Abstract
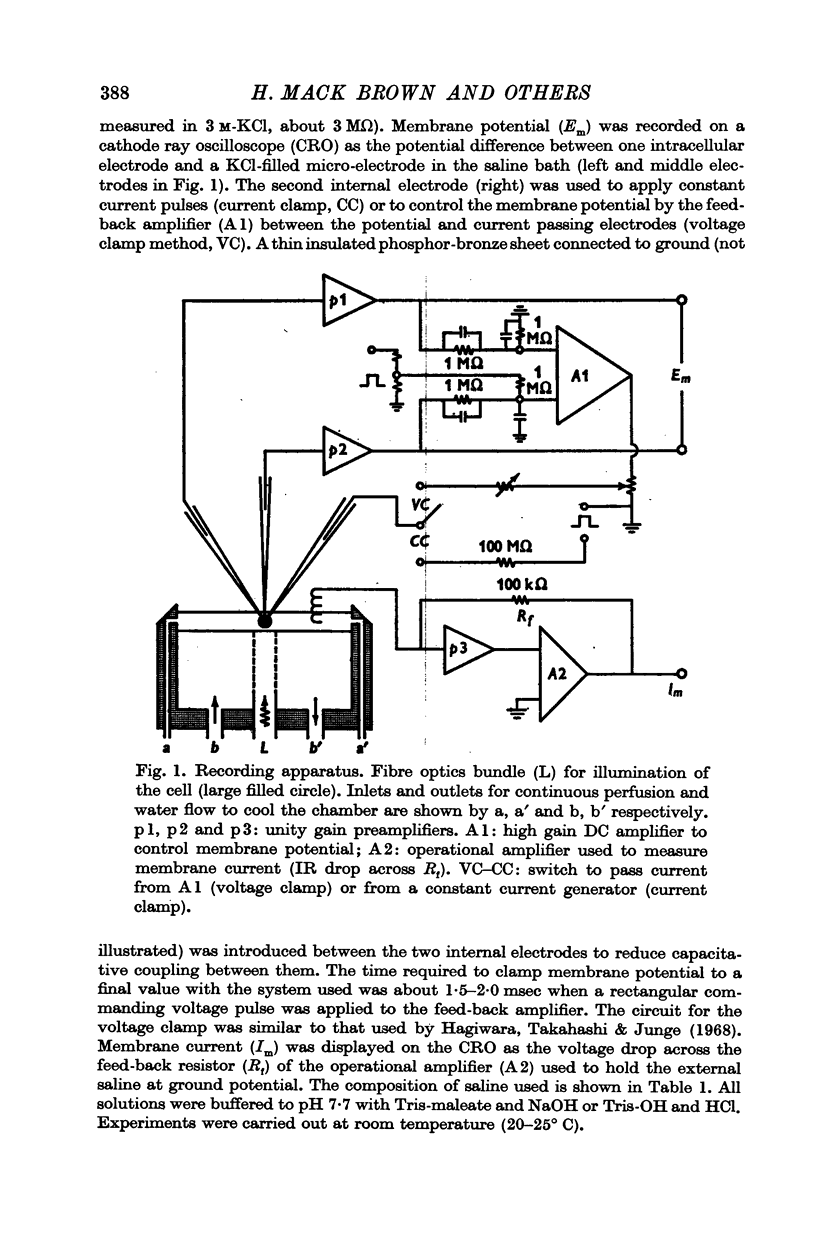
1. Electrical properties of the membrane of photoreceptor cells in the lateral ocelli of barnacles, Balanus amphitrite and B. eburneus were investigated by intracellular recording, polarization and voltage-clamp techniques.
