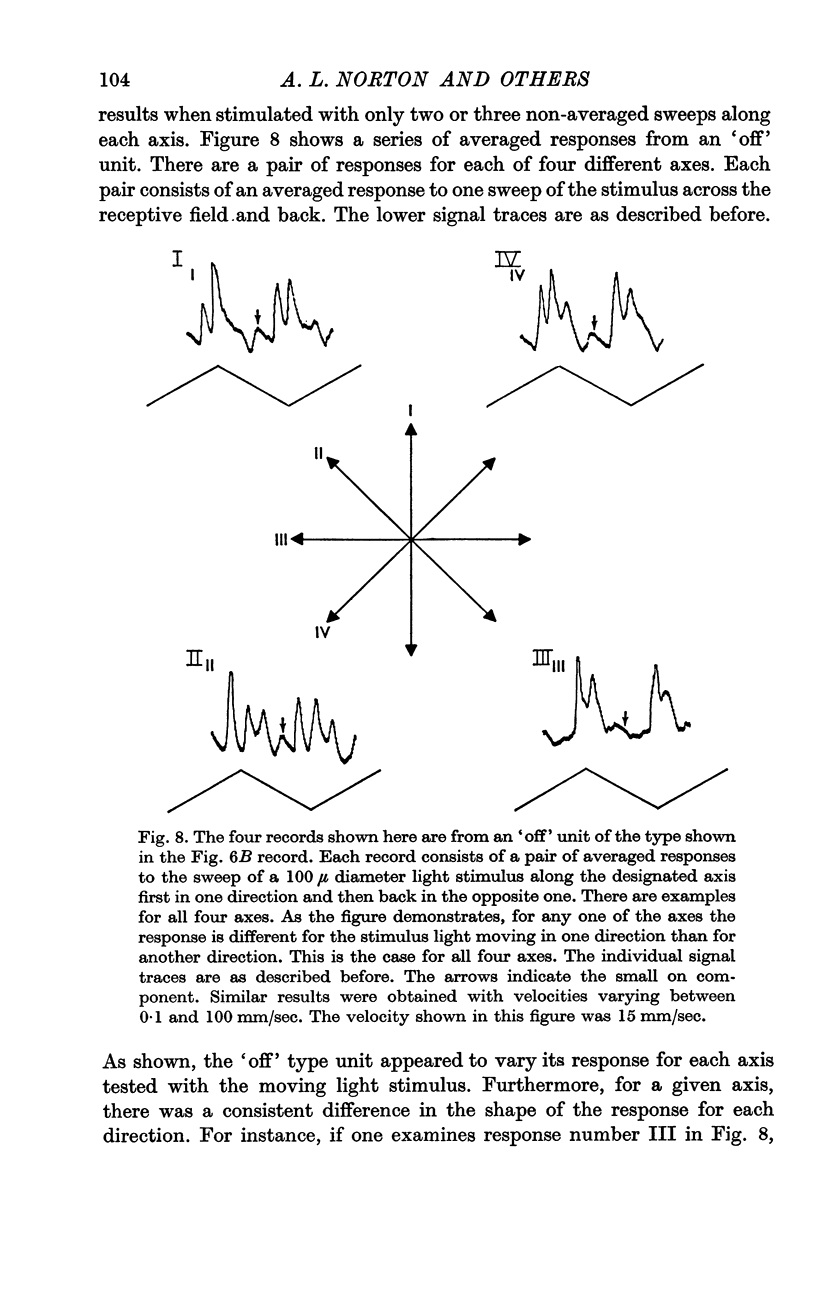
5. The transitory type of responses showed some centre-surround antagonistic organization. Some of these transitory units also appeared to show some discrimination in response as a function of the distribution of light on the retina.
6. No specific directional selectivity was found from units at the inner nuclear layer. This further excluded any mechanism of directional sensitivity which involves selectivity at the photoreceptor level.
7. It was concluded that although inner nuclear layer units may play a role in the mechanism of directional selectivity, no specific directionality was found at the first synaptic level of the retina.
