Abstract
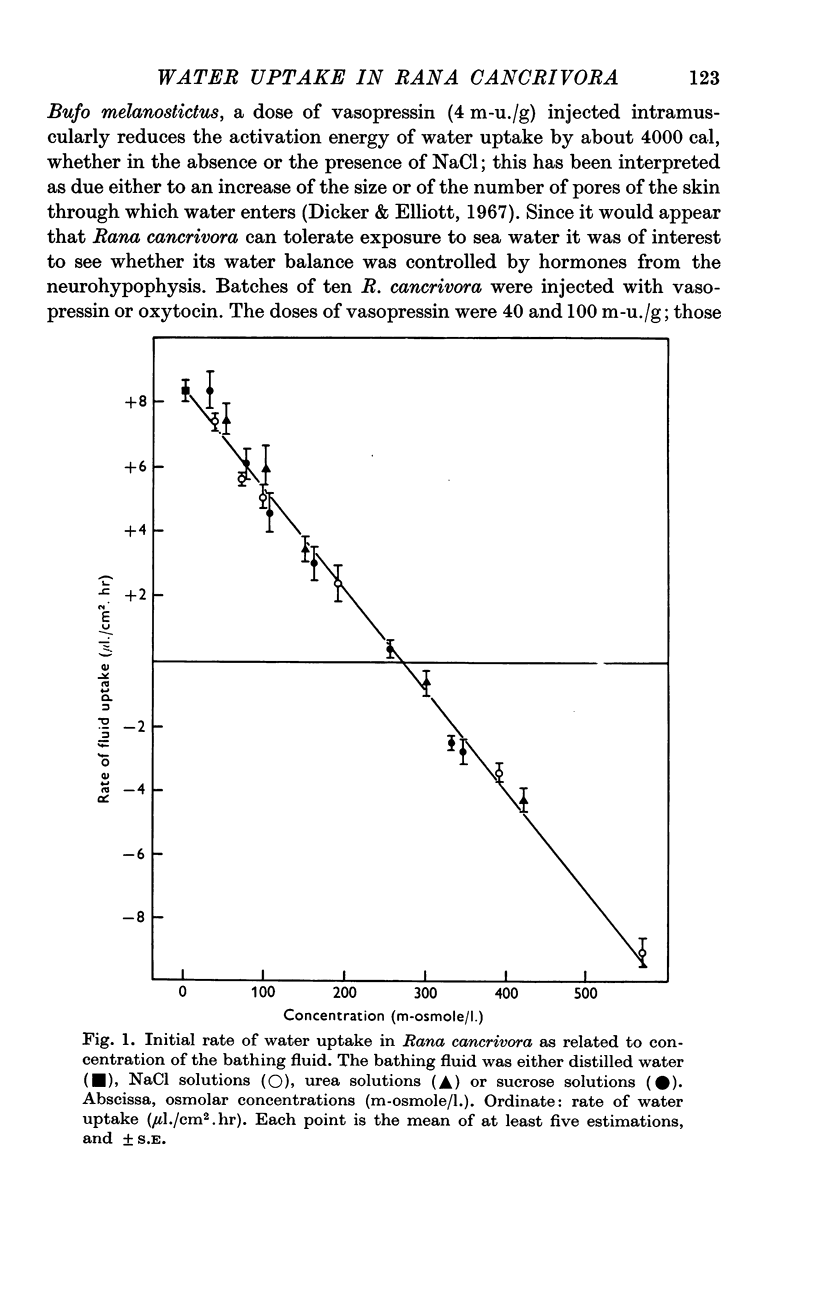
1. The rate of water uptake across the skin was investigated in live Rana cancrivora, an euryhaline frog which has been reported to tolerate sea water. When they were exposed to distilled water at 29° C, the rate of water uptake was 8·4 ± 0·4 μl./cm2.hr; when bathed in solutions ranging from 30 to 570 m-osmole/l., irrespective of whether the solute was sucrose, urea or NaCl, the rate of fluid uptake during the first day was inversely related to the osmolarity of the solution. No appreciable fluid movement was observed when the bathing solution had an osmolar concentration of 270 m-osmole/l.