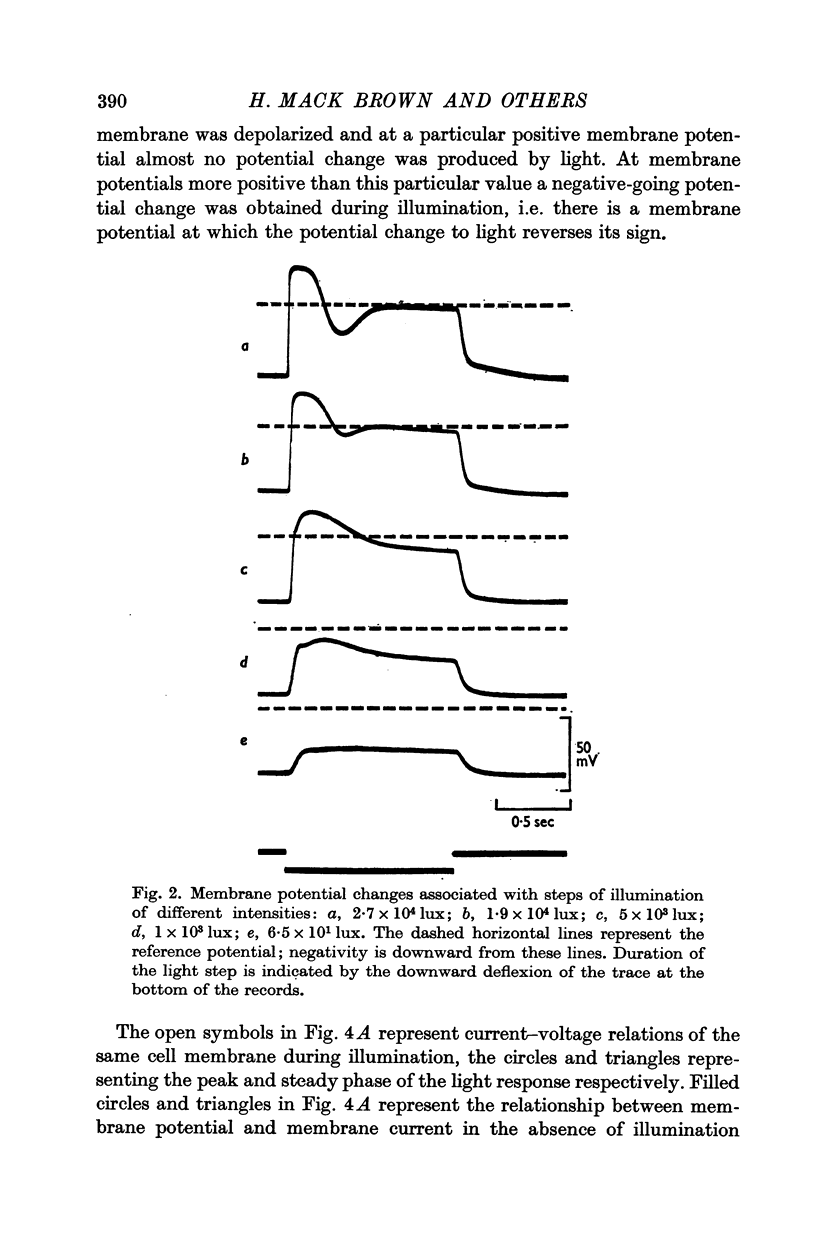
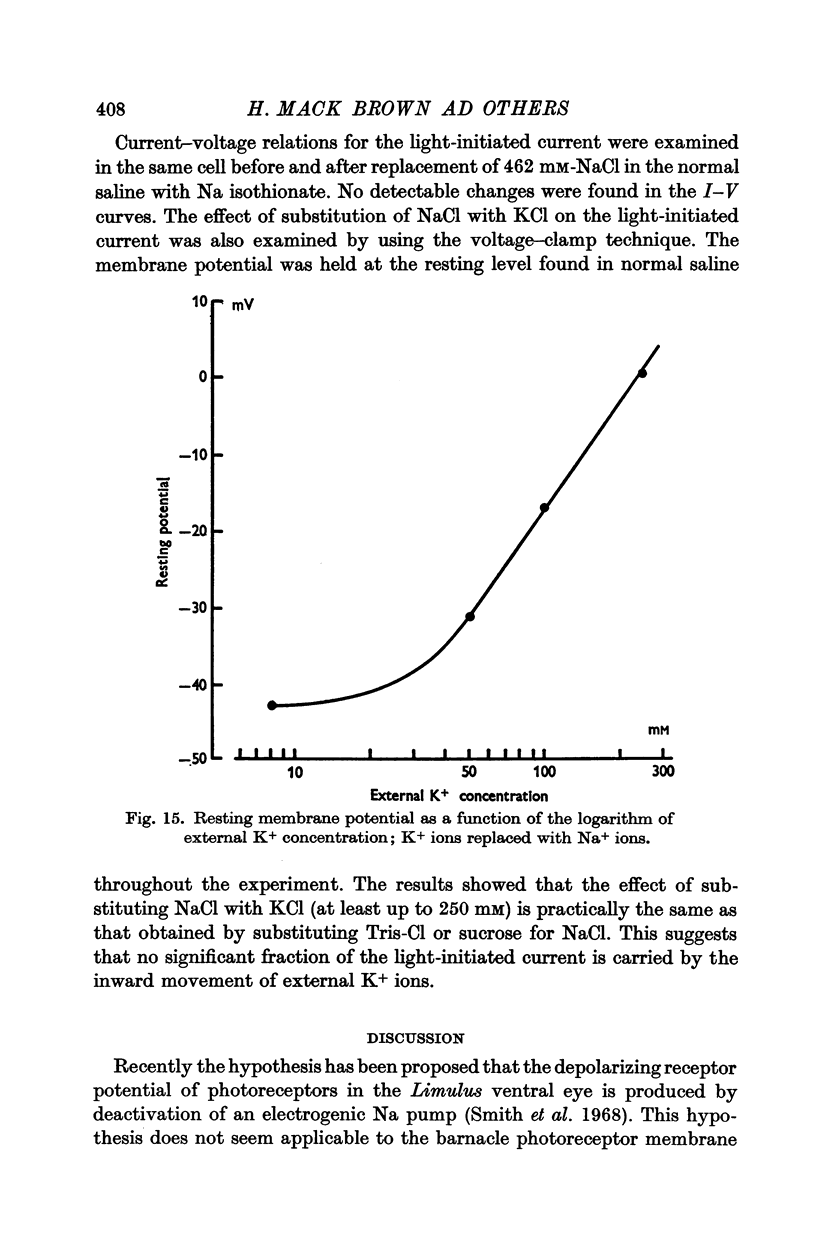
2. The resting potential of a dark adapted cell was 36·3 ± 6·6 mV (S.D.) and depended mainly on the external K+ concentration.
