Abstract
1. The evoked potential in response to a grating alternating in phase at 8 c/s was recorded as a function of contrast from the occiput of man.
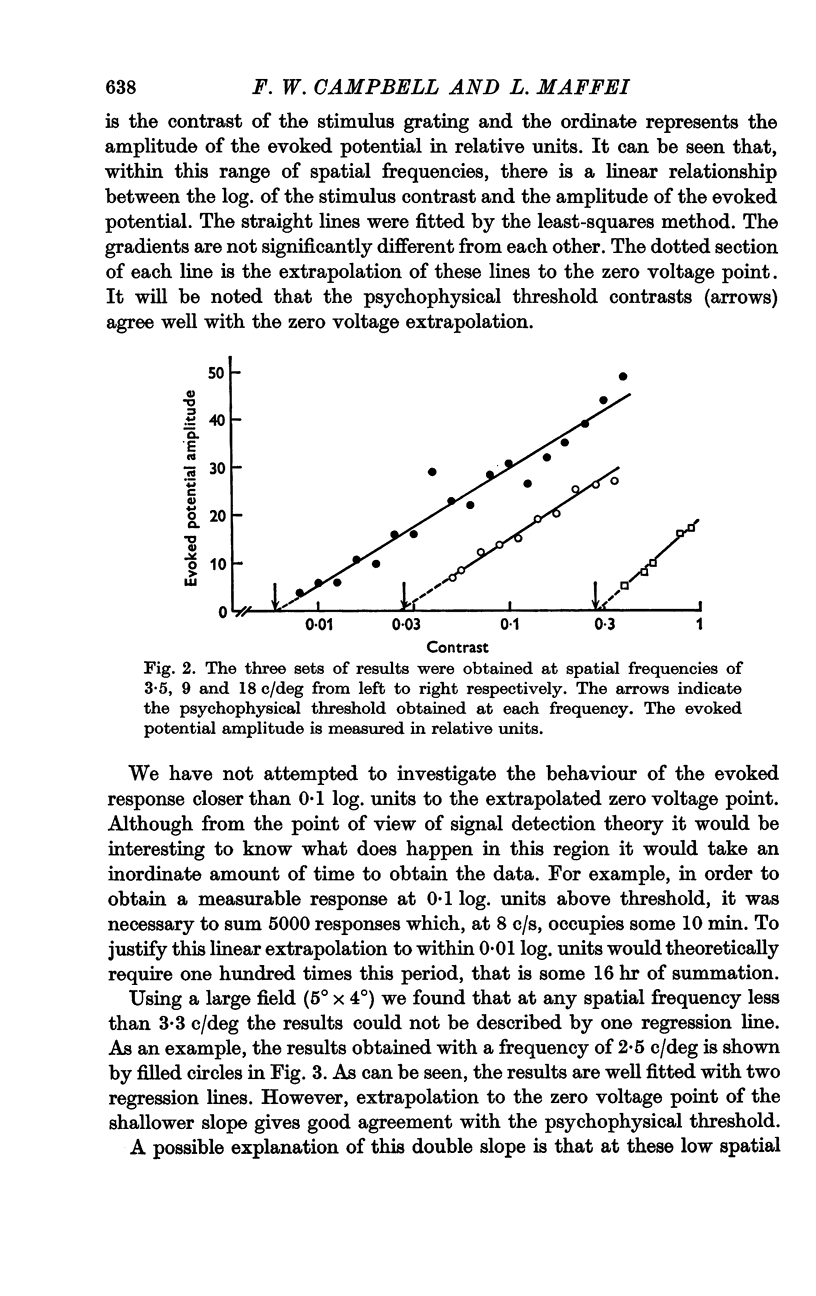
2. It was found that a linear relation exists between the log. of contrast and the amplitude of the evoked potential.
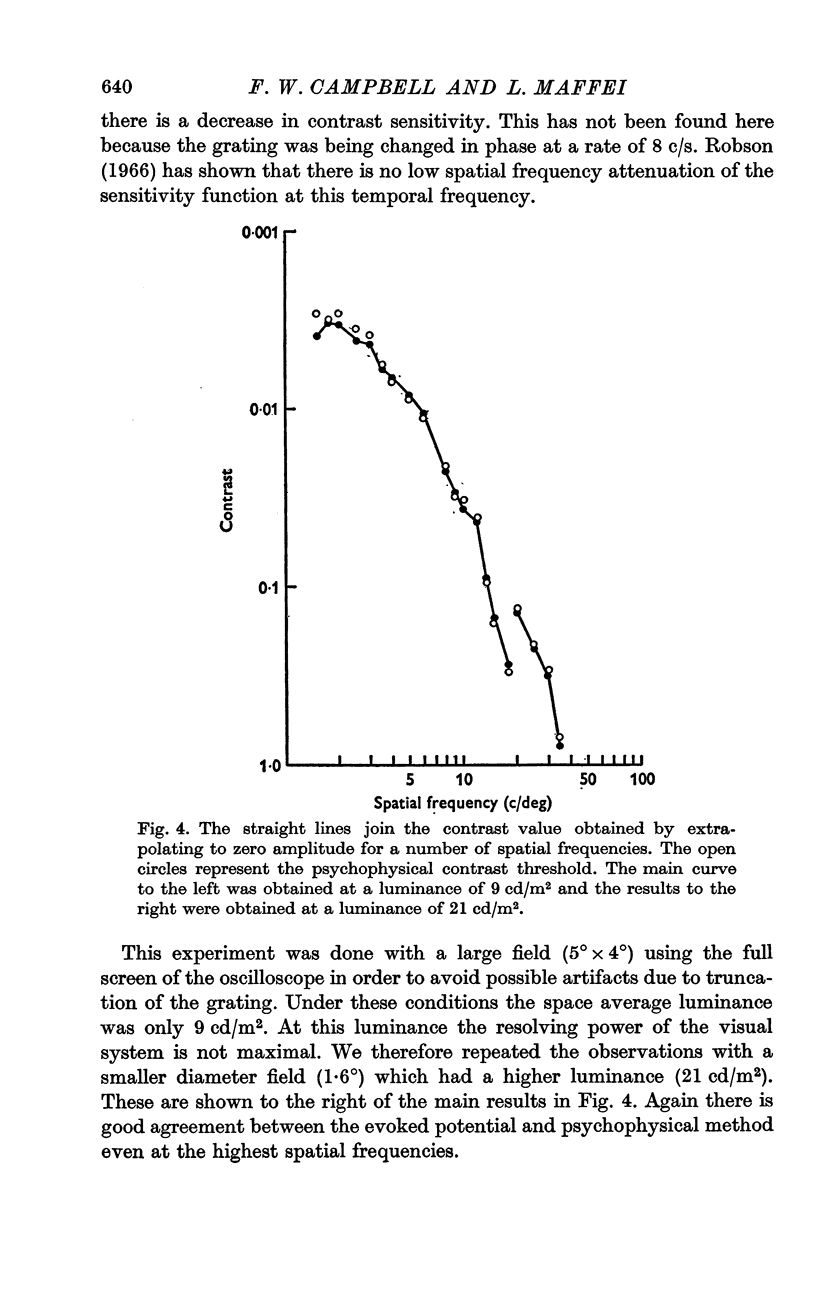
