Abstract
1. We have studied red and green cones by contrast flash inhibition and found them both to be very similar to rods in their response to flash energy, except that all light quantities must be some 100-fold greater in cones for the same effect.
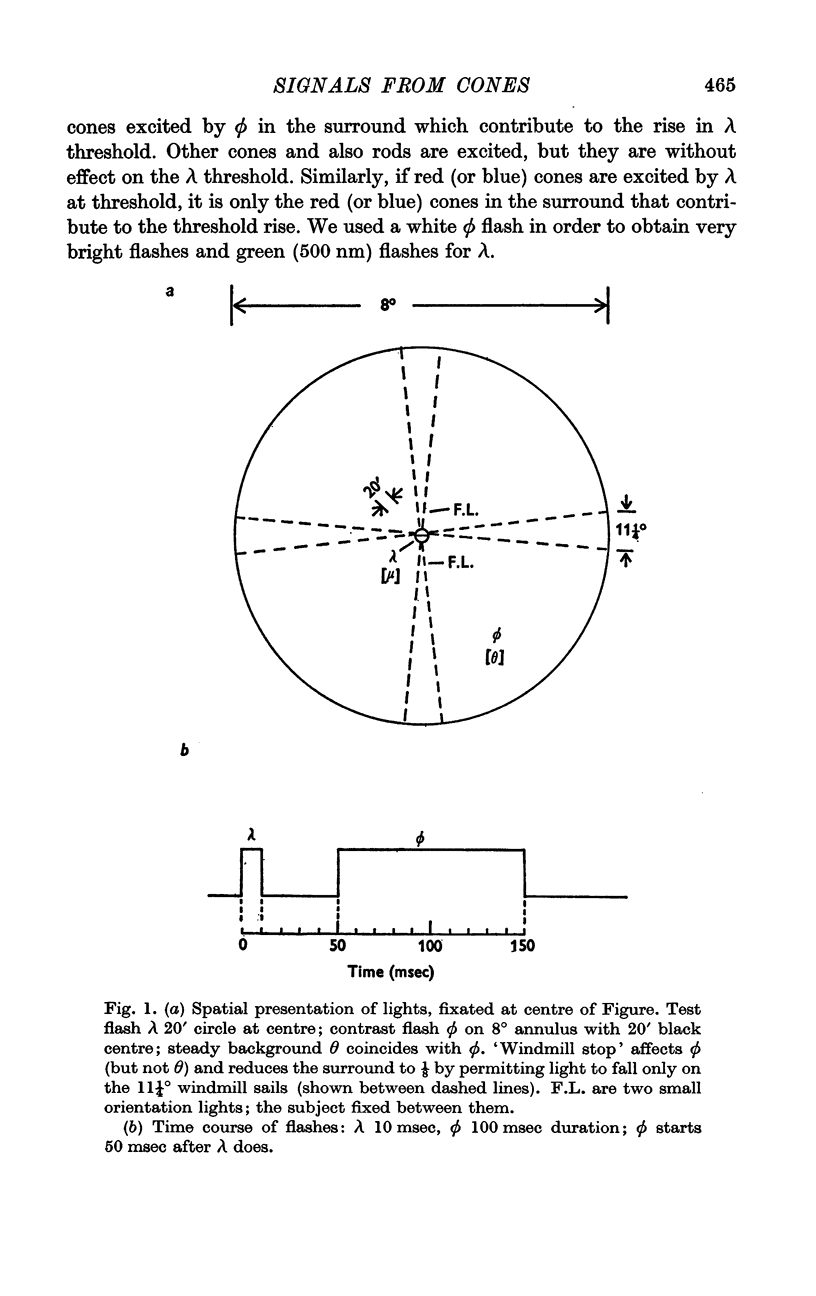
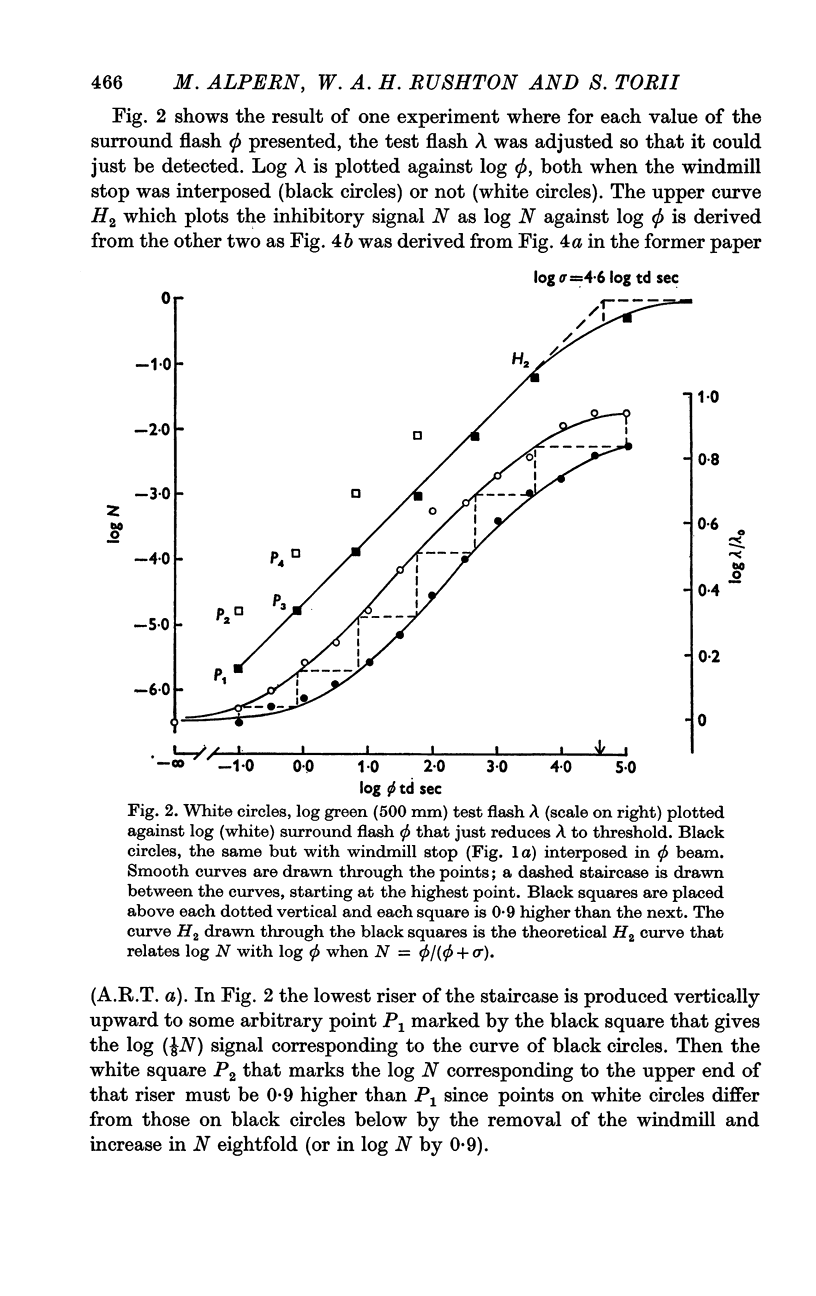
2. Using the methods of a previous paper (A.R.T. a) where no backgrounds were employed, we plotted log test flash λ against log surround flash ϕ with criterion that λ should just be detected. The experiment was repeated with a `windmill stop' interposed in the ϕ flash which reduced its area symmetrically to ⅛. From these two curves it is possible to extract the relation between N, the inhibitory signal, and ϕ, the test flash. It isN = ϕ/(ϕ+σ),where σ, the semi-saturation constant, is about 4·5 log td sec.