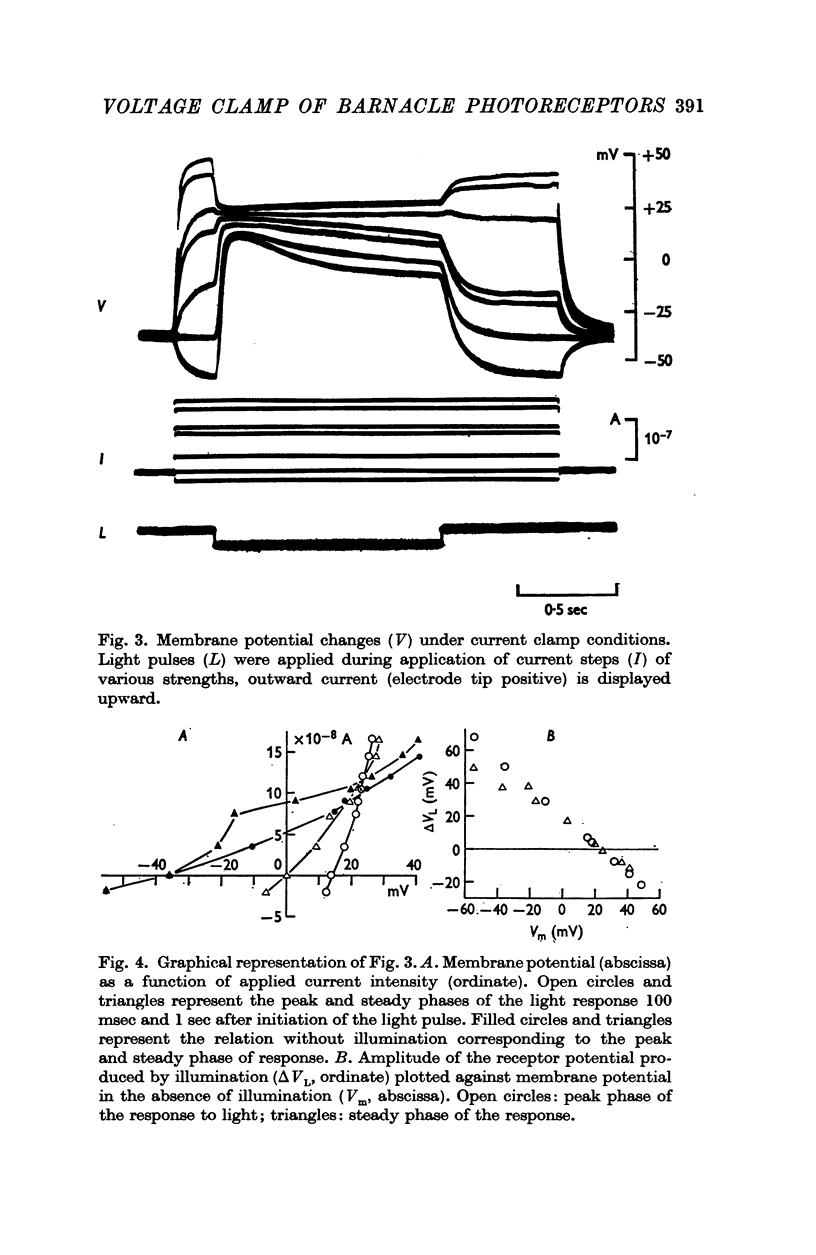
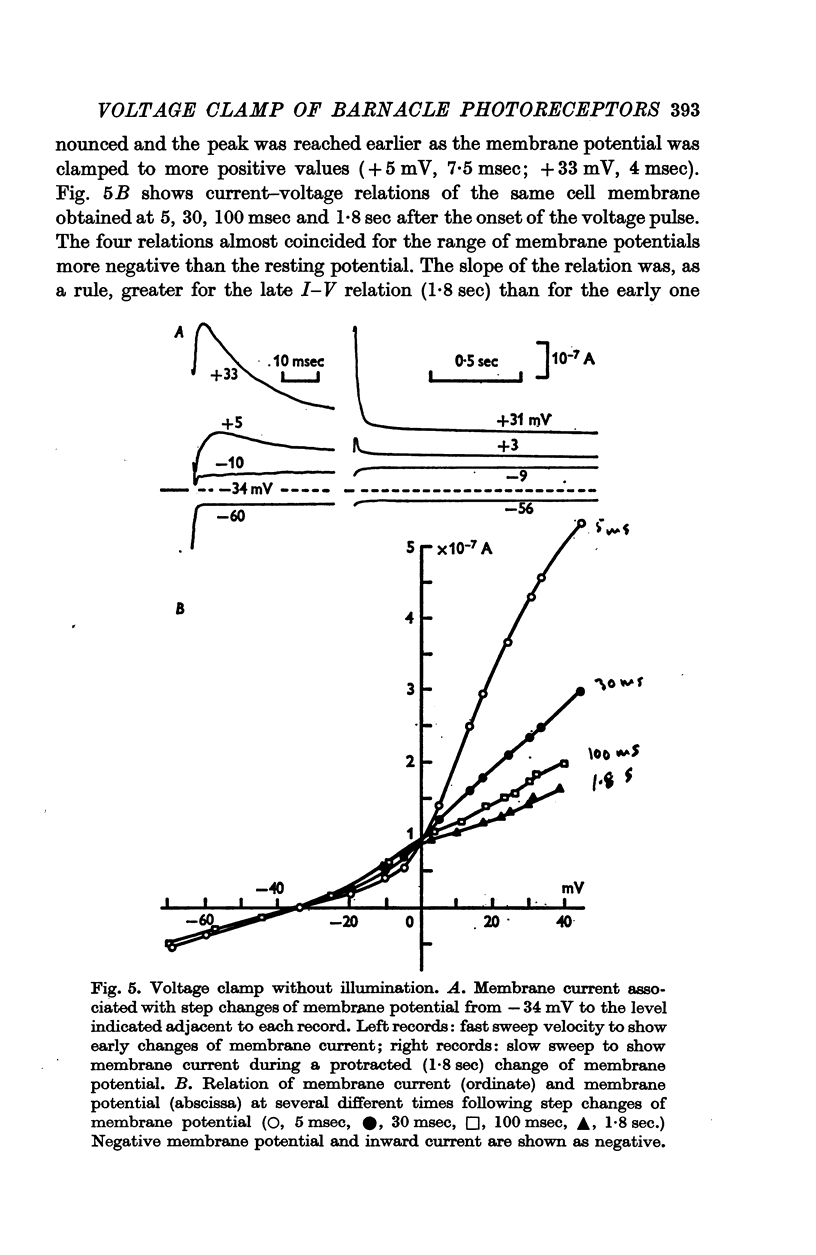
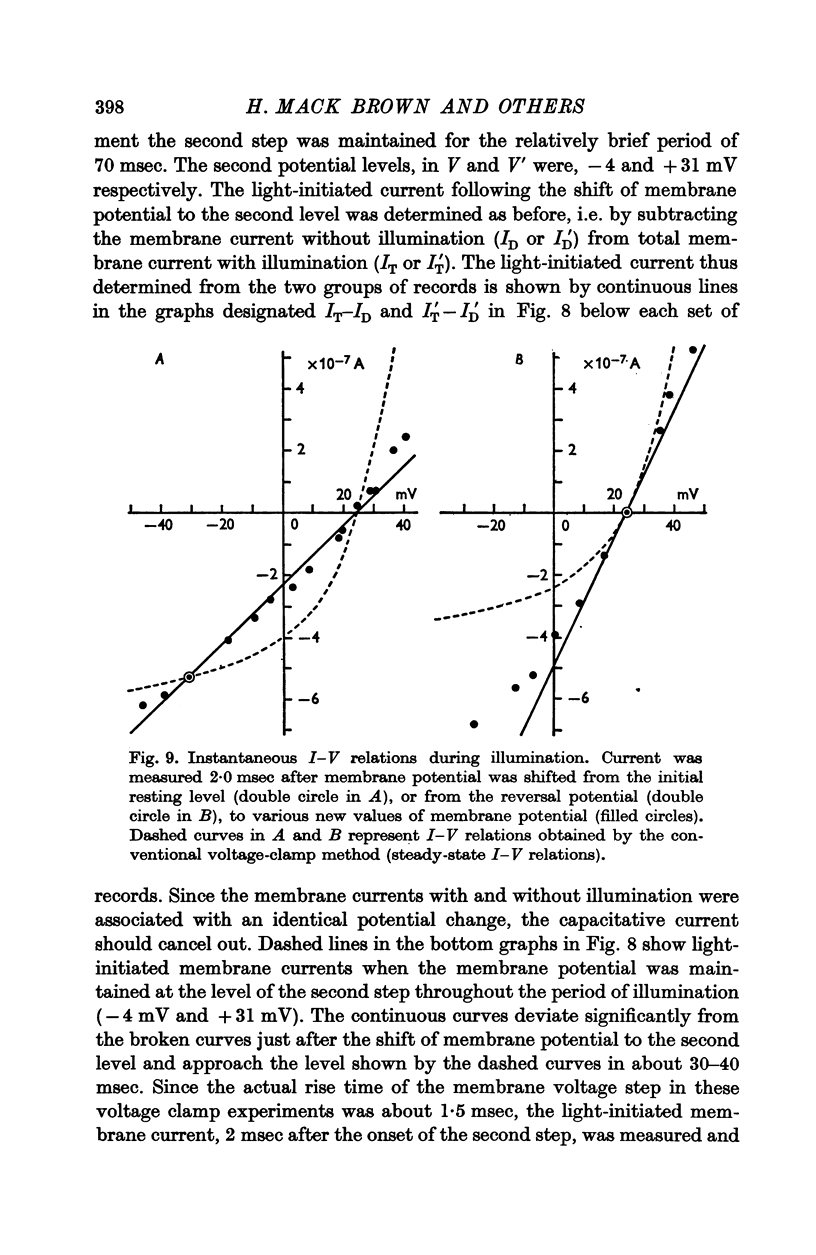
3. Current—voltage relations obtained from voltage—clamp experiments in the absence of light were non-linear and varied with time after the onset of a step change in membrane potential; the steady state was reached after about 0·5 sec.
