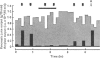
Full text
PDF



























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avakian O. V., Gillespie J. S. Uptake of noradrenaline by adrenergic nerves, smooth muscle and connective tissue in isolated perfused arteries and its correlation with the vasoconstrictor response. Br J Pharmacol Chemother. 1968 Jan;32(1):168–184. doi: 10.1111/j.1476-5381.1968.tb00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BDOLAH A., BEN-ZVI R., SCHRAMM M. THE MECHANISM OF ENZYME SECRETION BY THE CELL. II. SECRETION OF AMYLASE AND OTHER PROTEINS BY SLICES OF RAT PAROTID GLAND. Arch Biochem Biophys. 1964 Jan;104:58–66. doi: 10.1016/s0003-9861(64)80034-5. [DOI] [PubMed] [Google Scholar]
- Berliner R. W., Bennett C. M. Concentration of urine in the mammalian kidney. Am J Med. 1967 May;42(5):777–789. doi: 10.1016/0002-9343(67)90095-2. [DOI] [PubMed] [Google Scholar]
- Browse N. L. Response of lymphatics of canine hind limb to sympathetic nerve stimulation. J Physiol. 1968 Jul;197(1):25–36. doi: 10.1113/jphysiol.1968.sp008542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Harper A. A., Scratcherd T. Water and electrolyte secretion by the perfused pancreas of the cat. J Physiol. 1968 May;196(1):133–149. doi: 10.1113/jphysiol.1968.sp008499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Functional consequences of ultrastructural geometry in "backwards" fluid-transporting epithelia. J Cell Biol. 1968 Jun;37(3):694–702. doi: 10.1083/jcb.37.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker S. E. Release of vasopressin and oxytocin from isolated pituitary glands of adult and new-born rats. J Physiol. 1966 Jul;185(2):429–444. doi: 10.1113/jphysiol.1966.sp007994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. F. Narrow 'tuning' of cochlear nerve fibre responses in the guinea-pig. J Physiol. 1970 Feb;206(2):14P–15P. [PMC free article] [PubMed] [Google Scholar]
- Evans E. F., Rosenberg J., Wilson J. P. The effective bandwidth of cochlear nerve fibres. J Physiol. 1970 Apr;207(2):62P–63P. [PubMed] [Google Scholar]
- FOLKOW B. Nervous control of the blood vessels. Physiol Rev. 1955 Jul;35(3):629–663. doi: 10.1152/physrev.1955.35.3.629. [DOI] [PubMed] [Google Scholar]
- Findlay J. D., Thompson G. E. The effect of intraventricular injections of noradrenaline, 5-hydroxytryptamine, acetylcholine and tranylcypromine on the ox (Bos taurus) at different environmental temperatures. J Physiol. 1968 Feb;194(3):809–816. doi: 10.1113/jphysiol.1968.sp008436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Muir T. C. Species and tissue variation in extraneuronal and neuronal accumulation of noradrenaline. J Physiol. 1970 Mar;206(3):591–604. doi: 10.1113/jphysiol.1970.sp009032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLER H., LEDERIS K. Maturation of the hypothalamoneurohypophysial system. J Physiol. 1959 Sep 2;147:299–314. doi: 10.1113/jphysiol.1959.sp006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., GORDON A. M. Striation patterns in active and passive shortening of muscle. Nature. 1962 Jan 20;193:280–281. doi: 10.1038/193280b0. [DOI] [PubMed] [Google Scholar]
- Hai M. A., Thomas S. The time-course of changes in renal tissue composition during lysine vasopressin infusion in the rat. Pflugers Arch. 1969;310(4):297–317. doi: 10.1007/BF00587241. [DOI] [PubMed] [Google Scholar]
- Huxley A. F. Is resonance possible in the cochlea after all? Nature. 1969 Mar 8;221(5184):935–940. doi: 10.1038/221935a0. [DOI] [PubMed] [Google Scholar]
- Johnstone B. M., Boyle A. J. Basilar membrane vibration examined with the Mössbauer technique. Science. 1967 Oct 20;158(3799):389–390. doi: 10.1126/science.158.3799.389. [DOI] [PubMed] [Google Scholar]
- KATZ B. The efferent regulation of the muscle spindle in the frog. J Exp Biol. 1949 Aug;26(2):201–217. doi: 10.1242/jeb.26.2.201. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang N. Y., Sachs M. B., Peake W. T. Shapes of tuning curves for single auditory-nerve fibers. J Acoust Soc Am. 1967 Dec;42(6):1341–1342. doi: 10.1121/1.1910723. [DOI] [PubMed] [Google Scholar]
