Abstract
1. The ability of arterial and non-arterial smooth muscle in five tissues (vas deferens, heart, bladder, colon, spleen) in four species (mouse, rabbit, rat, guinea-pig) to accumulate and retain noradrenaline (NA) was measured in thin tissue slices exposed to NA for 30 min, then washed in cold saline solution for 30 min. NA accumulation was assessed histochemically by measuring the fluorescence brightness of the tissue with the Leitz MPV microphotometer. In addition, similar measurements were made on smooth muscle in the cat spleen, on cardiac muscle and on the terminal adrenergic nerves.
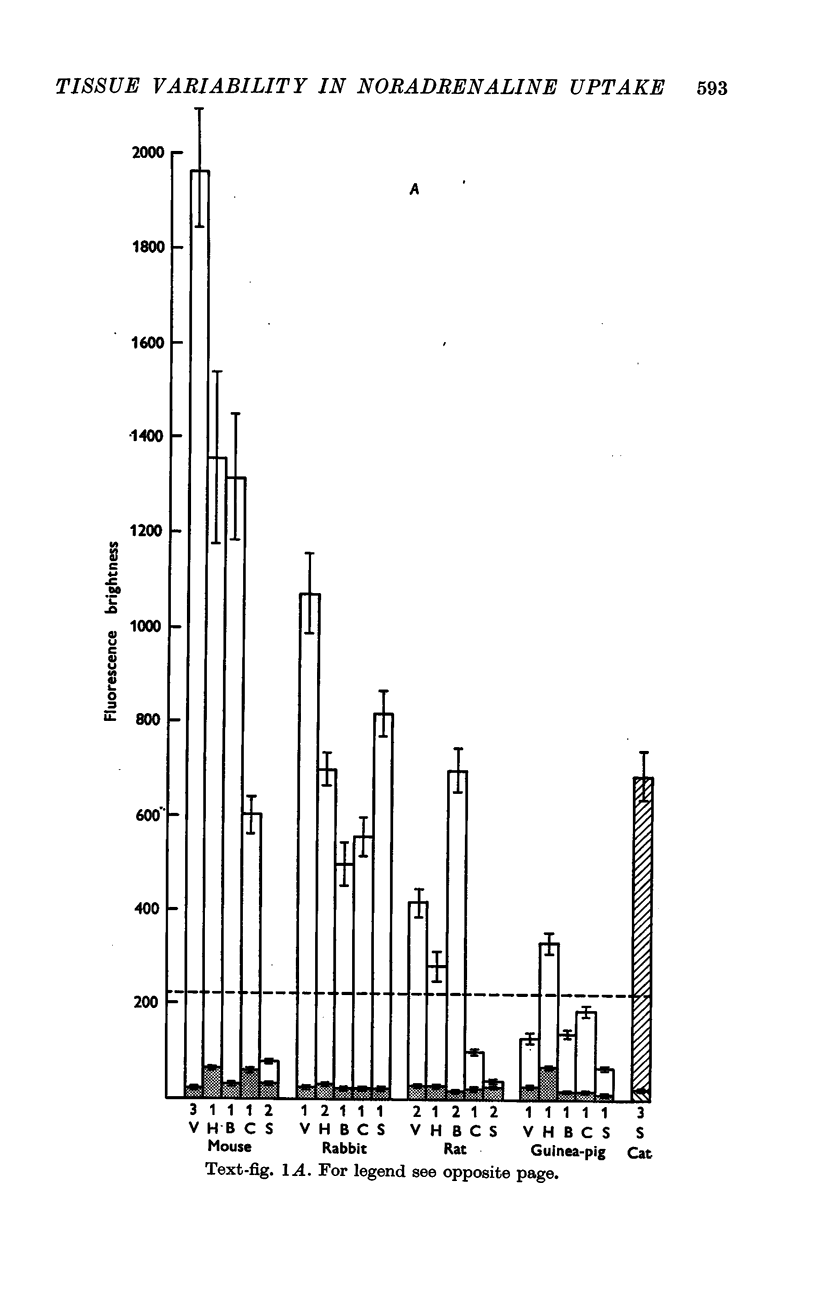
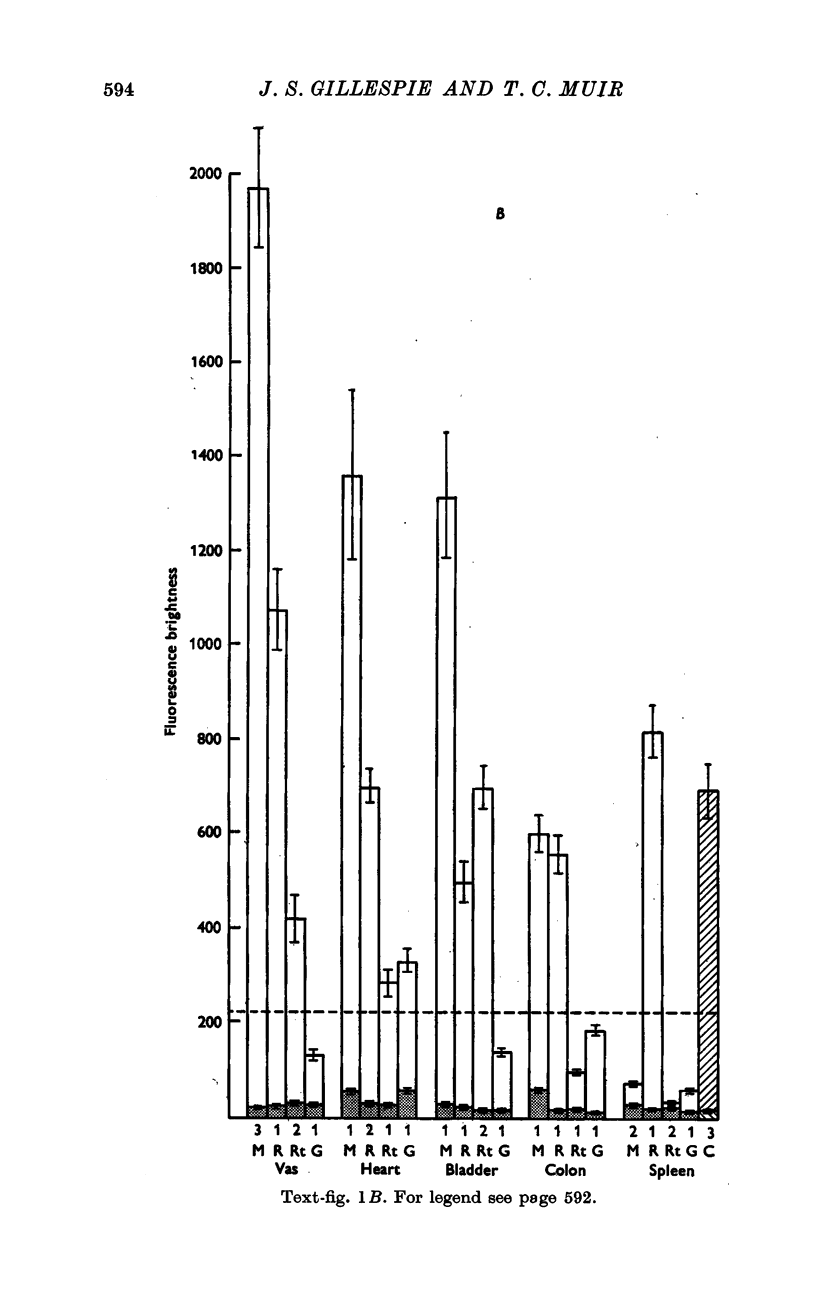
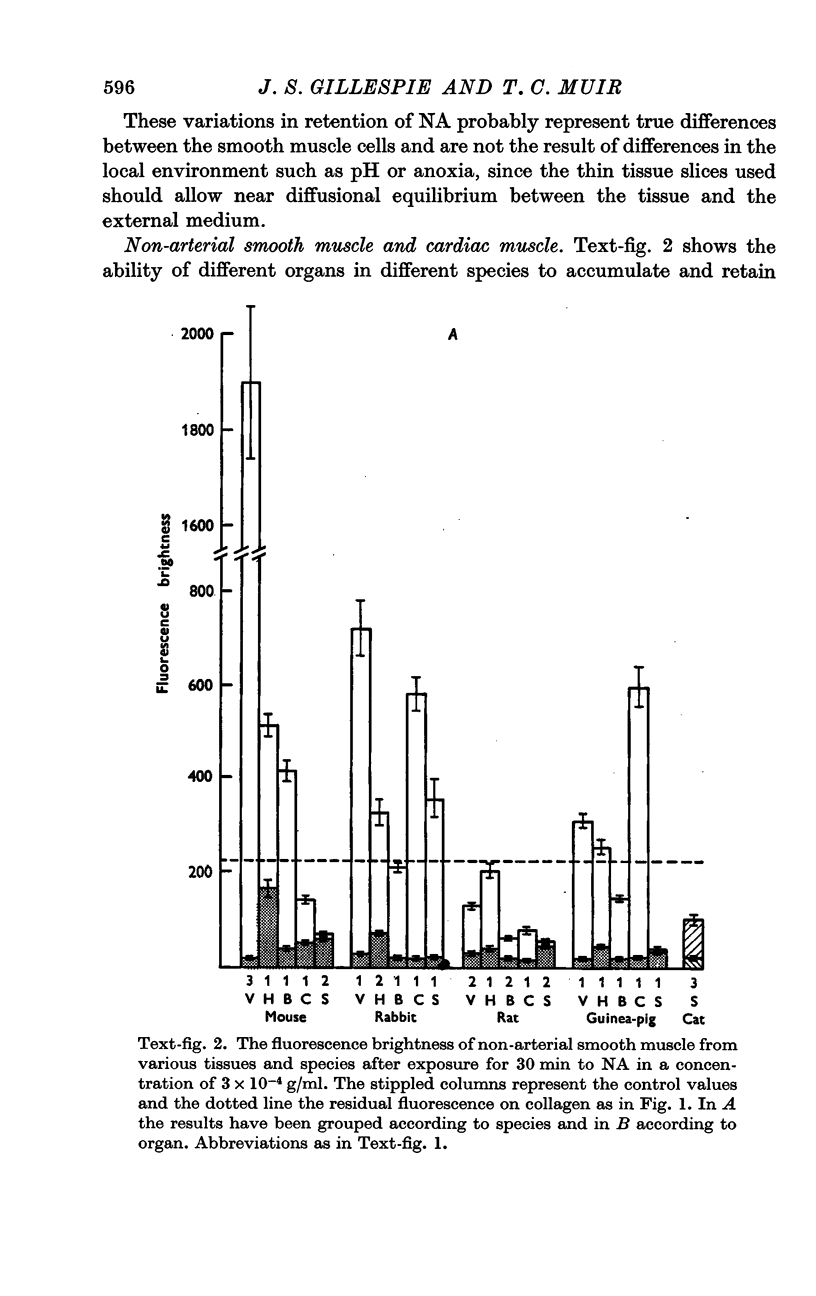
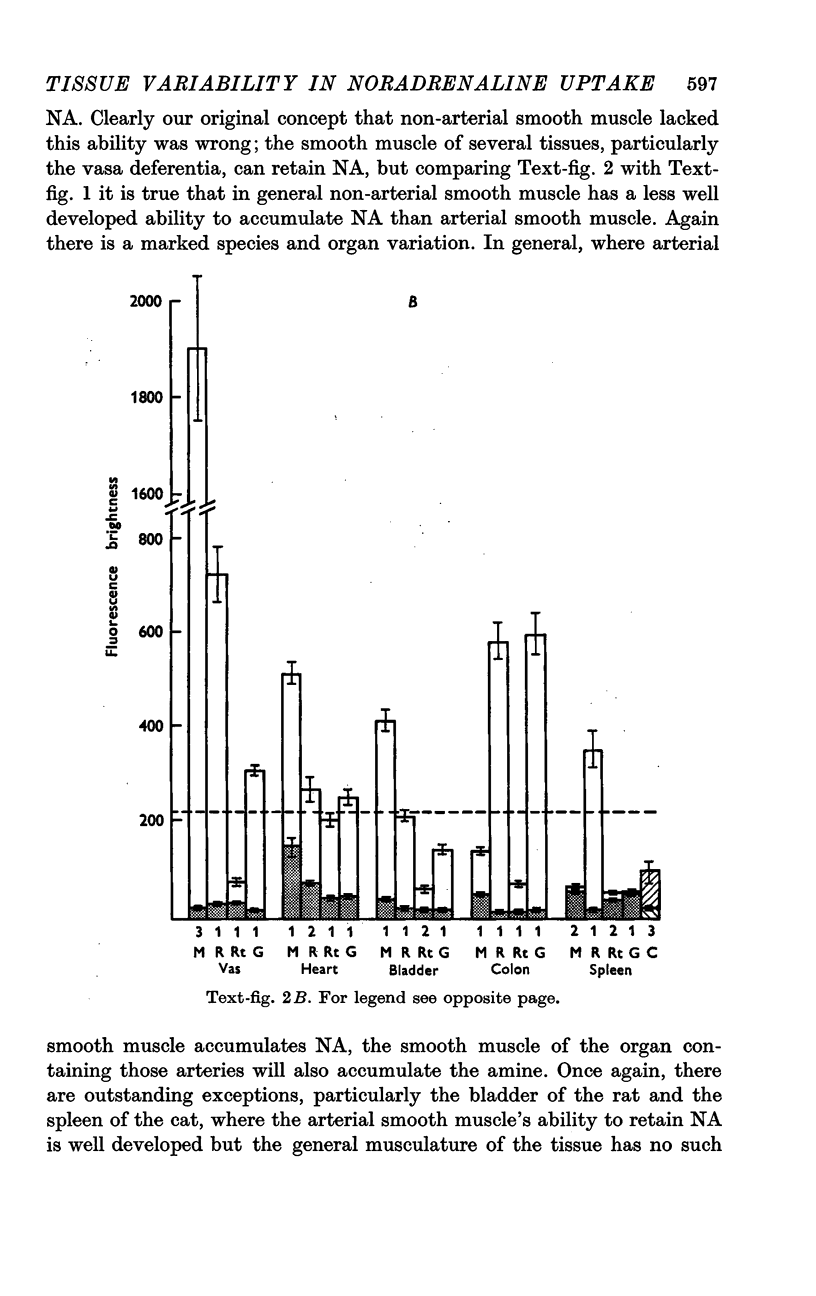