3. Extrapolation to zero amplitude voltage of the regression line between the amplitude of the evoked potential and log. contrast predicts the psychophysical threshold. This law was found to hold over the wide range of spatial frequencies tested.
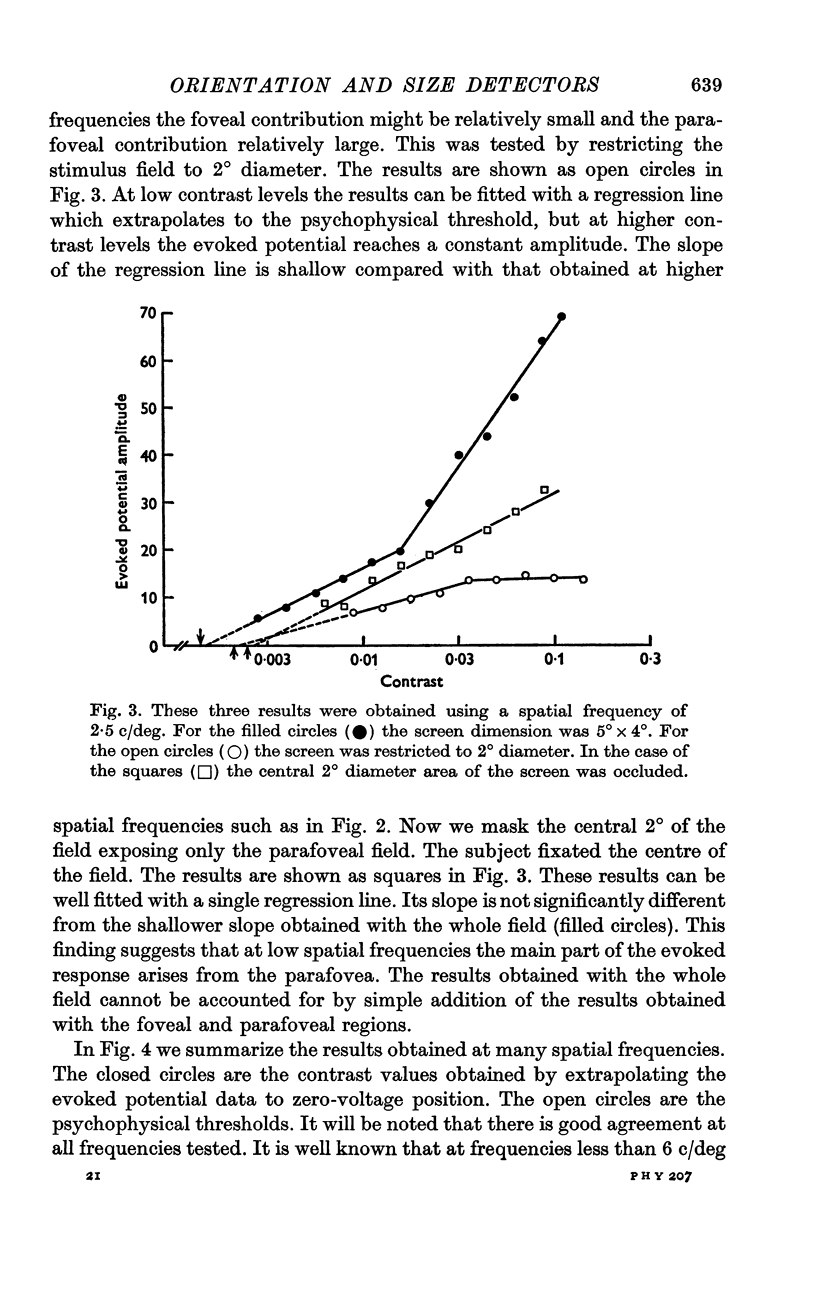
4. Below 3 c/deg the results are best fitted with two regression lines; one of these is generated from the foveal and the other from the parafoveal representation in the cortex.
