Abstract
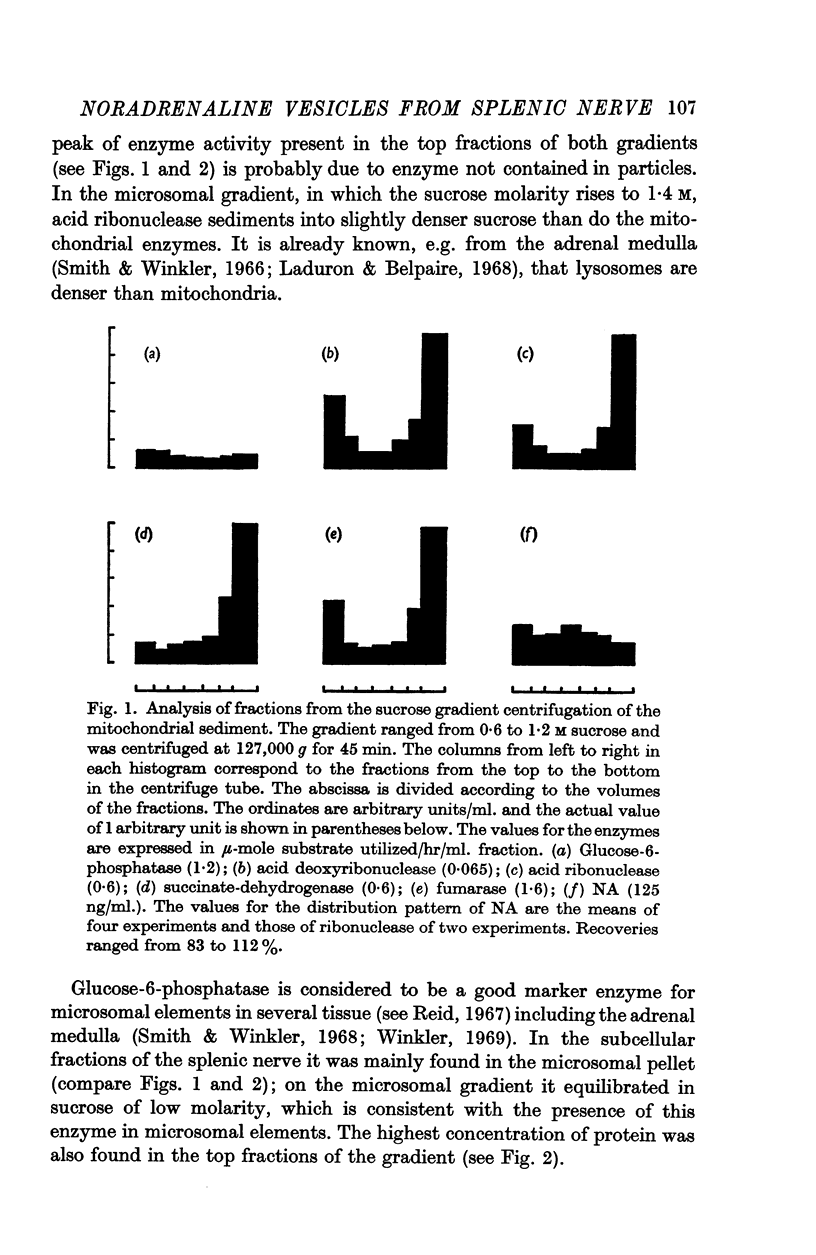
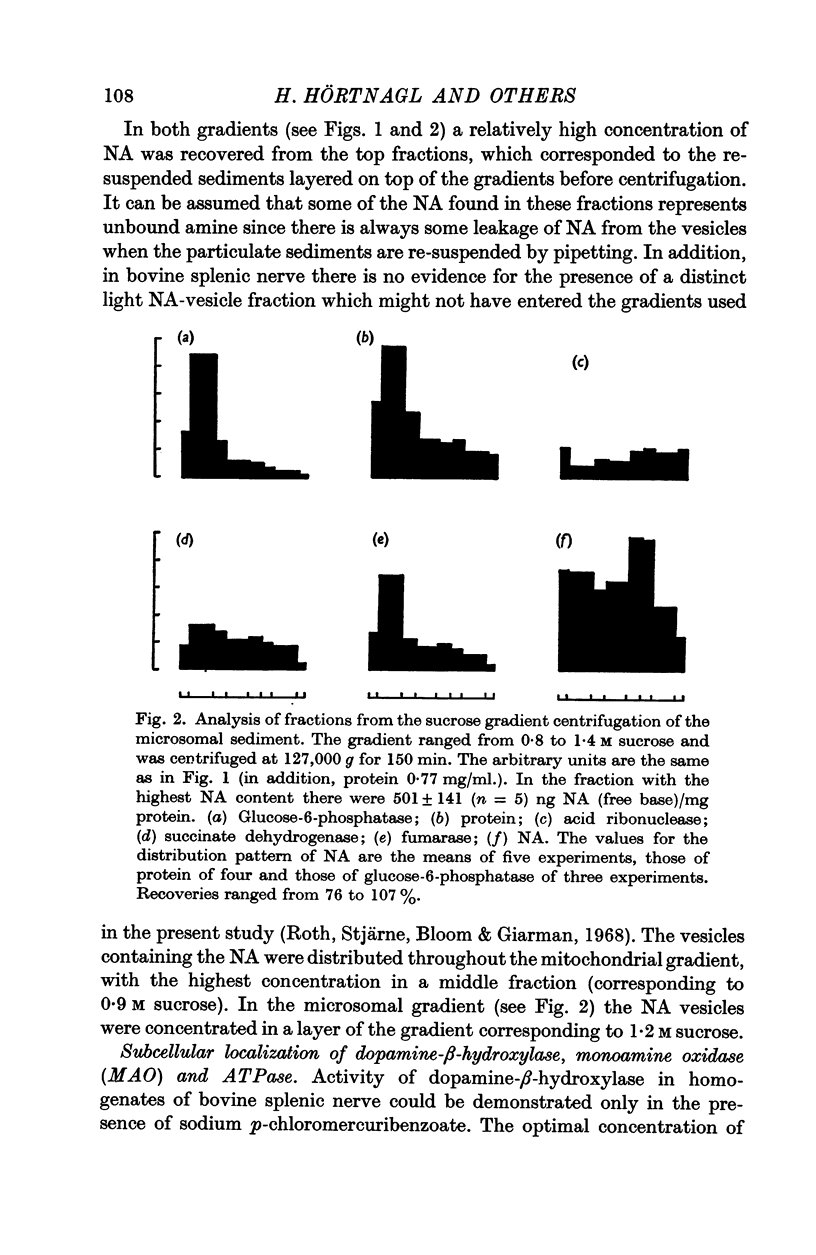
1. Homogenates of bovine splenic nerves were subjected to differential and sucrose density gradient centrifugation. From the low-speed supernatant a high-speed sediment (mitochondria, lysosomes, microsomes and noradrenaline (NA) vesicles) was obtained. By density gradient centrifugation of this sediment it was shown that NA vesicles are slightly less dense than mitochondria, but denser than microsomes.
2. In further experiments a mitochondrial and a microsomal sediment were obtained. The mitochondrial sediment was fractionated with a short centrifugation time over a density gradient ranging from 0·6 to 1·2 M sucrose. Mitochondria (fumarase and succinate-dehydrogenase) and lysosomes (acid ribonuclease and deoxyribonuclease) sedimented to the bottom of the tube. The highest concentration of NA vesicles was found in a medium position. There was only a small amount of microsomes (glucose-6-phosphatase) present.
3. The microsomal sediment was centrifuged for 150 min over a density gradient ranging from 0·8 to 1·4 M sucrose. The microsomes remained on the top of the gradient. There were also some mitochondria and lysosomes present. The NA vesicles were found in highest concentration in the middle of the gradient (at about 1·2 M sucrose).