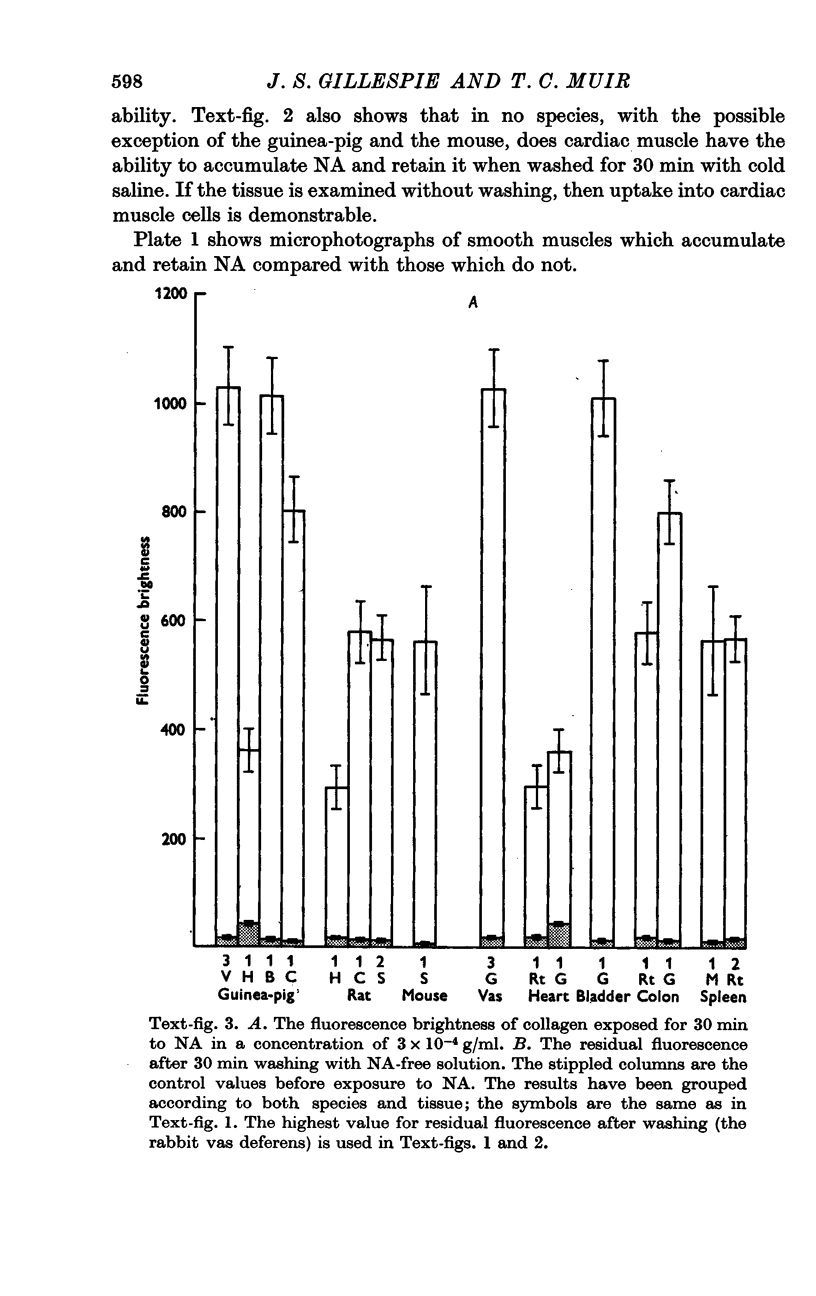
2. In general, arterial smooth muscle had a greater capacity to accumulate and retain NA than non-arterial smooth muscle, but there was a great species and organ variability. The ability to accumulate and retain NA was best developed in the mouse, followed by the rabbit, rat and guinea-pig in that order. Among organs the artery to the vas and the coronary arteries showed the greatest retention. Among non-arterial smooth muscle the mouse vas and the rabbit colon were notable.