2. The rate of fluid uptake was not affected by injections of vasopressin, oxytocin or of extracts of amphibian or rat pituitary glands, irrespective of whether R. cancrivora were bathed in distilled water or in solutions of NaCl or sucrose.
3. In Bufo melanostictus, in contrast with R. cancrivora, injections of neurohypophysial extracts produced a marked increase of the rate of fluid uptake.
4. In the laboratory, R. cancrivora could be acclimatized stepwise to tolerate NaCl solutions up to 700 m-osmole/l. for 7 days.
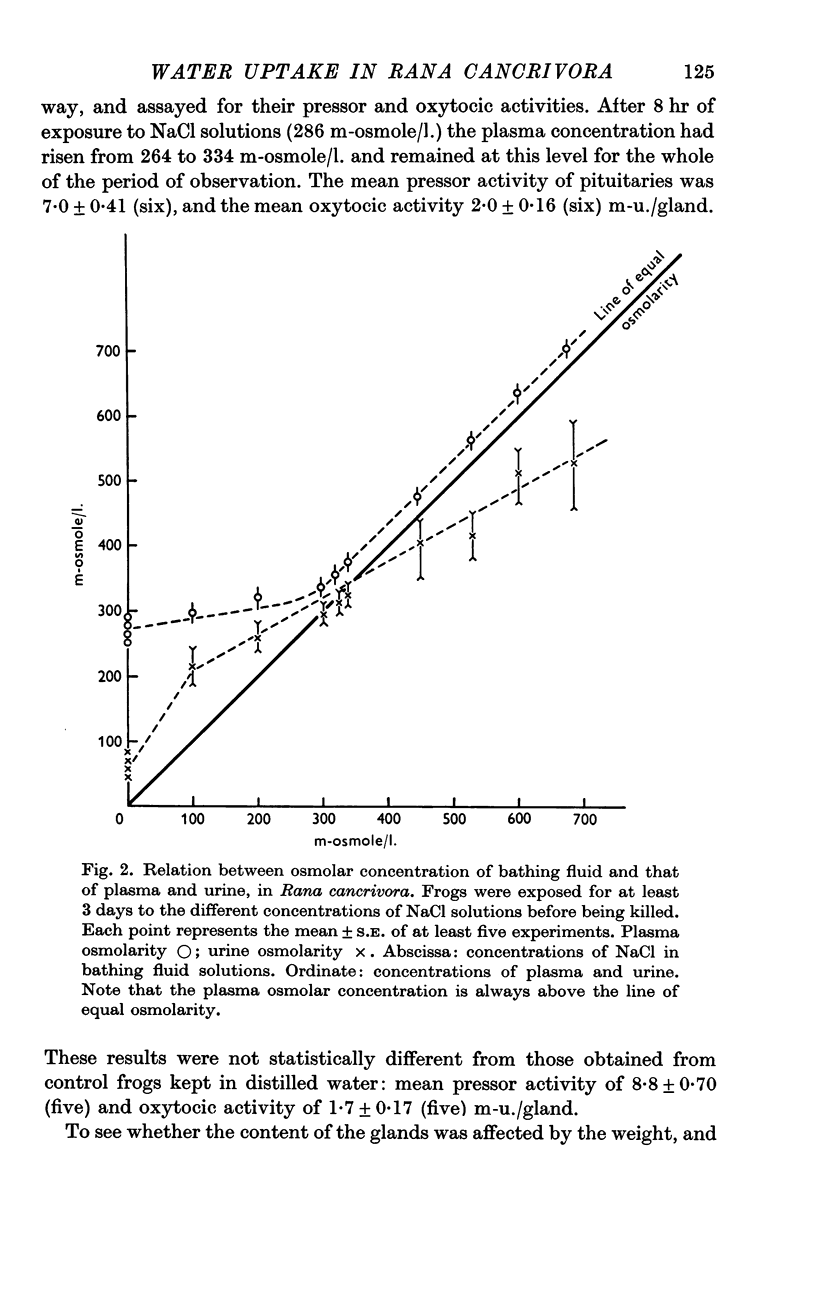
5. After 24 hr exposure either to distilled water or to NaCl solutions from 100 to 670 m-osmole/l., the osmolar concentration of the plasma of R. cancrivora was always higher than that of the bathing fluid. In R. pipiens or R. temporaria plasma osmolar concentration was higher than that of the bathing fluid only when the latter did not exceed 300 m-osmole/l.