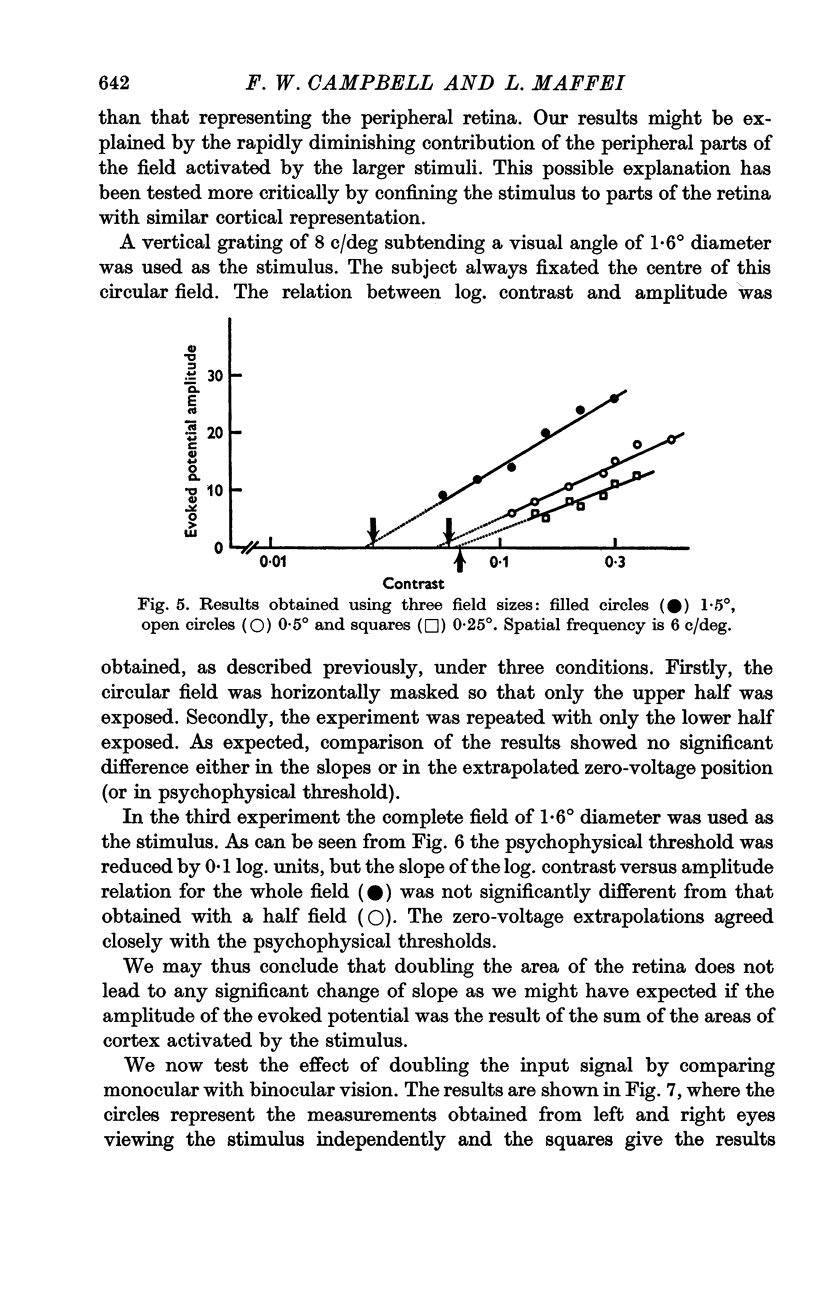
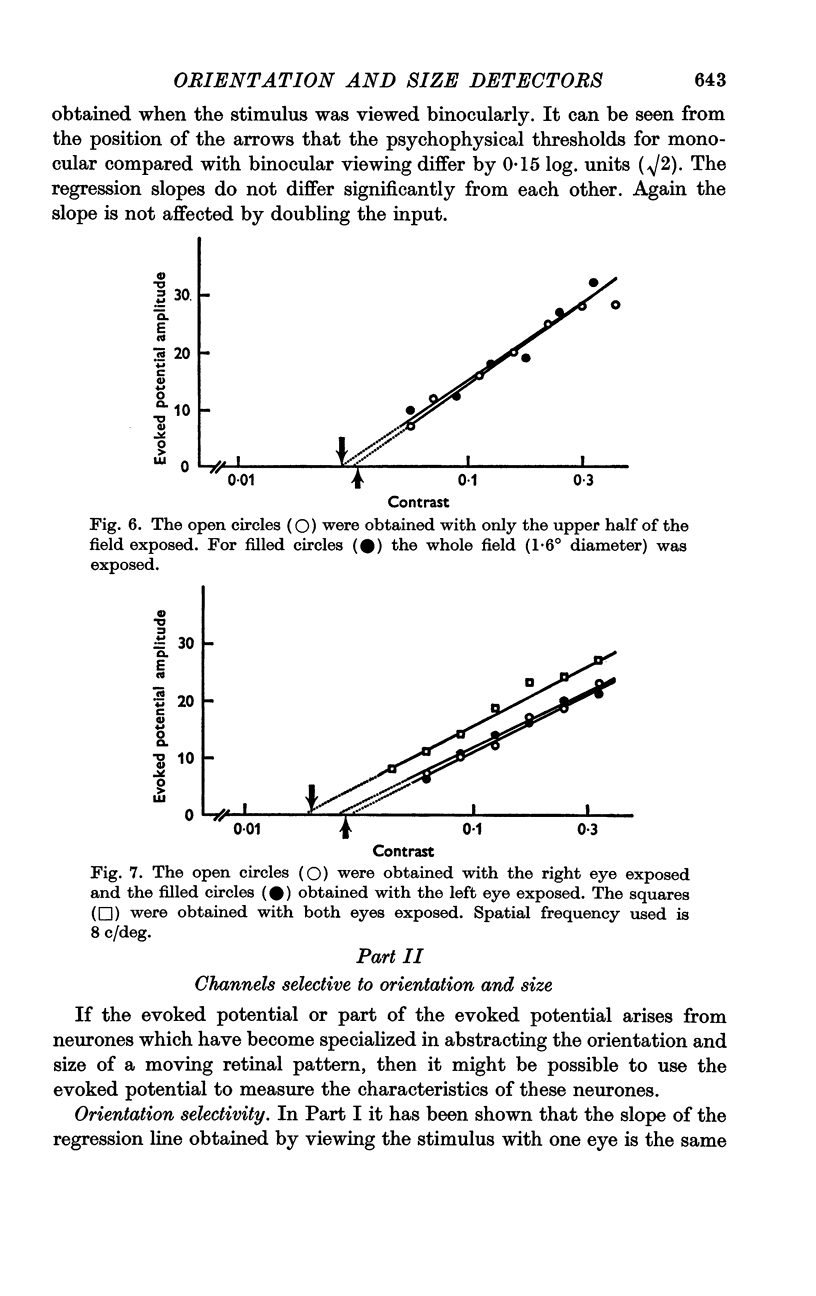
5. The slope of the regression lines was found to be almost