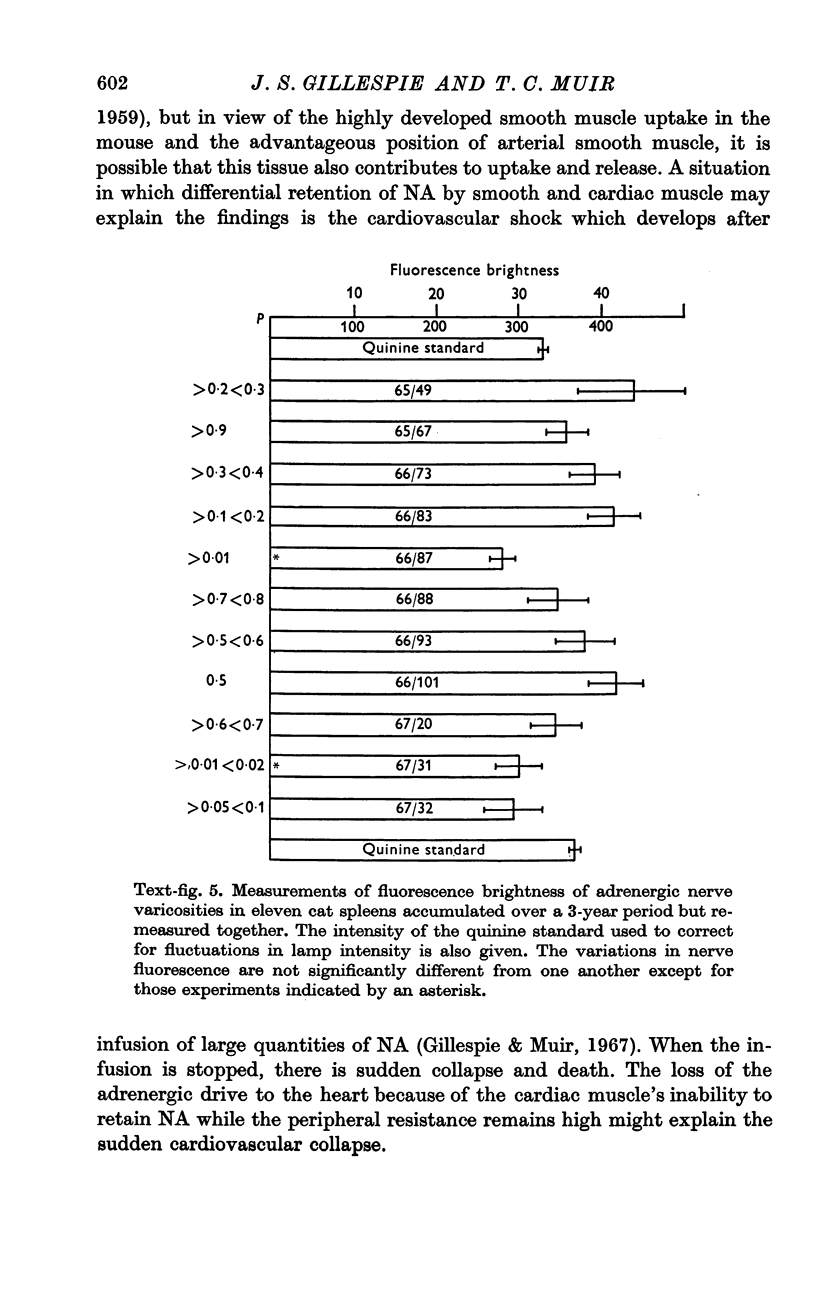
3. Cardiac muscle accumulates NA during exposure to the amine but, unlike smooth muscle, cannot retain it when washed with NA-free solution.