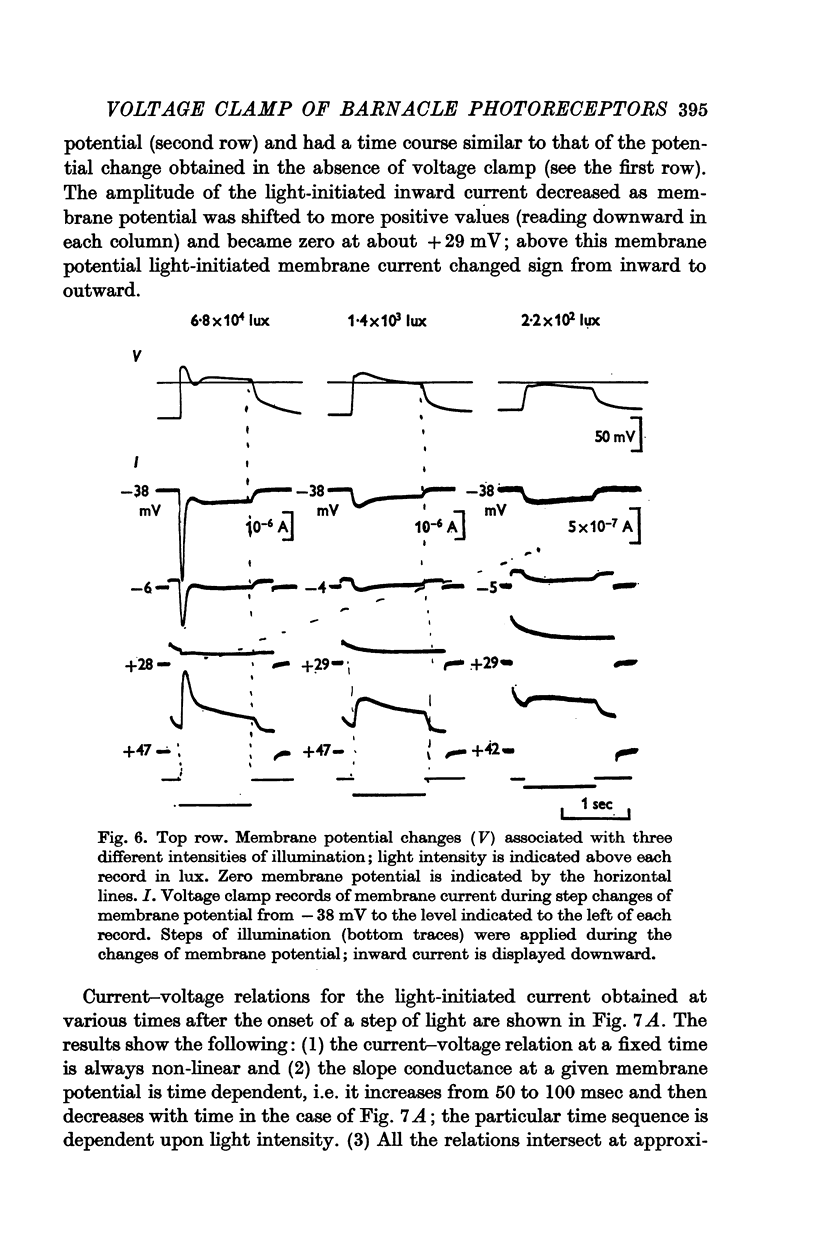
4. Illumination resulted in a membrane potential change under current clamp and in a change of membrane current (light-initiated membrane current (L.I.C.): total membrane current with illumination minus current without illumination) under voltage—clamp conditions. Amplitudes and time