6. Under all conditions investigated, the osmolar concentration of the urine of R. cancrivora was always lower than that of the plasma.
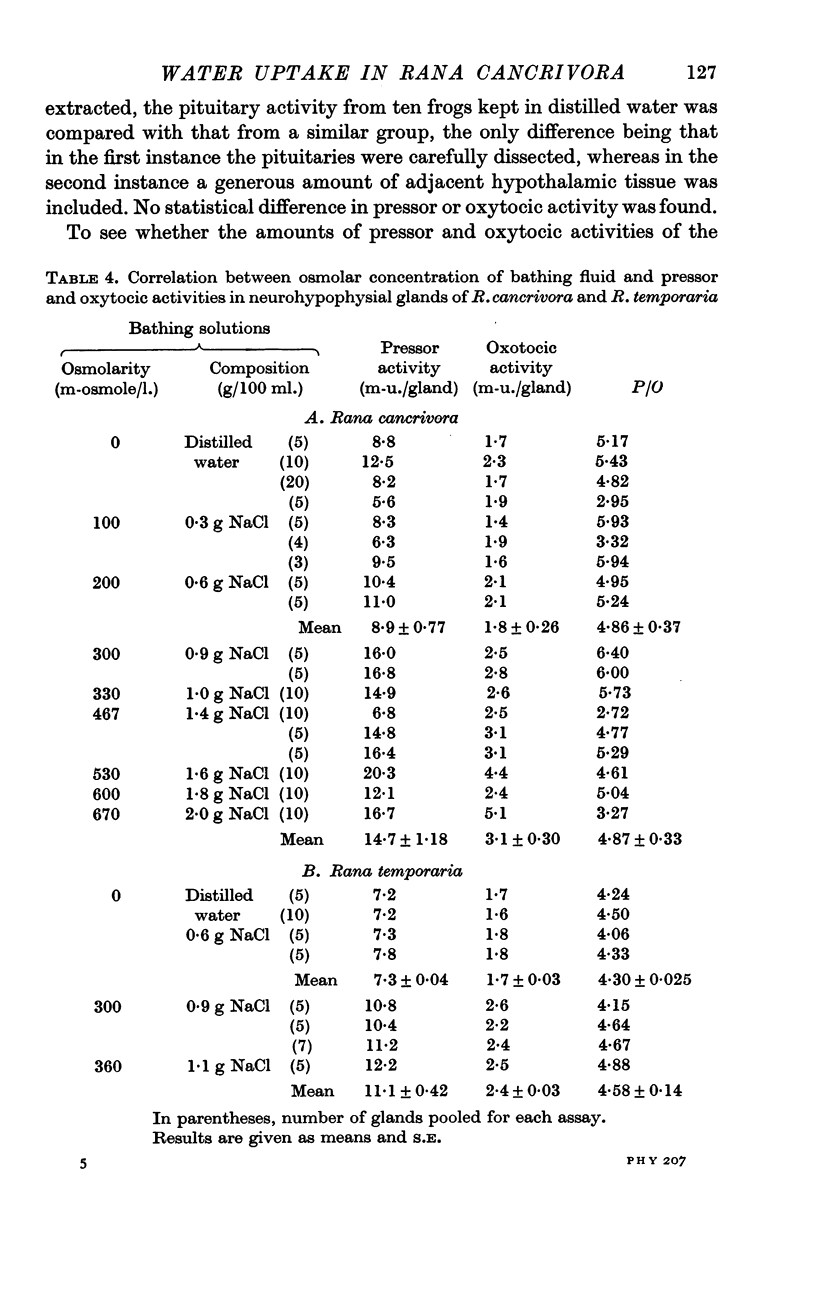
7. The amounts of pressor and oxytocic activities of pituitary glands of R. cancrivora kept in distilled water or in NaCl solutions up to 300 m-osmole/l. were 8·9 ± 0·8 and 1·8 ± 0·3 m-u./gland, irrespective of sex or body weight within the range 30-50 g. After 3 days exposure to hypertonic NaCl solutions, the amounts of pressor and oxytocic activities were 14·7 ± 1·2 and 3·1 ± 0·3 m-u./gland. In both instances the pressor/oxytocic ratio was 4·9. Pituitary glands of R. temporaria similarly showed increased pressor and oxytocic activities after exposure to NaCl solutions of 300-360 m-osmole/l.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHER R., CHAUVET J., CHAUVET M. T., CRERY D. PHYLOG'ENIE DES PEPTIDES NEUROHYPOPHYSAIRES: ISOLEMENT DE LA MESOTOCINE (ILEU 8-OCYTOCINE) DE LA GRENOUILLE, INTERM'EDIAIRE ENTRE LA SER-4-ILEU 8-OCYTOCINE DES POISSONS OSSEUX ET L'OCYTOCINE DES MAMMIF'ERES. Biochim Biophys Acta. 1964 Sep 4;90:613–615. [PubMed] [Google Scholar]
- BENTLEY P. J., HELLER H. THE ACTION OF NEUROHYPOPHYSIAL HORMONES ON THE WATER AND SODIUM METABOLISM OF URODELE AMPHIBIANS. J Physiol. 1964 Jun;171:434–453. doi: 10.1113/jphysiol.1964.sp007389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKER S. E., NUNN J. The role of the antiduretic hormone during water deprivation in rats. J Physiol. 1957 Apr 30;136(2):235–248. doi: 10.1113/jphysiol.1957.sp005756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker S. E., Elliott A. B. Water uptake by Bufo melanostictus, as affected by osmotic gradients, vasopressin and temperature. J Physiol. 