independent of either the spatial frequency or the area of the stimulus grating.
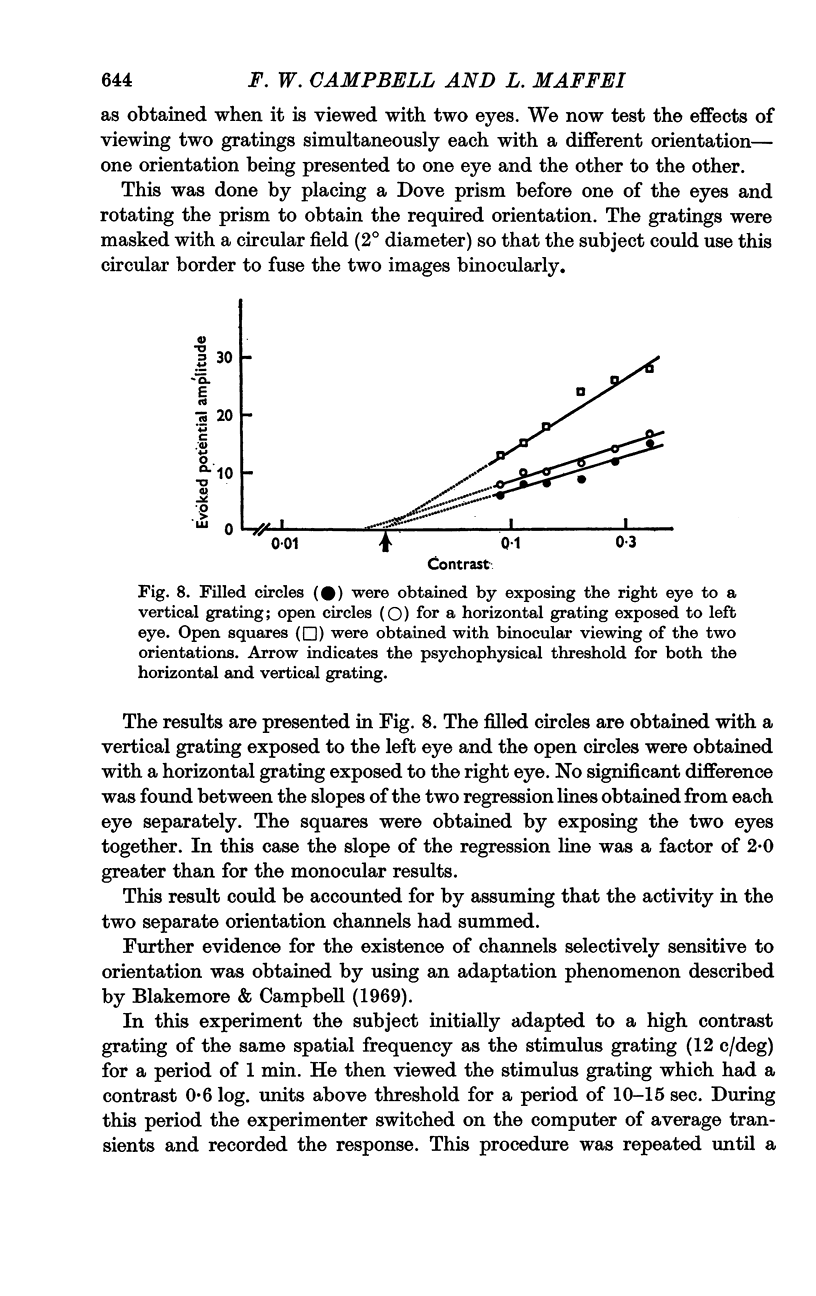
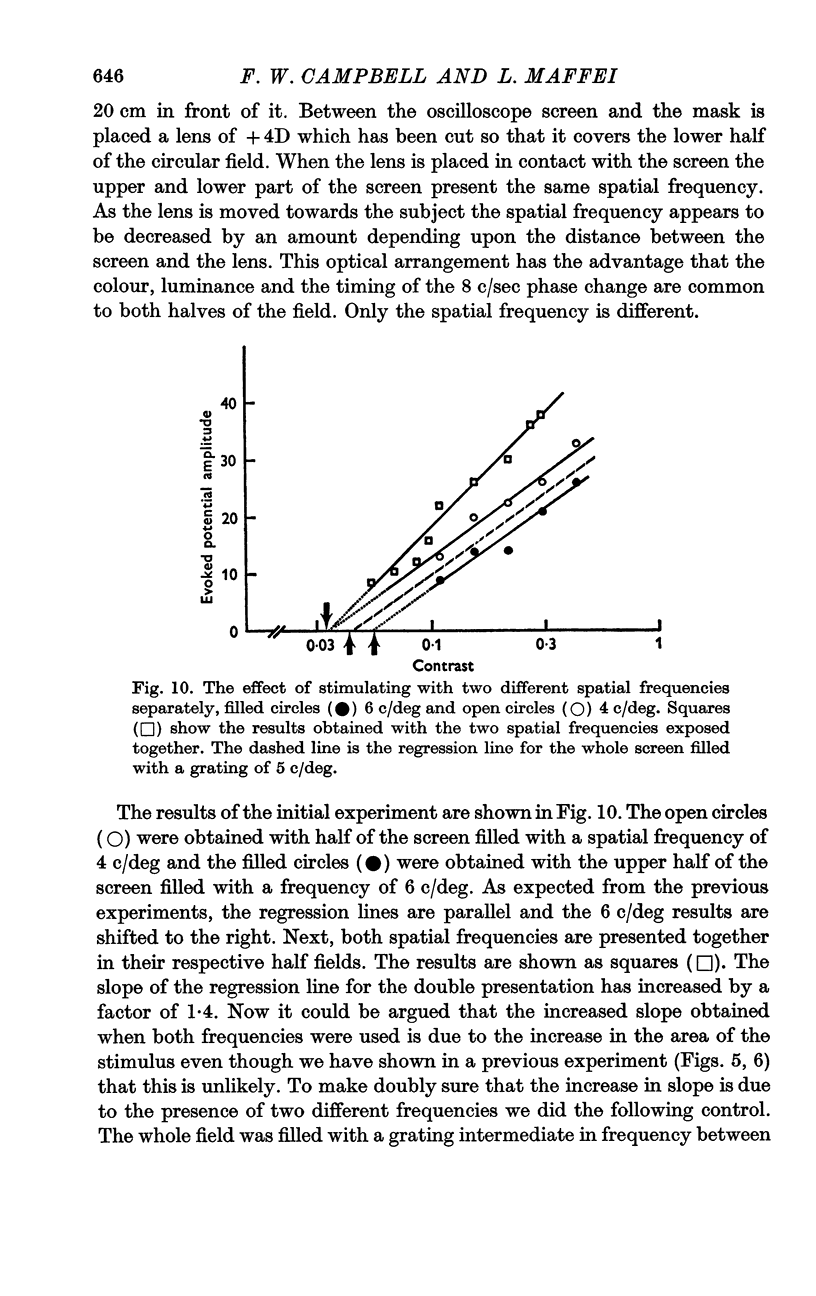