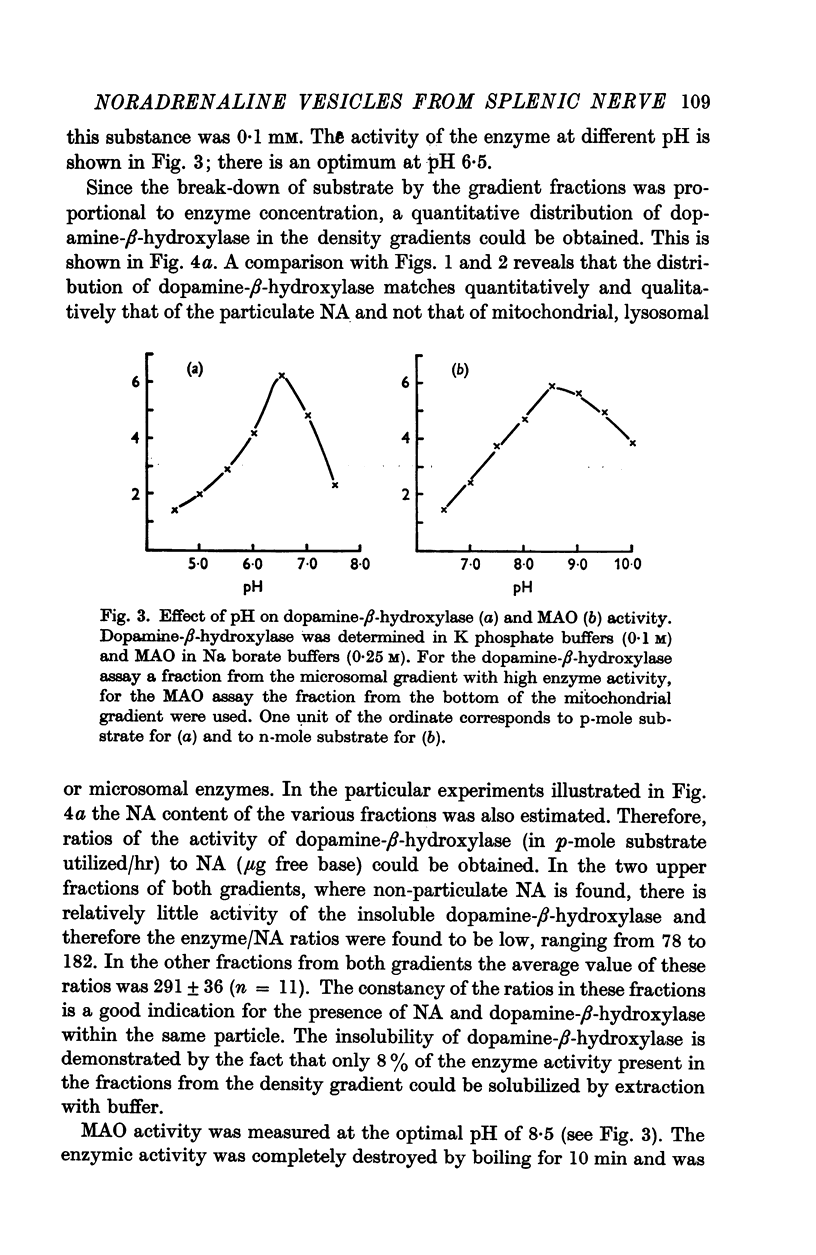
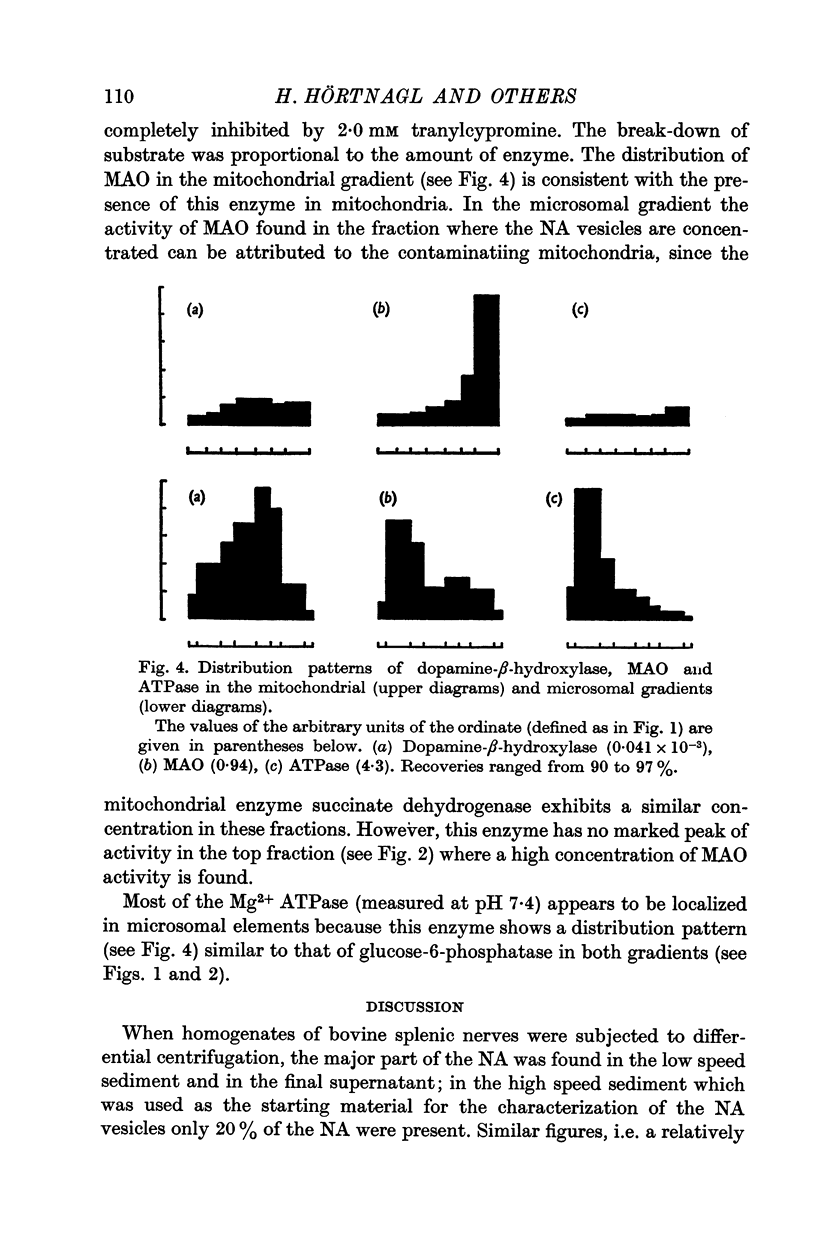
4. With the use of these two density gradients, the subcellular distribution of dopamine-β-hydroxylase, monoamine oxidase and ATPase was studied. Dopamine-β-hydroxylase was found to be localized in the NA vesicles. Monoamine oxidase was mainly recovered in mitochondria; a small part of the enzyme appeared to be microsomal. ATPase was present in microsomal elements.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin L., Chubb I. W., Livett B. G. The subcellular localization of catecholamines in nerve terminals in smooth muscle tissue. J Neurochem. 1967 May;14(5):473–478. doi: 10.1111/j.1471-4159.1967.tb09546.x. [DOI] [PubMed] [Google Scholar]
- BLASCHKO H., HAGEN J. M., HAGEN P. Mitochondrial enzymes and chromaffin granules. J Physiol. 1957 Dec 3;139(2):316–322. doi: 10.1113/jphysiol.1957.sp005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger A., Philippu A., Schümann H. J. ATP-Spaltung und Aminaufnahme durch Milznervengranula. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1969;262(2):208–220. [PubMed] [Google Scholar]
- CAMPOS H. A., STITZEL R. E., SHIDEMAN F. E. ACTIONS OF TYRAMINE AND COCAINE ON CATECHOLAMINE LEVELS IN SUBCELLULAR FRACTIONS OF THE ISOLATED CAT HEART. J Pharmacol Exp Ther. 1963 Sep;141:290–300. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Friedman S., Kaufman S. 3,4-dihydroxyphenylethylamine beta-hydroxylase. Physical properties, copper content, and role of copper in the catalytic acttivity. J Biol Chem. 1965 Dec;240(12):4763–4773. [PubMed] [Google Scholar]
- Glassman P. M., Angelakos E. T., McNary W. F. Catecholamine-containing fractions of dog heart homogenates. Life Sci. 1965 Sep;4(18):1727–1734. doi: 10.1016/0024-3205(65)90233-x. [DOI] [PubMed] [Google Scholar]
- IVERSEN L. L., WHITBY L. C. The subcellular distributijon of catecholamines in normal and tyramine-depleted mouse hearts. Biochem Pharmacol. 1963 Jun;12:582–584. doi: 10.1016/0006-2952(63)90135-7. [DOI] [PubMed] [Google Scholar]