1967 May;190(2):359–370. doi: 10.1113/jphysiol.1967.sp008214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLLETT B. K., HELLER H. THE NEUROHYPOPHYSIAL HORMONES OF LUNGFISHES AND AMPHIBIANS. J Physiol. 1964 Jul;172:92–106. doi: 10.1113/jphysiol.1964.sp007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLER H. OSMOREGULATION IN AMPHIBIA. Arch Anat Microsc Morphol Exp. 1965 Jan-Mar;54:471–489. [PubMed] [Google Scholar]
- Jones I. C., Chan D. K., Rankin J. C. Renal function in the European ell (Anguilla anguilla L.): changes in blood pressure and renal function of the freshwater eel transferred to sea-water. J Endocrinol. 1969 Jan;43(1):9–19. doi: 10.1677/joe.0.0430009. [DOI] [PubMed] [Google Scholar]
- Munsick R. A. Chromatographic and pharmacologic characterization of the neurohypophysial hormones of an amphibian and a reptile. Endocrinology. 1966 Mar;78(3):591–599. doi: 10.1210/endo-78-3-591. [DOI] [PubMed] [Google Scholar]
- PICKERING B. T., HELLER H. Chromatographic and biological characteristics of fish and frog neuro-hypophysial extracts. Nature. 1959 Nov 7;184:1463–1464. doi: 10.1038/1841463a0. [DOI] [PubMed] [Google Scholar]
- SAWYER W. H., MUNSICK R. A., VANDYKE H. B. Pharmacological evidence for the presence of arginine vasotocin and oxytocin in neurohypophysial extracts from cold-blooded vertebrates. Nature. 1959 Nov 7;184:1464–1465. doi: 10.1038/1841464a0. [DOI] [PubMed] [Google Scholar]
- SCHMIDT-NIELSEN K., LEE P. Kidney function in the crab-eating frog (Rana cancrivora). J Exp Biol. 1962 Mar;39:167–177. doi: 10.1242/jeb.39.1.167. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ I. L., LIVINGSTON L. M. CELLULAR AND MOLECULAR ASPECTS OF THE ANTIDIURETIC ACTION OF VASOPRESSINS AND RELATED PEPTIDES. Vitam Horm. 1964;22:261–358. doi: 10.1016/s0083-6729(08)60341-6. [DOI] [PubMed] [Google Scholar]