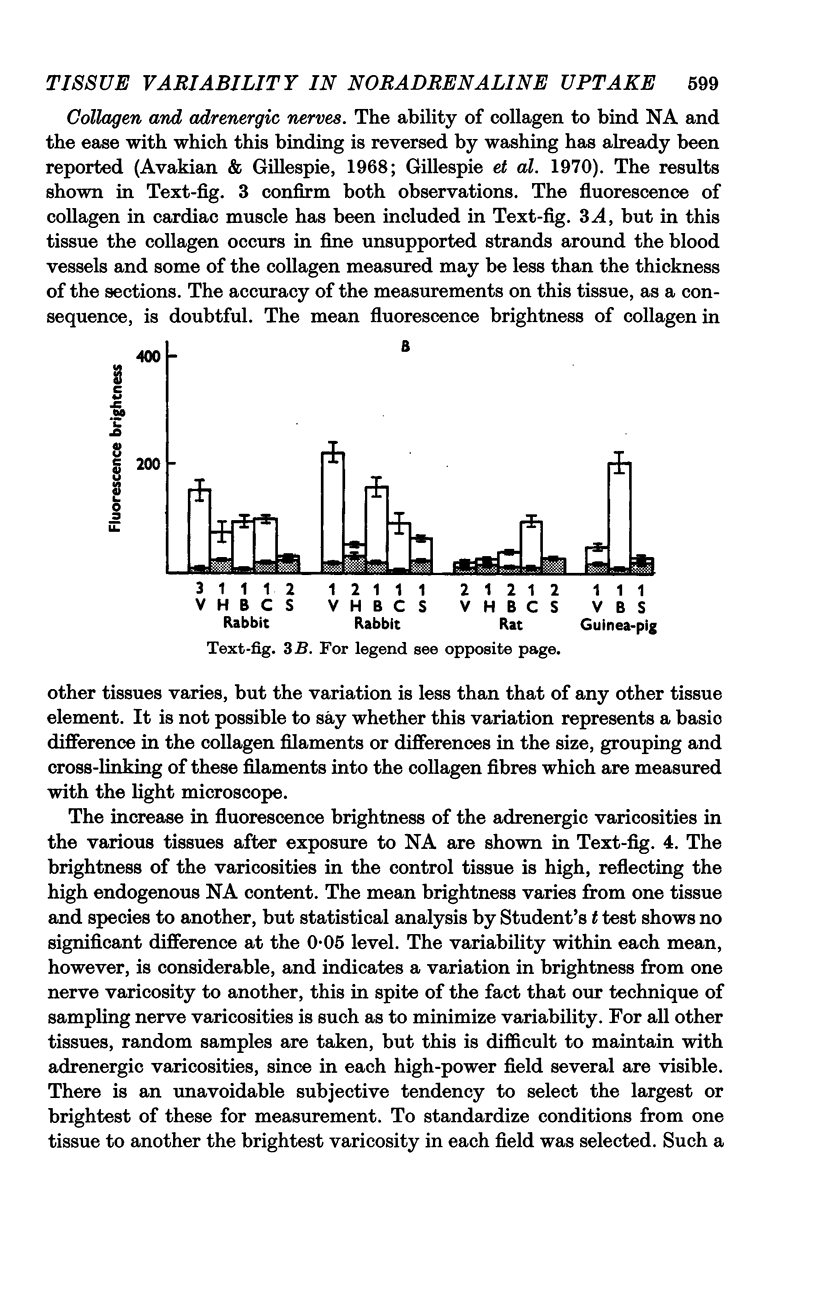
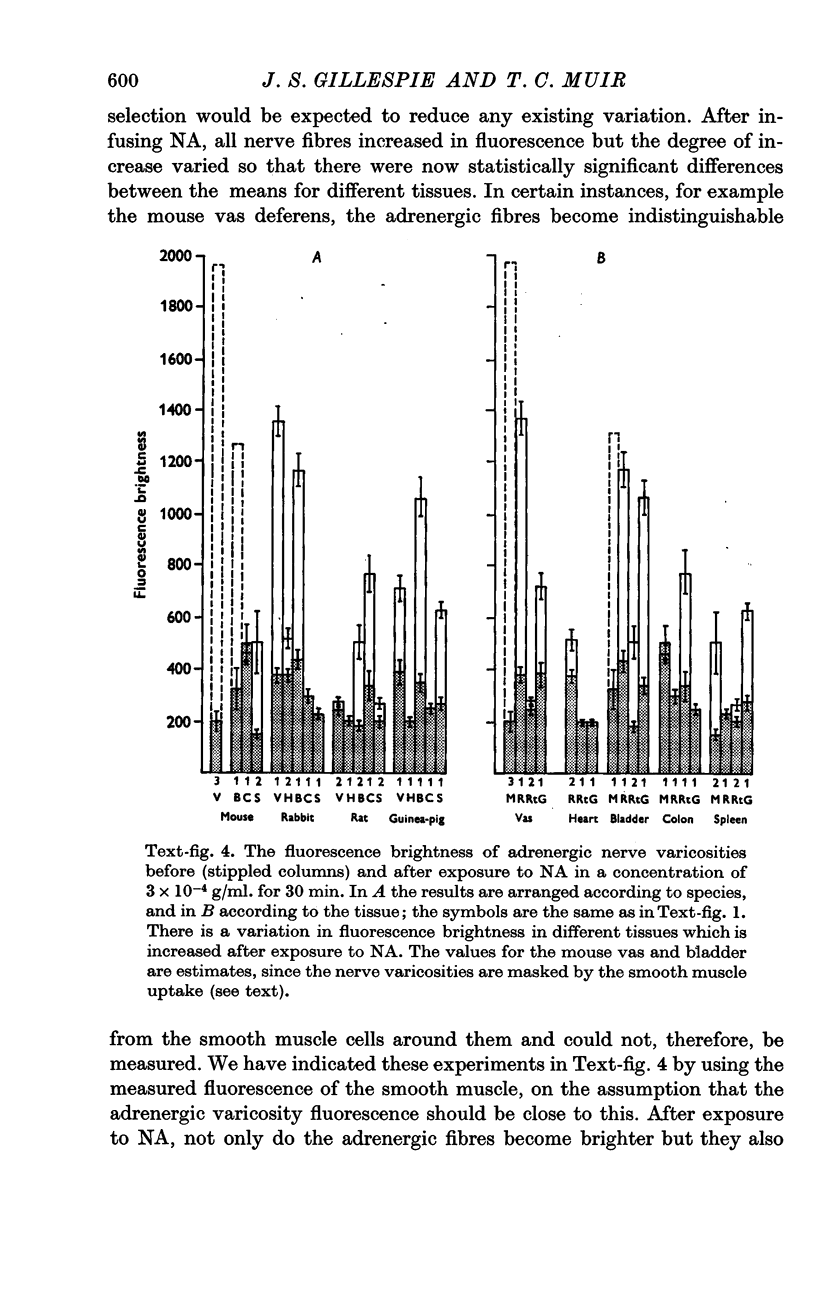
4. Terminal adrenergic nerves in different tissues show some variability in fluorescence intensity, and this is increased after exposure to NA. This may indicate a variable capacity of these cells to accumulate and retain NA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J., WEIL-MALHERBE H., TOMCHICK R. The physiological disposition of H3-epinephrine and its metabolite metanephrine. J Pharmacol Exp Ther. 1959 Dec;127:251–256. [PubMed] [Google Scholar]
- Avakian O. V., Gillespie J. S. Uptake of noradrenaline by adrenergic nerves, smooth muscle and connective tissue in isolated perfused arteries and its correlation with the vasoconstrictor response. Br J Pharmacol Chemother. 1968 Jan;32(1):168–184. doi: 10.1111/j.1476-5381.1968.tb00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E., Boullin D. J., Hammer W., Vogel W., Brodie B. B. Interactions of drugs with adrenergic neurons. Pharmacol Rev. 1966 Mar;18(1):577–597. [PubMed] [Google Scholar]
- Gillespie J. S., Hamilton D. N., Hosie J. A. The extraneuronal uptake and localization of noradrenaline in the cat spleen and the effect on this of some drugs, of cold and of denervation. J Physiol. 1970 Mar;206(3):563–590. doi: 10.1113/jphysiol.1970.sp009031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Kirpekar S. M. The histological localization of noradrenaline in the cat spleen. J Physiol. 1966 Nov;187(1):69–79. doi: 10.1113/jphysiol.1966.sp008076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Muir T. C. The origin of the decline in the vasopressor response to infused noradrenaline in the pithed rat. Br J Pharmacol Chemother. 1967 May;30(1):88–98. doi: 10.1111/j.1476-5381.1967.tb02115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]