- Kirshner N., Kirshner A. G., Kamin D. L. Adenosine triphosphatase activity of adrenal medulla catecholamine granules. Biochim Biophys Acta. 1966 Feb 14;113(2):332–335. doi: 10.1016/s0926-6593(66)80072-3. [DOI] [PubMed] [Google Scholar]
- Laduron P., Belpaire F. Tissue fractionation and catecholamines. II. Intracellular distribution patterns of tyrosine hydroxylase, dopa decarboxylase, dopamine-beta-hydroxylase, phenylethanolamine N-methyltransferase and monoamine oxidase in adrenal medulla. Biochem Pharmacol. 1968 Jul;17(7):1127–1140. doi: 10.1016/0006-2952(68)90048-8. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., Kuzuya H., Hidaka H. Inhibition of dopamine beta-hydroxylase by sulfhydryl compounds and the nature of the natural inhibitors. Biochim Biophys Acta. 1967 Jul 11;139(2):319–327. doi: 10.1016/0005-2744(67)90035-6. [DOI] [PubMed] [Google Scholar]
- PORTEOUS J. W., CLARK B. THE ISOLATION AND CHARACTERIZATION OF SUBCELLULAR COMPONENTS OF THE EPITHELIAL CELLS OF RABBIT SMALL INTESTINE. Biochem J. 1965 Jul;96:159–171. doi: 10.1042/bj0960159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POTTER L. T., AXELROD J. SUBCELLULAR LOCALIZATION OF CATECHOLAMINES IN TISSUES OF THE RAT. J Pharmacol Exp Ther. 1963 Dec;142:291–298. [PubMed] [Google Scholar]
- Potter L. T. Storage of norepinephrine in sympathetic nerves. Pharmacol Rev. 1966 Mar;18(1):439–451. [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Roth R. H., Stjärne L., Bloom F. E., Giarman N. J. Light and heavy norepinephrine storage particles in the rat heart and in bovine splenic nerve. J Pharmacol Exp Ther. 1968 Aug;162(2):203–212. [PubMed] [Google Scholar]
- SCHUEMANN H. J., SCHNELL K., PHILIPPU A. SUBCELLULAERE VERTEILUNG VON NORADRENALIN UND ADRENALIN IM MEERSCHWEINCHENHERZEN. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964 Oct 23;249:251–266. [PubMed] [Google Scholar]