course of L.I.C. depended on the light intensity as well as membrane potential.
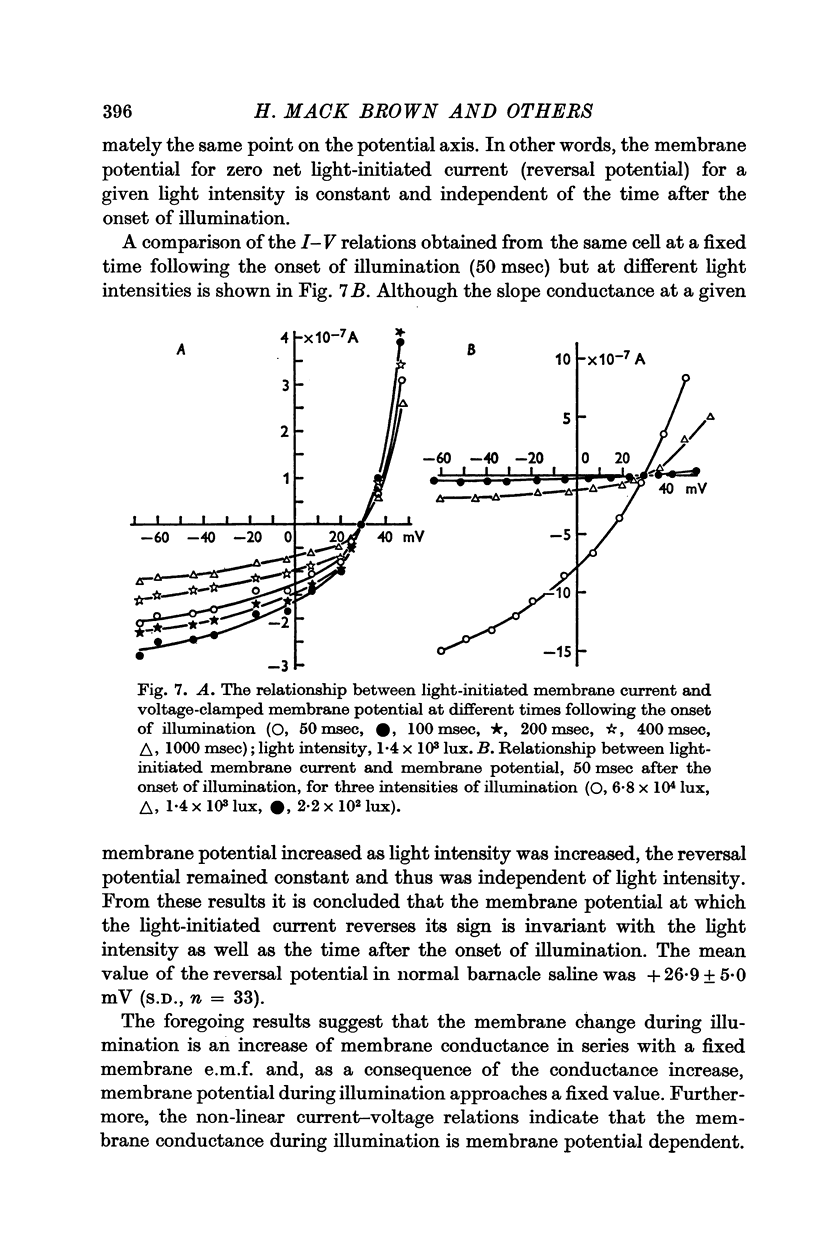
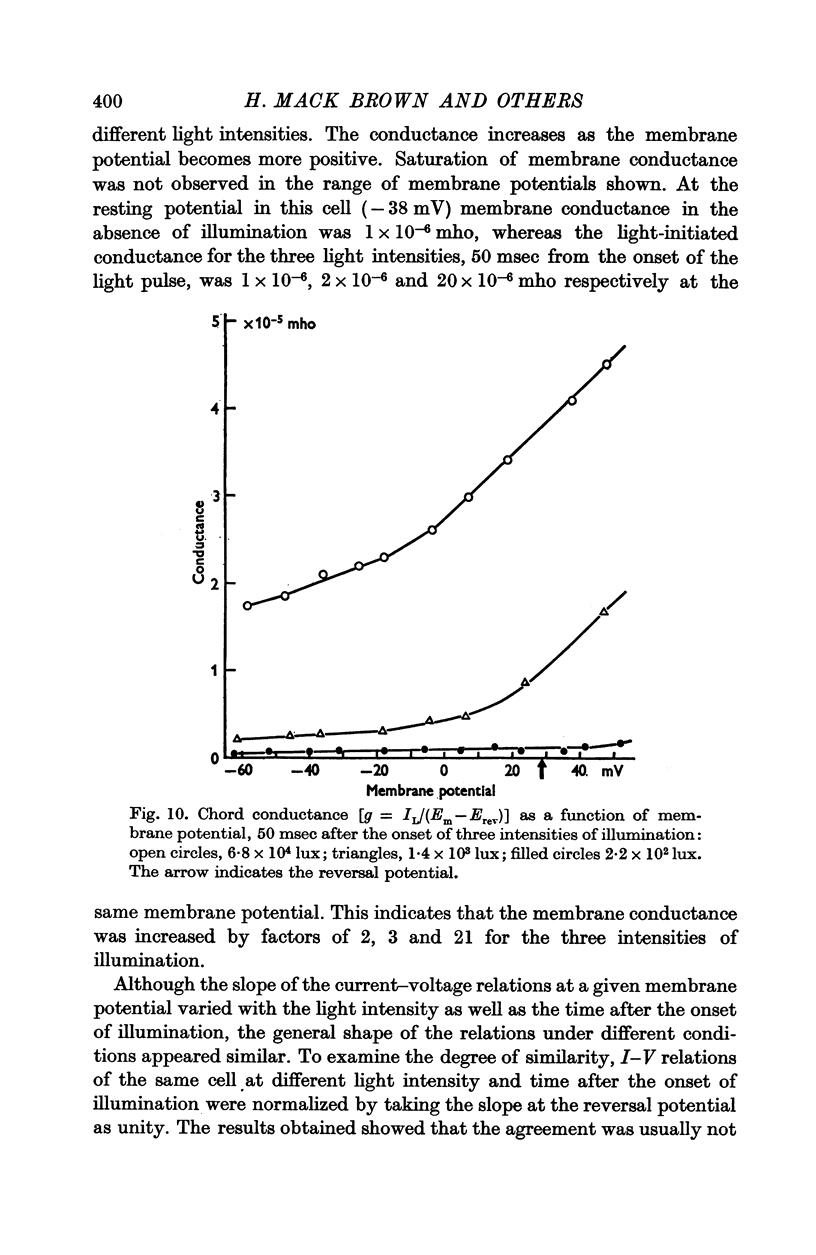
5. The L.I.C.-voltage relation was non-linear and corresponded with a slope conductance increase with increasing positive membrane potential.