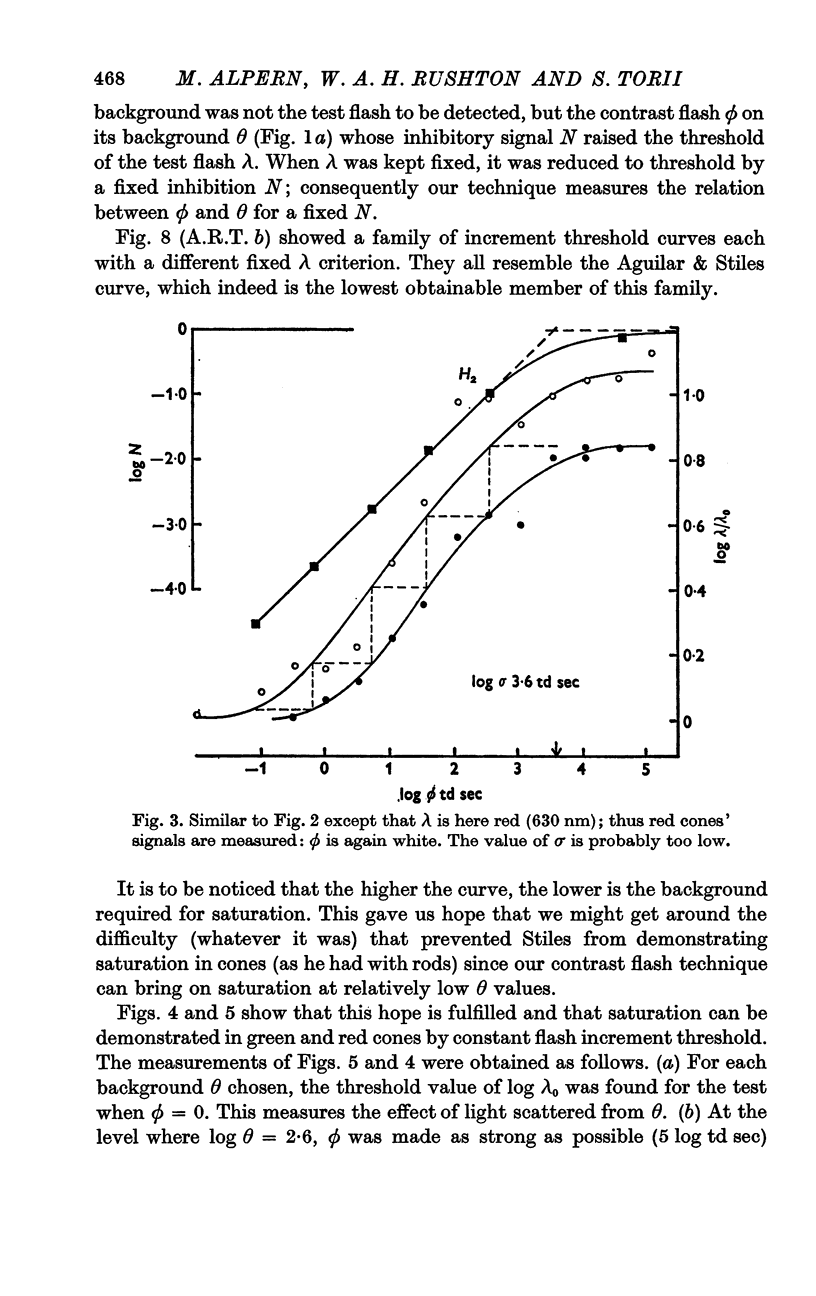
3. Using the methods of (A.R.T. b) where backgrounds were studied, we measured the increment threshold for the surround flash ϕ against its background θ using as criterion not that ϕ should just be seen but that it should generate a fixed inhibitory signal N so that the fixed test flash λ could just be seen.
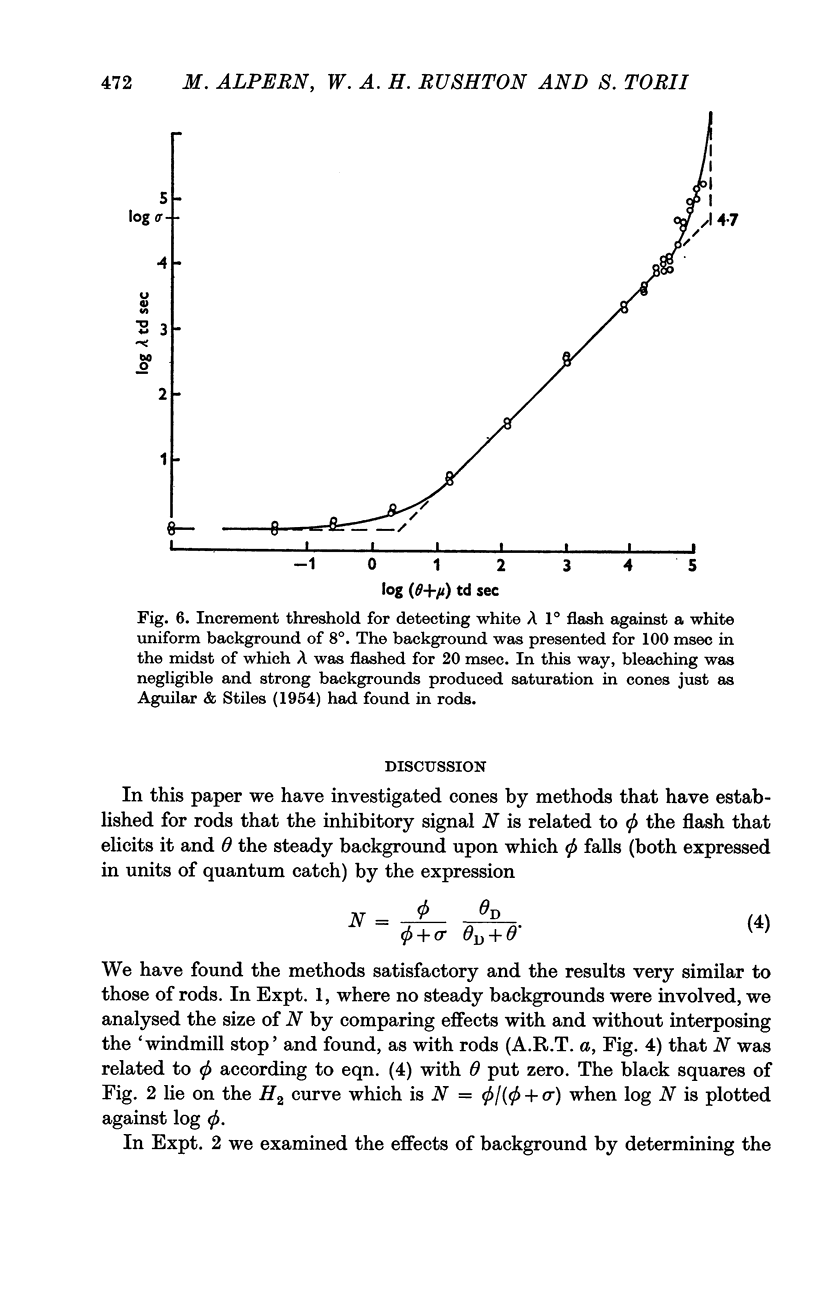
4. This increment threshold curve resembled the Aguilar & Stiles (1954) curve for rods, showed saturation and a complete symmetry about the 45° line through the point with co-ordinates (log θD, log σ).
5. These results imply that the cone signal N is related to flash ϕ and steady background θ by [Formula: see text], where θD is receptor noise (= eigengrau), and ϕ and θ are expressed in units of quantum catch.
6. The ordinary increment threshold for cones does not show saturation because a steady saturating background bleaches all the pigment away. When the background is presented for only 100 msec with dark pauses between, no great bleaching occurs and saturation is seen.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPERN M. ROD-CONE INDEPENDENCE IN THE AFTER-FLASH EFFECT. J Physiol. 1965 Feb;176:462–472. doi: 10.1113/jphysiol.1965.sp007561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALPERN M., RUSHTON W. A. THE SPECIFICITY OF THE CONE INTERACTION IN THE AFTER-FLASH EFFECT. J Physiol. 