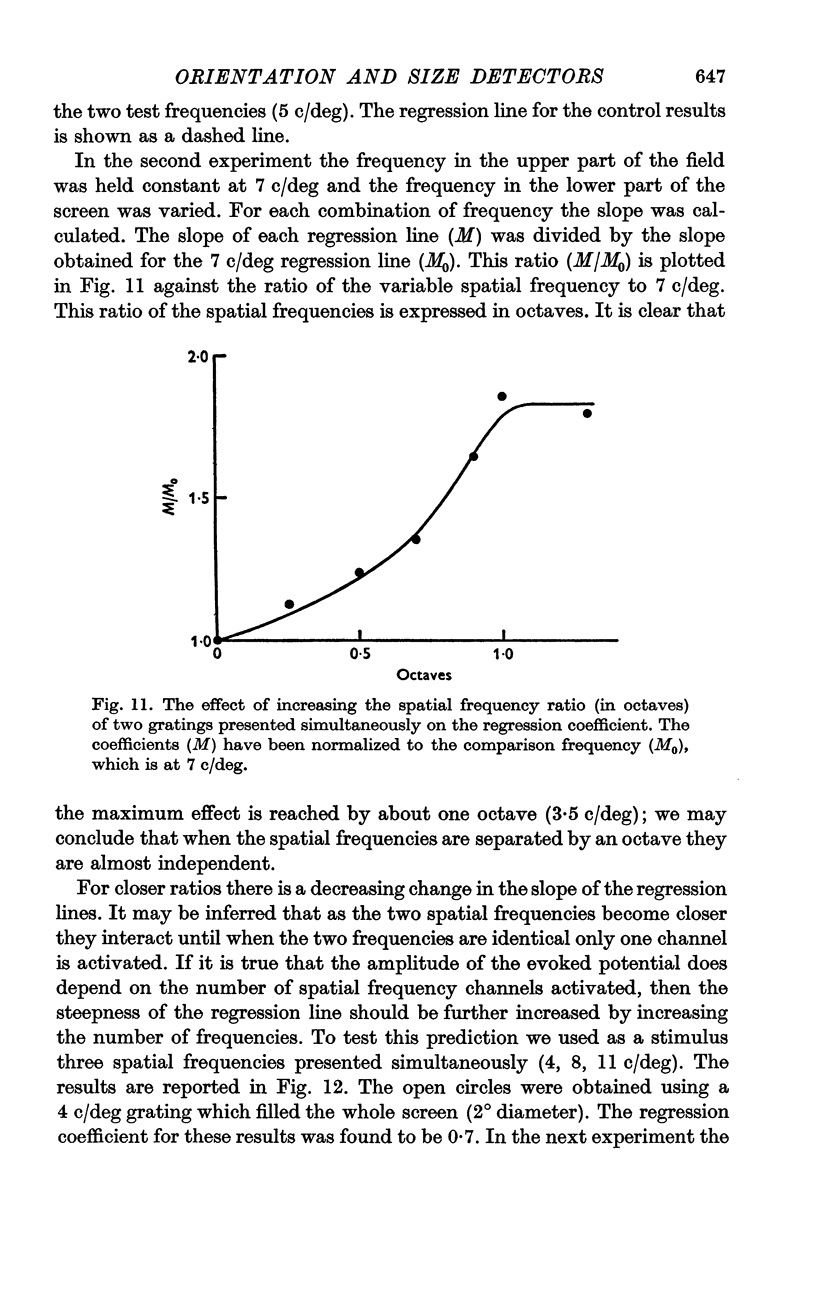
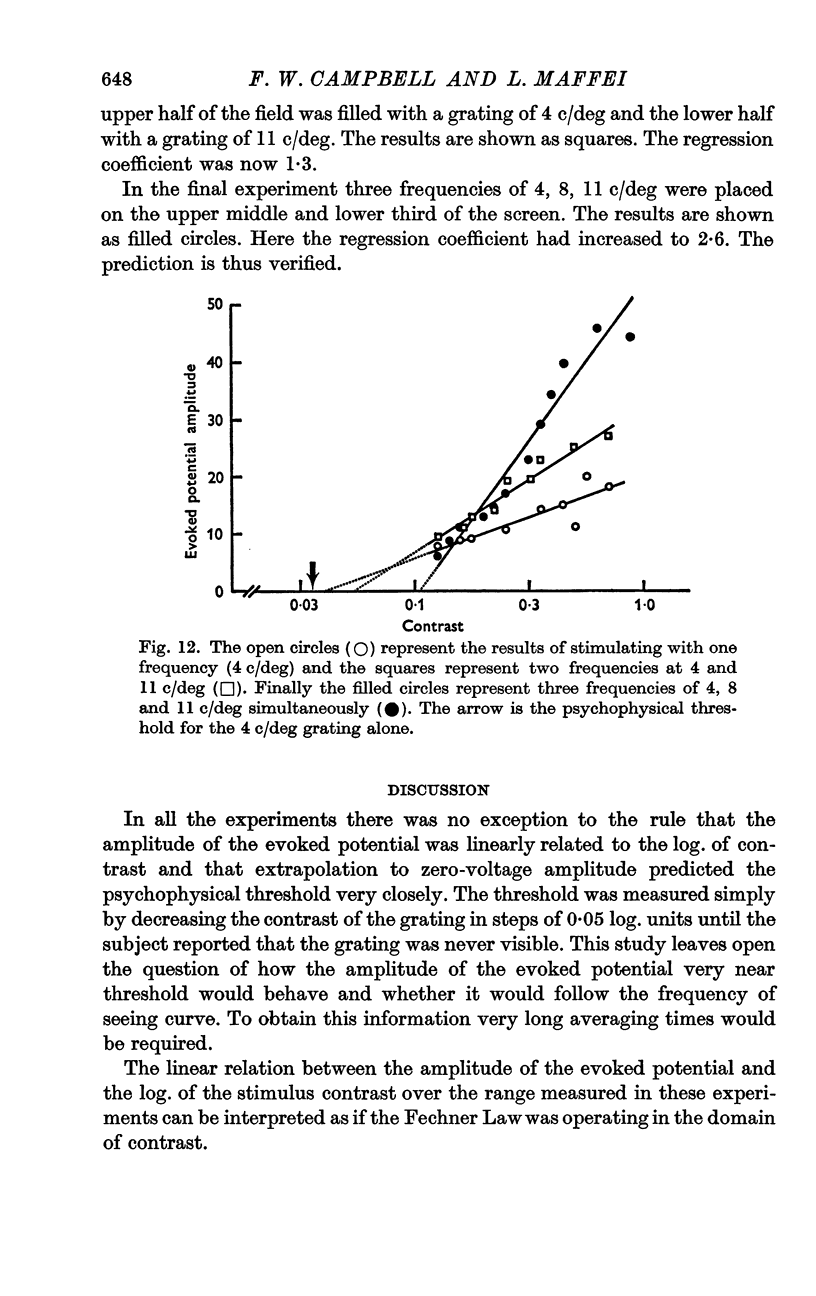
6. The slope of the regression lines could be markedly increased by using as a stimulus either two different spatial frequencies, or two different orientations, presented simultaneously.