6. The reversal potential of L.I.C. was independent of the light intensity and the time after onset of illumination; the average value obtained in normal saline was +26·9 ± 5·0 mV.
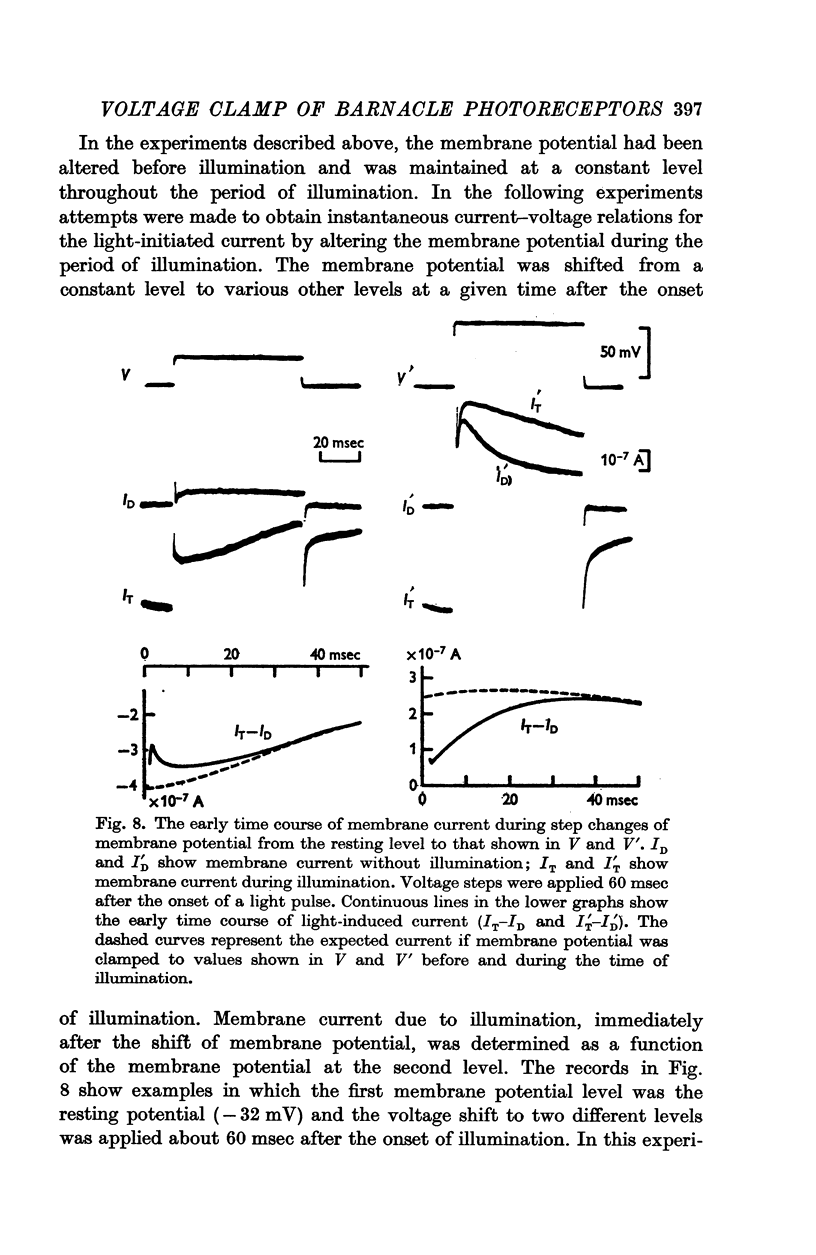
7. The membrane conductance estimated from instantaneous L.I.C.-voltage relations agreed with the chord conductance of the non-linear L.I.C.-voltage relation.