1965 Feb;176:473–482. doi: 10.1113/jphysiol.1965.sp007562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern M., Rushton W. A., Torii S. The attenuation of rod signals by backgrounds. J Physiol. 1970 Jan;206(1):209–227. doi: 10.1113/jphysiol.1970.sp009007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern M., Rushton W. A., Torii S. The attenuation of rod signals by bleachings. J Physiol. 1970 Apr;207(2):449–461. doi: 10.1113/jphysiol.1970.sp009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern M., Rushton W. A., Torii S. The size of rod signals. J Physiol. 1970 Jan;206(1):193–208. doi: 10.1113/jphysiol.1970.sp009006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Croz J. J., Rushton W. A. The separation of cone mechanisms in dark adaptation. J Physiol. 1966 Mar;183(2):481–496. doi: 10.1113/jphysiol.1966.sp007878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. CONE PIGMENT KINETICS IN THE PROTANOPE. J Physiol. 1963 Sep;168:374–388. doi: 10.1113/jphysiol.1963.sp007198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. Kinetics of cone pigments measured objectively on the living human fovea. Ann N Y Acad Sci. 1959 Nov 12;74(2):291–304. doi: 10.1111/j.1749-6632.1958.tb39552.x. [DOI] [PubMed] [Google Scholar]
- Rushton W. A., Henry G. H. Bleaching and regeneration of cone pigments in man. Vision Res. 1968 Jun;8(6):617–631. doi: 10.1016/0042-6989(68)90040-0. [DOI] [PubMed] [Google Scholar]