Full text
PDF


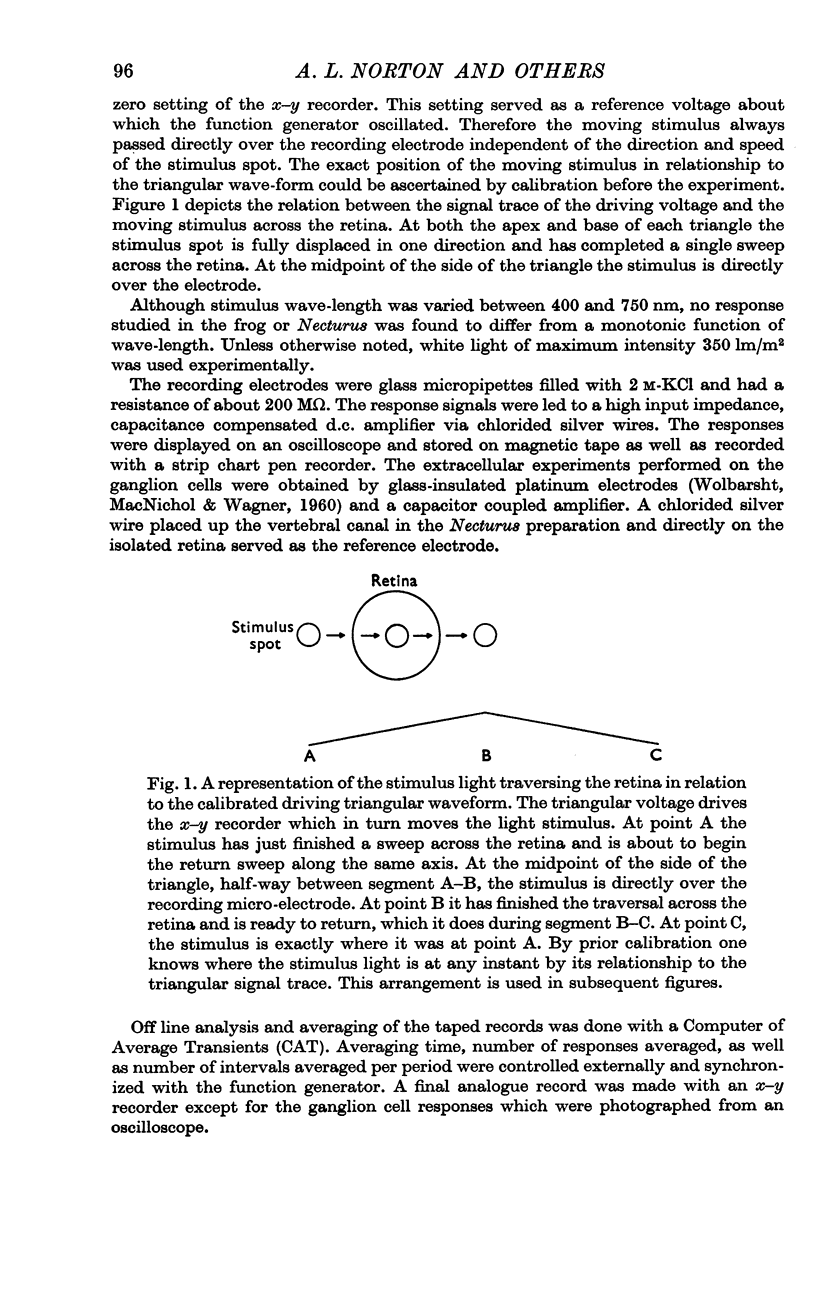
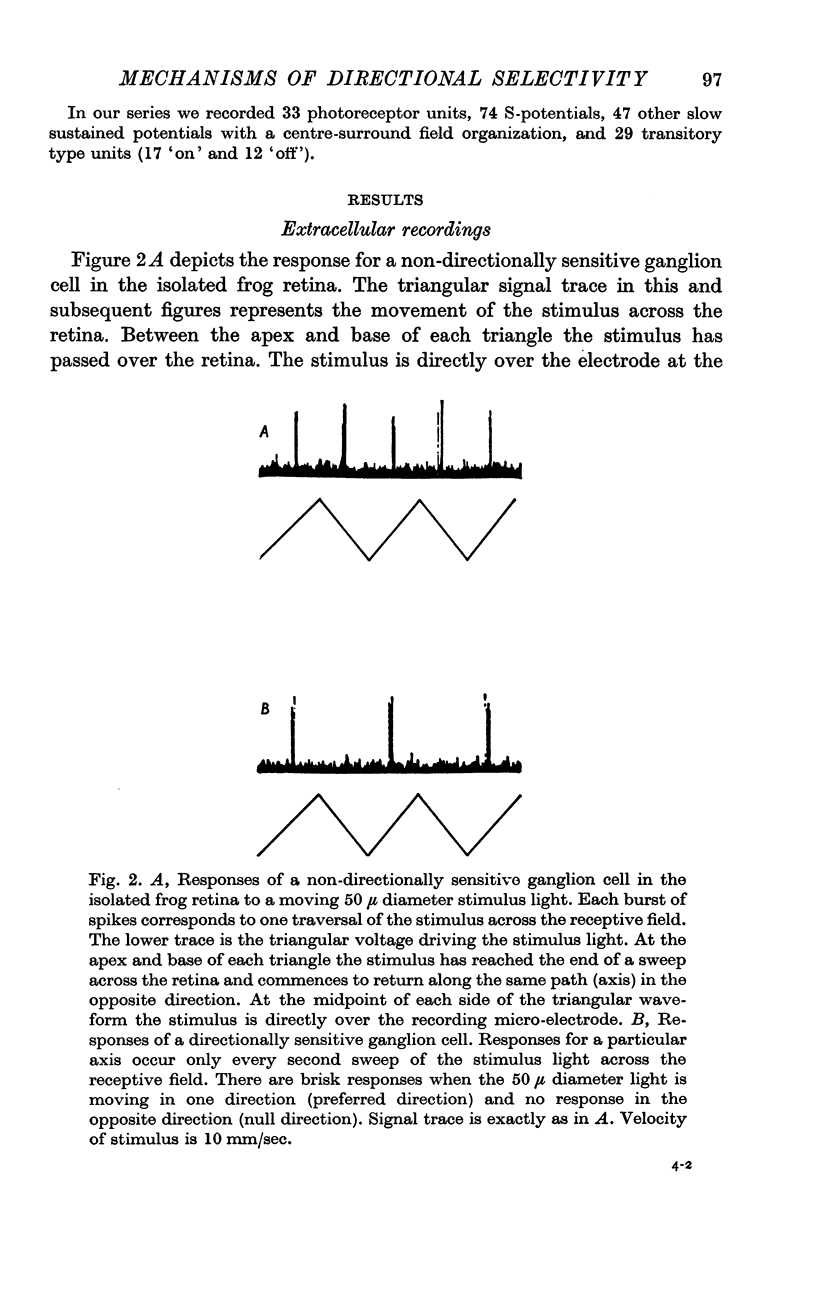
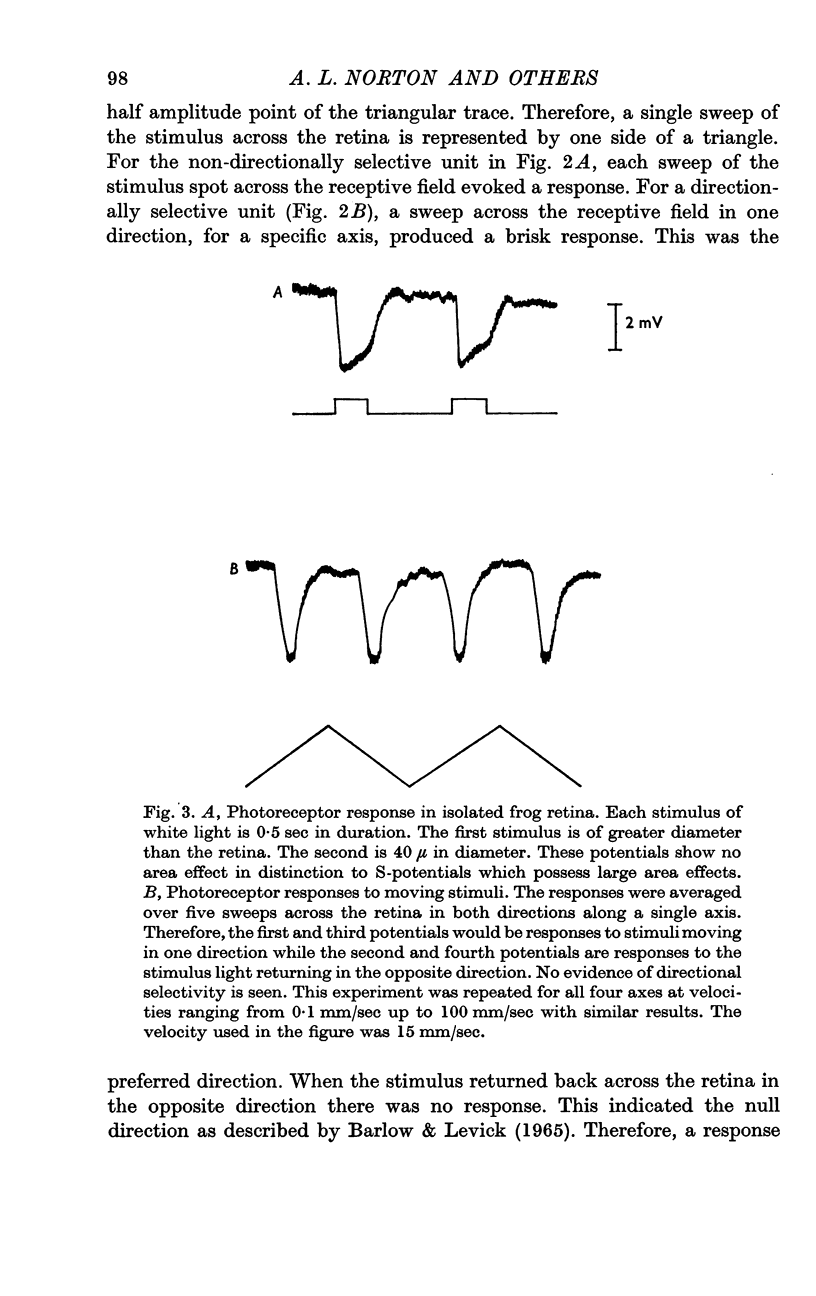


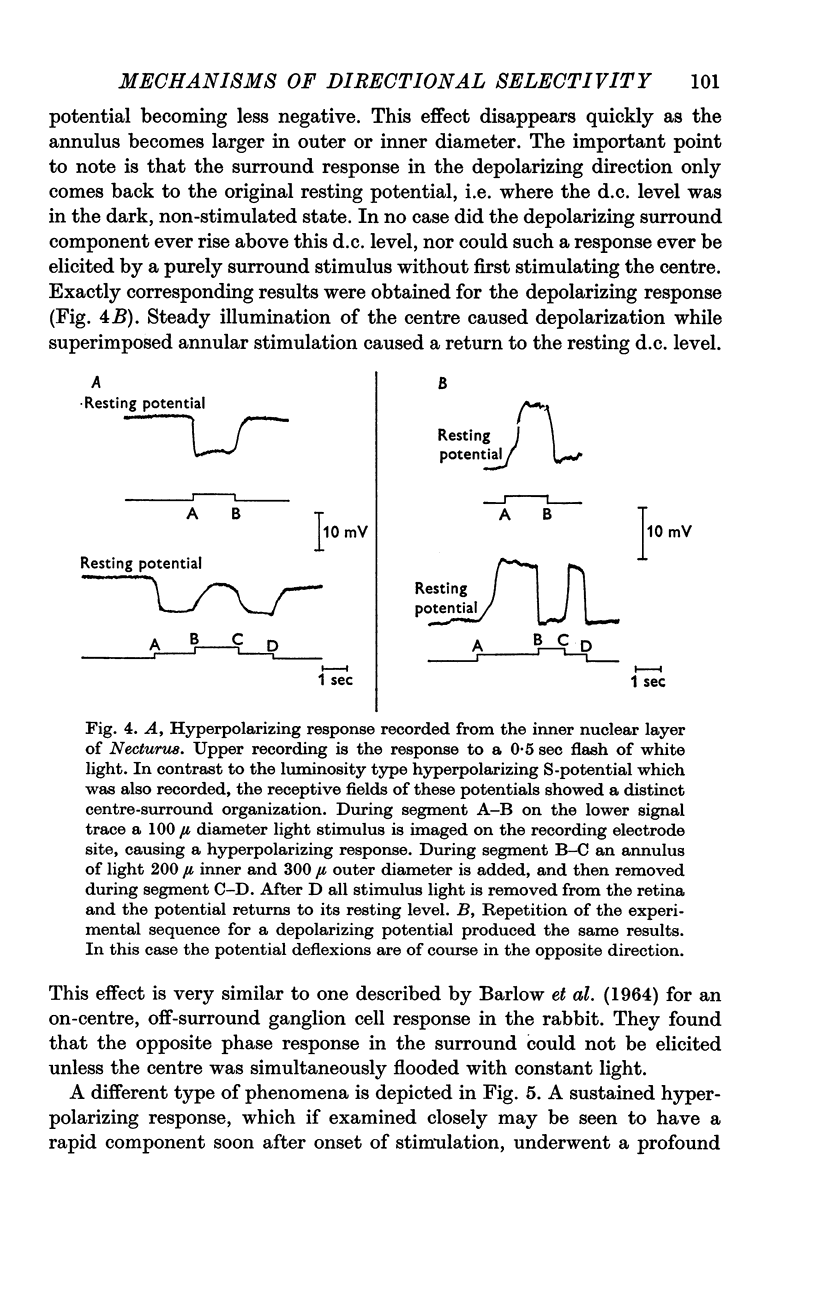



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965 Jun;178(3):477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoff A. Localization of slow potential responses in the Necturus retina. Vision Res. 1964 Dec;4(11):627–635. doi: 10.1016/0042-6989(64)90048-3. [DOI] [PubMed] [Google Scholar]
- Byzov A. L., Trifonov J. A. The response to electric stimulation of horizontal cells in the carp retina. Vision Res. 1968 Jul;8(7):817–822. doi: 10.1016/0042-6989(68)90132-6. [DOI] [PubMed] [Google Scholar]
- CRONLY-DILLON J. R. UNITS SENSITIVE TO DIRECTION OF MOVEMENT IN GOLDFISH OPTIC TECTUM. Nature. 1964 Jul 11;203:214–215. doi: 10.1038/203214a0. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Electrophysiological study of single neurons in the inner nuclear layer of the carp retina. Vision Res. 1969 Jan;9(1):37–55. doi: 10.1016/0042-6989(69)90030-3. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Localization of spike-producing cells in the frog retina. Vision Res. 1968 Mar;8(3):259–262. doi: 10.1016/0042-6989(68)90013-8. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Recording site of the single cone response determined by an electrode marking technique. Vision Res. 1967 Nov;7(11):847–851. doi: 10.1016/0042-6989(67)90005-3. [DOI] [PubMed] [Google Scholar]
- MACNICHOL E. J., SVAETICHIN G. Electric responses from the isolated retinas of fishes. Am J Ophthalmol. 1958 Sep;46(3 Pt 2):26–46. doi: 10.1016/0002-9394(58)90053-9. [DOI] [PubMed] [Google Scholar]
- MATURANA H. R., FRENK S. DIRECTIONAL MOVEMENT AND HORIZONTAL EDGE DETECTORS IN THE PIGEON RETINA. Science. 1963 Nov 15;142(3594):977–979. doi: 10.1126/science.142.3594.977. [DOI] [PubMed] [Google Scholar]
- MATURANA H. R., LETTVIN J. Y., MCCULLOCH W. S., PITTS W. H. Anatomy and physiology of vision in the frog (Rana pipiens). J Gen Physiol. 1960 Jul;43(6):129–175. doi: 10.1085/jgp.43.6.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael C. R. Receptive fields of directionally selective units in the optic nerve of the ground squirrel. Science. 1966 May 20;152(3725):1092–1095. doi: 10.1126/science.152.3725.1092. [DOI] [PubMed] [Google Scholar]
- Michael C. R. Receptive fields of single optic nerve fibers in a mammal with an all-cone retina. 3. Opponent color units. J Neurophysiol. 1968 Mar;31(2):268–282. doi: 10.1152/jn.1968.31.2.268. [DOI] [PubMed] [Google Scholar]
- Norton A. L., Spekreijse H., Wolbarsht M. L., Wagner H. G. Receptive field organization of the S-potential. Science. 1968 May 31;160(3831):1021–1022. doi: 10.1126/science.160.3831.1021. [DOI] [PubMed] [Google Scholar]
- OIKAWA T., OGAWA T., MOTOKAWA K. Origin of so-called cone action potential. J Neurophysiol. 1959 Jan;22(1):102–111. doi: 10.1152/jn.1959.22.1.102. [DOI] [PubMed] [Google Scholar]
- TOMITA T., MURAKAMI M., SATO Y., HASHIMOTO Y. Further study on the origin of the so-called cone action potential (s-potential); its histological determination. Jpn J Physiol. 1959 Mar 25;9(1):63–68. doi: 10.2170/jjphysiol.9.63. [DOI] [PubMed] [Google Scholar]
- Tomita T., Kaneko A., Murakami M., Pautler E. L. Spectral response curves of single cones in the carp. Vision Res. 1967 Jul;7(7):519–531. doi: 10.1016/0042-6989(67)90061-2. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Wolbarsht M. L., Macnichol E. F., Jr, Wagner H. G. Glass Insulated Platinum Microelectrode. Science. 1960 Nov 4;132(3436):1309–1310. doi: 10.1126/science.132.3436.1309. [DOI] [PubMed] [Google Scholar]