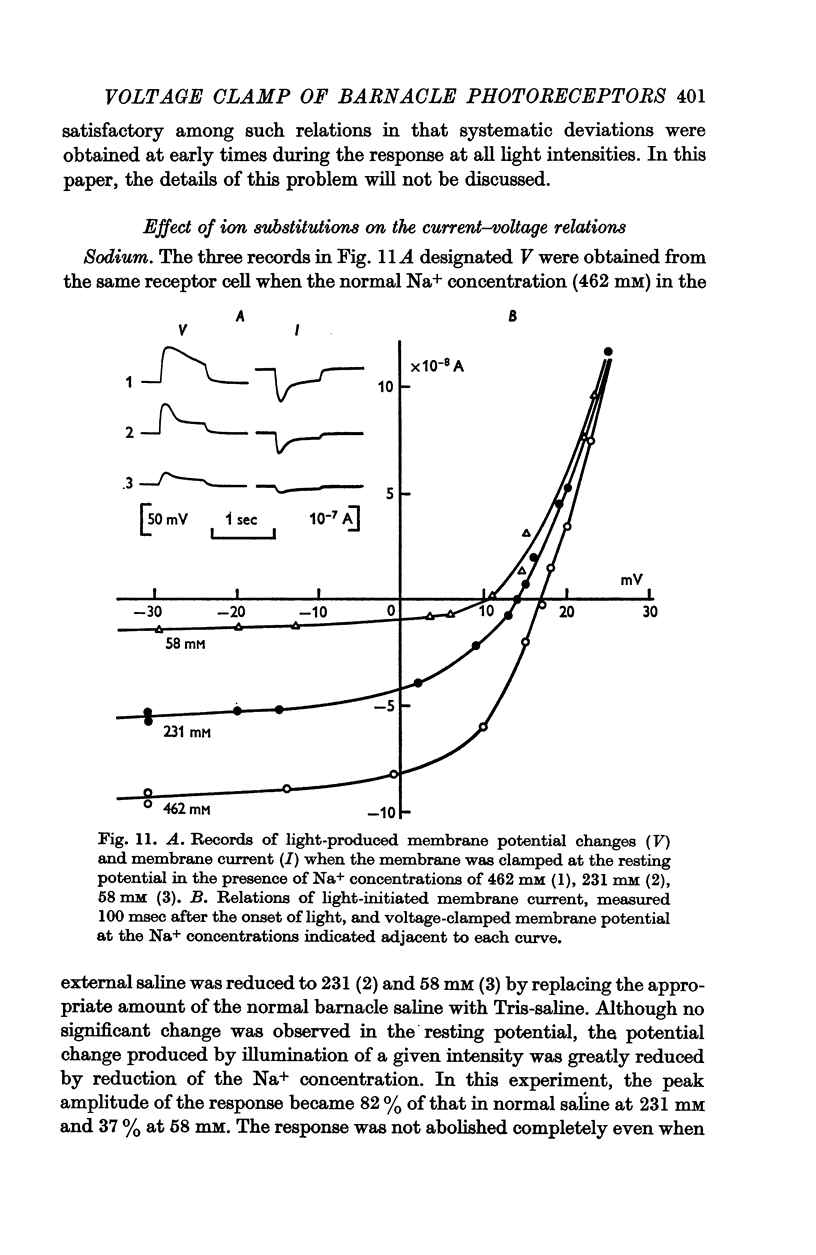
8. Decreasing external Na+ concentration decreased the inward component of L.I.C. but not the outward component.