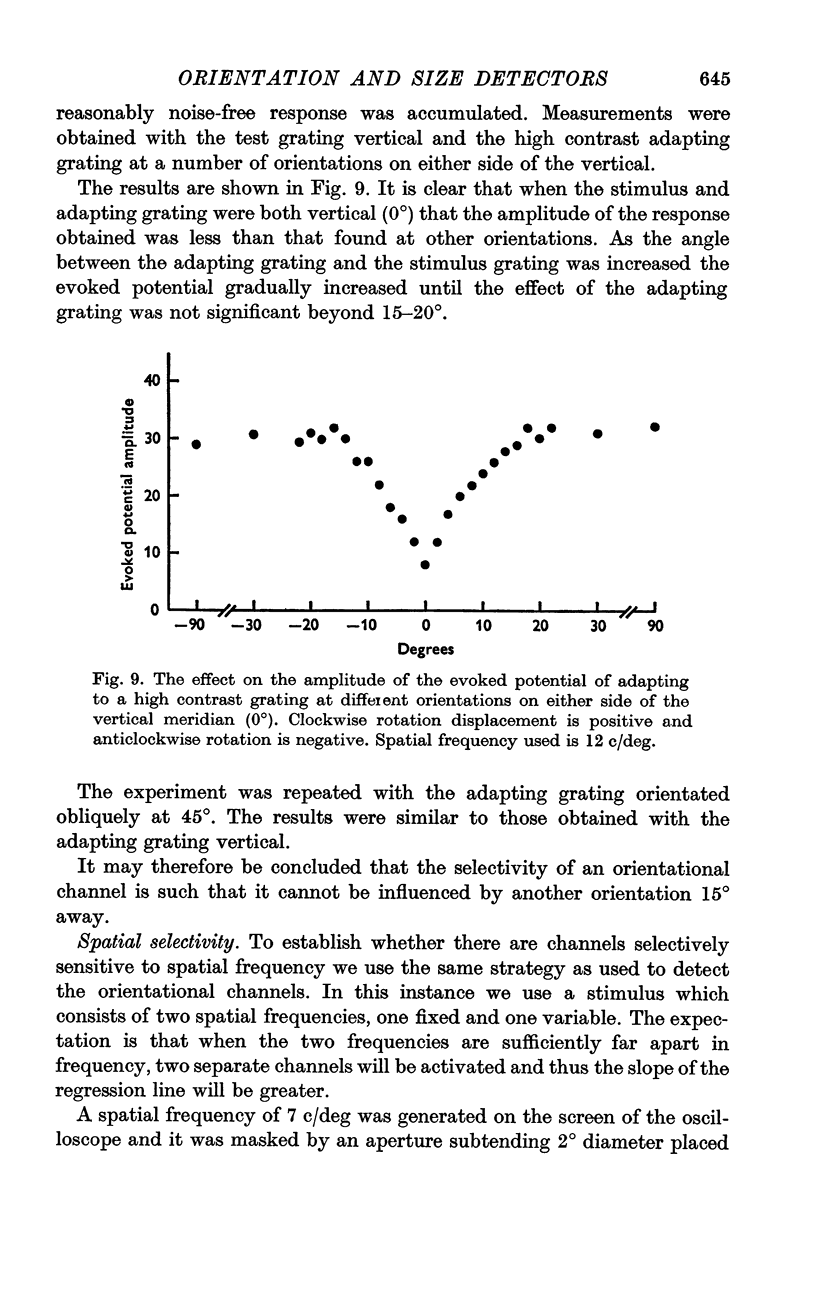
7. Using the evoked potential the selectivity to orientation was found to be so high that a channel was not influenced by another orientation 15° away.
8. The channels selectively sensitive to spatial frequency were highly selective and were not influenced by another spatial frequency one octave removed in spatial frequency.