- Schümann H. J., Schmidt K., Philippu A. Storage of norepinephrine in sympathetic ganglia. Life Sci. 1966 Oct;5(19):1809–1815. doi: 10.1016/0024-3205(66)90057-9. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. Lysosomal phospholipases A1 and A2 of bovine adrenal medulla. Biochem J. 1968 Aug;108(5):867–874. doi: 10.1042/bj1080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. The localization of lysosomal enzymes in chromaffin tissue. J Physiol. 1966 Mar;183(1):179–188. doi: 10.1113/jphysiol.1966.sp007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjärne L., Roth R. J., Giarman N. J. Microsomal monoamine oxidase in sympathetically innervated tissues. Biochem Pharmacol. 1968 Sep;17(9):2008–2012. doi: 10.1016/0006-2952(68)90120-2. [DOI] [PubMed] [Google Scholar]
- Stjärne L. Studies of noradrenaline biosynthesis in nerve tissue. Acta Physiol Scand. 1966 Jul-Aug;67(3):441–454. doi: 10.1111/j.1748-1716.1966.tb03331.x. [DOI] [PubMed] [Google Scholar]
- Taylor P. W., Jr, Chidsey C. A., Richardson K. C., Cooper T., Michaelson I. A. Subcellular distribution of norepinephrine in the normal and surgically denervated cat heart. Biochem Pharmacol. 1966 Jun;15(6):681–689. doi: 10.1016/0006-2952(66)90002-5. [DOI] [PubMed] [Google Scholar]
- VON EULERU, LISHAJKO F. FREE AND BOUND NORADRENALINE IN THE RABBIT HEART. Nature. 1965 Jan 9;205:179–180. doi: 10.1038/205179a0. [DOI] [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]
- Winkler H. Isolierung und Charakterisierung von chromaffinen Noradrenalin-Granula aus Schweine-Nebennierenmark. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1969;263(2):340–357. [PubMed] [Google Scholar]
- von EULER U., LISHAJKO F. Noradrenaline release from isolated nerve granules. Acta Physiol Scand. 1961 Feb-Mar;51:193–203. doi: 10.1111/j.1748-1716.1961.tb02145.x. [DOI] [PubMed] [Google Scholar]
- von EULER U., LISHAJKO F. The estimation of catechol amines in urine. Acta Physiol Scand. 1959 Mar 31;45:122–132. doi: 10.1111/j.1748-1716.1959.tb01684.x. [DOI] [PubMed] [Google Scholar]