- LATNER A. L., HODSON A. W., SMITH P. A. Intestinal absorption of coenzyme B12 in the normal and fluoroacetate-poisoned rat. Lancet. 1962 Aug 4;2(7249):230–230. doi: 10.1016/s0140-6736(62)92318-8. [DOI] [PubMed] [Google Scholar]
- LEDSOME J. R., LINDEN R. J. A REFLEX INCREASE IN HEART RATE FROM DISTENSION OF THE PULMONARY-VEIN-ATRIAL JUNCTIONS. J Physiol. 1964 Apr;170:456–473. doi: 10.1113/jphysiol.1964.sp007343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledsome J. R., Linden R. J. The effect of distending a pouch of the left atrium on the heart rate. J Physiol. 1967 Nov;193(1):121–129. doi: 10.1113/jphysiol.1967.sp008346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnell J. C., Hoffbrand A. V., Peters T. J., Matthews D. M. Estimation of individual plasma B 12 compounds in normal subjects and patients with deranged B 12 metabolism. J Clin Pathol. 1969 Nov;22(6):742–742. doi: 10.1136/jcp.22.6.742-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnell J. C., Mackenzie H. M., Mattews D. M. Normal values for individual plasma cobalamins. J Clin Pathol. 1969 Jul;22(4):506–506. doi: 10.1136/jcp.22.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSOUD A., TAYLOR G. BYSSINOSIS: ANTIBODY TO COTTON ANTIGENS IN NORMAL SUBJECTS AND IN COTTON CARD-ROOM WORKERS. Lancet. 1964 Sep 19;2(7360):607–610. doi: 10.1016/s0140-6736(64)90506-9. [DOI] [PubMed] [Google Scholar]
- MCGREGOR D. D. THE EFFECT OF SYMPATHETIC NERVE STIMULATION OF VASOCONSTRICTOR RESPONSES IN PERFUSED MESENTERIC BLOOD VESSELS OF THE RAT. J Physiol. 1965 Mar;177:21–30. doi: 10.1113/jphysiol.1965.sp007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik K. U., Ling G. M. The effect of 1,1-dimethyl-4-phenyl-piperazinium on the response of mesenteric arteries to sympathetic nerve stimulation. J Pharm Pharmacol. 1969 Aug;21(8):514–519. doi: 10.1111/j.2042-7158.1969.tb08304.x. [DOI] [PubMed] [Google Scholar]
- Matthews P. B., Stein R. B. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol. 1969 Feb;200(3):723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommaerts W. F. Energetics of muscular contraction. Physiol Rev. 1969 Jul;49(3):427–508. doi: 10.1152/physrev.1969.49.3.427. [DOI] [PubMed] [Google Scholar]
- NEWMAN P. P., WOLSTENCROFT J. H. Medullary responses to stimulation of orbital cortex. J Neurophysiol. 1959 Sep;22:516–523. doi: 10.1152/jn.1959.22.5.516. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Quinlan A., Hoffbrand A. V. Subcellular localization of radioactive vitamin B12 during absorption by guinea-pig ileum. Clin Sci. 1969 Oct;37(2):568–569. [PubMed] [Google Scholar]
- Provini L., Redman S., Strata P. Mossy and climbing fibre organization on the anterior lobe of the cerebellum activated by forelimb and hindlimb areas of the sensorimotor cortex. Exp Brain Res. 1968;6(3):216–233. doi: 10.1007/BF00235125. [DOI] [PubMed] [Google Scholar]
- RODECK H. Neurosekretion und Wasserhaushalt bei Neugeborenen und Sauglingen. Arch Kinderheilkd Suppl. 1958;36:1–62. [PubMed] [Google Scholar]
- Ridgway E. B., Ashley C. C. Calcium transients in single muscle fibers. Biochem Biophys Res Commun. 1967 Oct 26;29(2):229–234. doi: 10.1016/0006-291x(67)90592-x. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. INVESTIGATIONS ON RESPIRATORY MOTONEURONES OF THE THORACIC SPINAL CORD. Prog Brain Res. 1964;12:259–273. doi: 10.1016/s0079-6123(08)60627-5. [DOI] [PubMed] [Google Scholar]
- SHIMOMURA O., JOHNSON F. H., SAIGA Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962 Jun;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- SLOPER J. C., ARNOTT D. J., KING B. C. Sulphur metabolism in the pituitary and hypothalamus of the rat: a study of radioisotope-uptake after the injection of 35S DL-cysteine, methionine, and sodium sulphate. J Endocrinol. 1960 Feb;20:9–23. doi: 10.1677/joe.0.0200009. [DOI] [PubMed] [Google Scholar]