9. It is concluded that in man there exist neurones highly selective to both orientation and spatial frequency.
Full text
PDF

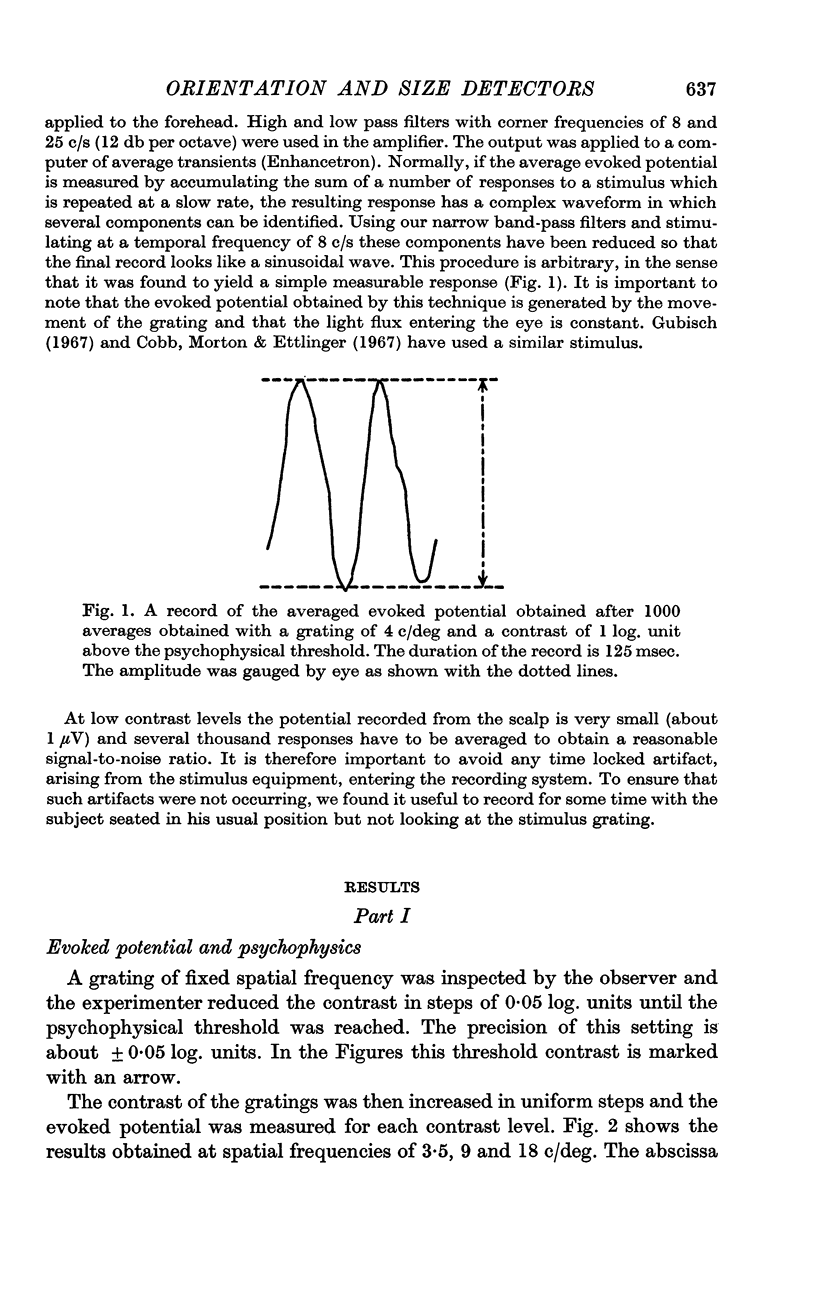





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore C., Campbell F. W. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. J Physiol. 1969 Jul;203(1):237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COWEY A. PROJECTION OF THE RETINA ON TO STRIATE AND PRESTRIATE CORTEX IN THE SQUIRREL MONKEY, SAIMIRI SCIUREUS. J Neurophysiol. 1964 May;27:366–393. doi: 10.1152/jn.1964.27.3.366. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Cleland B. G., Cooper G. F., Enroth-Cugell C. The angular selectivity of visual cortical cells to moving gratings. J Physiol. 1968 Sep;198(1):237–250. doi: 10.1113/jphysiol.1968.sp008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Cooper G. F., Enroth-Cugell C. The spatial selectivity of the visual cells of the cat. J Physiol. 1969 Jul;203(1):223–235. doi: 10.1113/jphysiol.1969.sp008861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Green D. G. Monocular versus binocular visual acuity. Nature. 1965 Oct 9;208(5006):191–192. doi: 10.1038/208191a0. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Green D. G. Optical and retinal factors affecting visual resolution. J Physiol. 1965 Dec;181(3):576–593. doi: 10.1113/jphysiol.1965.sp007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Kulikowski J. J., Levinson J. The effect of orientation on the visual resolution of gratings. J Physiol. 1966 Nov;187(2):427–436. doi: 10.1113/jphysiol.1966.sp008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Kulikowski J. J. Orientational selectivity of the human visual system. J Physiol. 1966 Nov;187(2):437–445. doi: 10.1113/jphysiol.1966.sp008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Robson J. G. Application of Fourier analysis to the visibility of gratings. J Physiol. 1968 Aug;197(3):551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb W. A., Morton H. B., Ettlinger G. Cerebral potentials evoked by pattern reversal and their suppression in visual rivalry. Nature. 1967 Dec 16;216(5120):1123–1125. doi: 10.1038/2161123b0. [DOI] [PubMed] [Google Scholar]
- DANIEL P. M., WHITTERIDGE D. The representation of the visual field on the cerebral cortex in monkeys. J Physiol. 1961 Dec;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959 Oct;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L., Campbell F. W. Neurophysiological localization of the vertical and horizontal visual coordinates in man. Science. 1970 Jan 23;167(3917):386–387. doi: 10.1126/science.167.3917.386. [DOI] [PubMed] [Google Scholar]