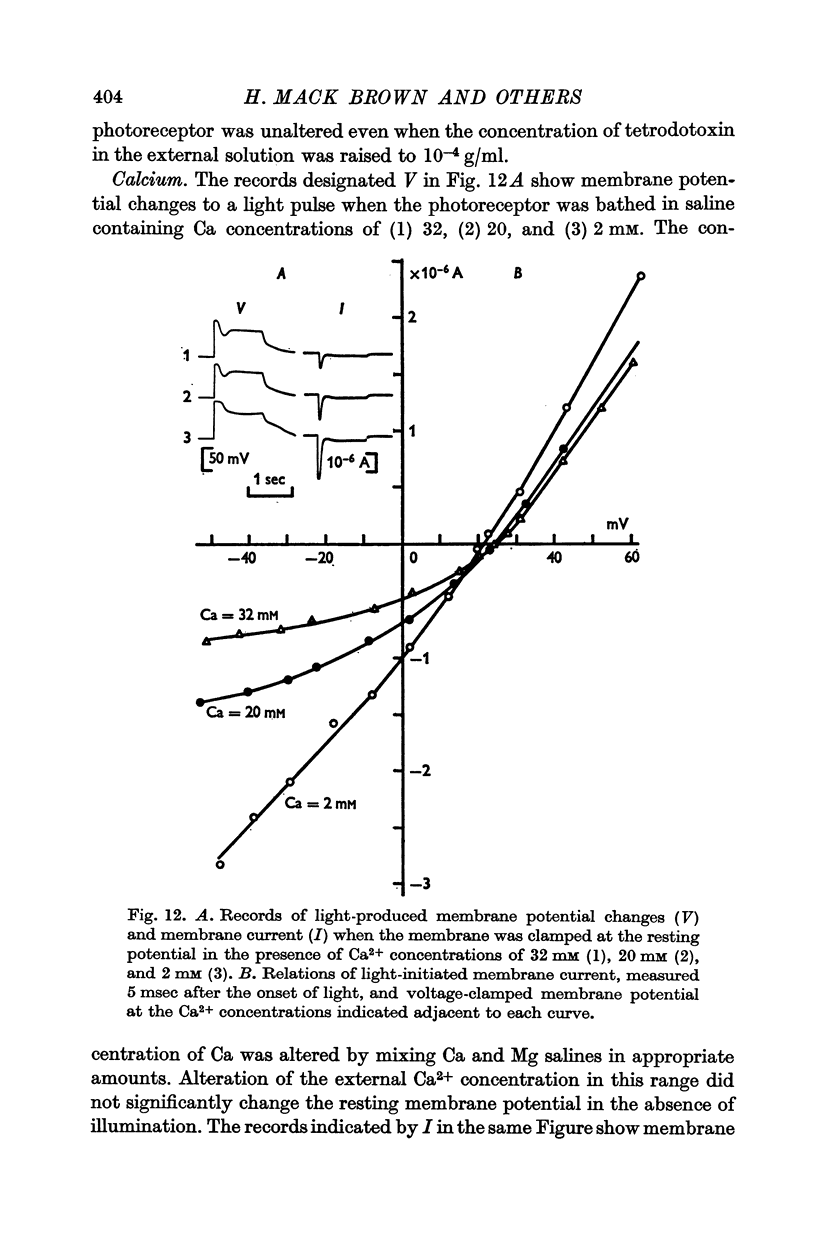
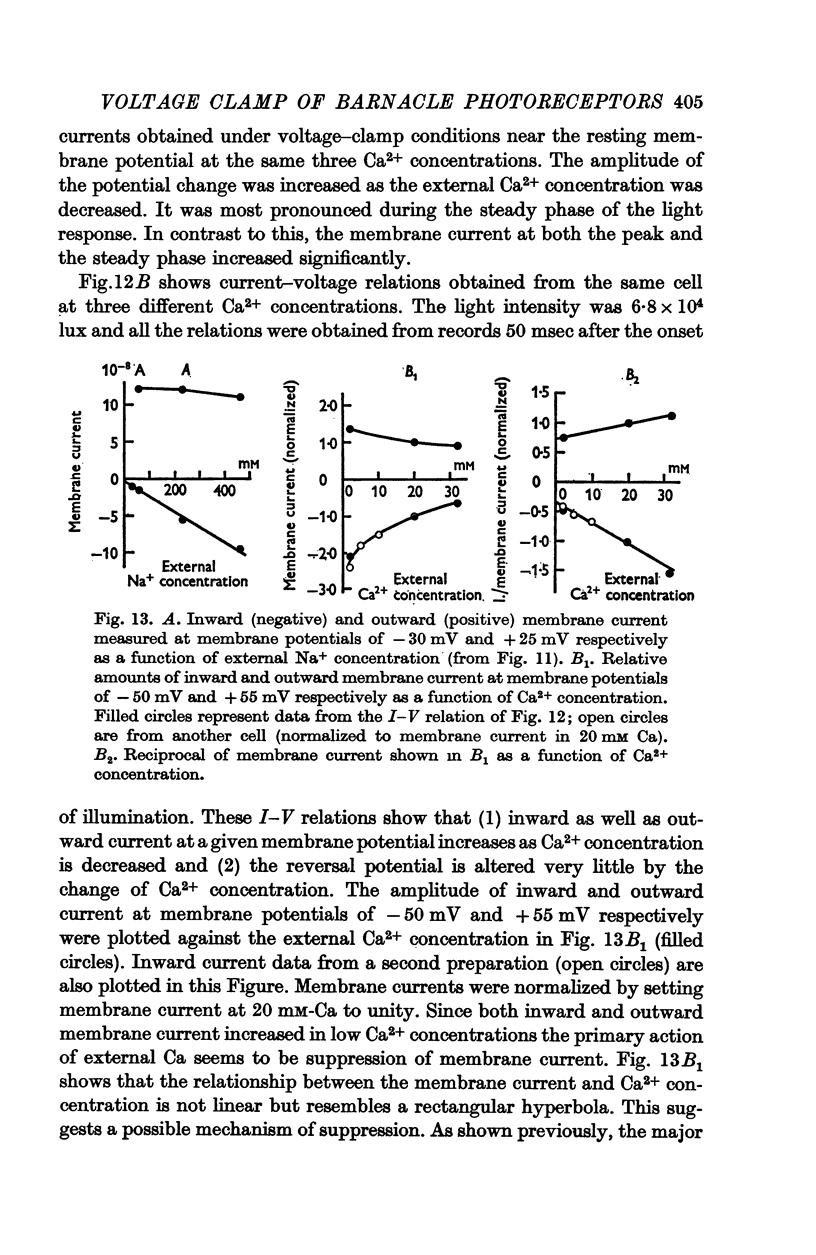
9. Decreasing external Ca2+ concentration increased the inward as well as the outward component of L.I.C.
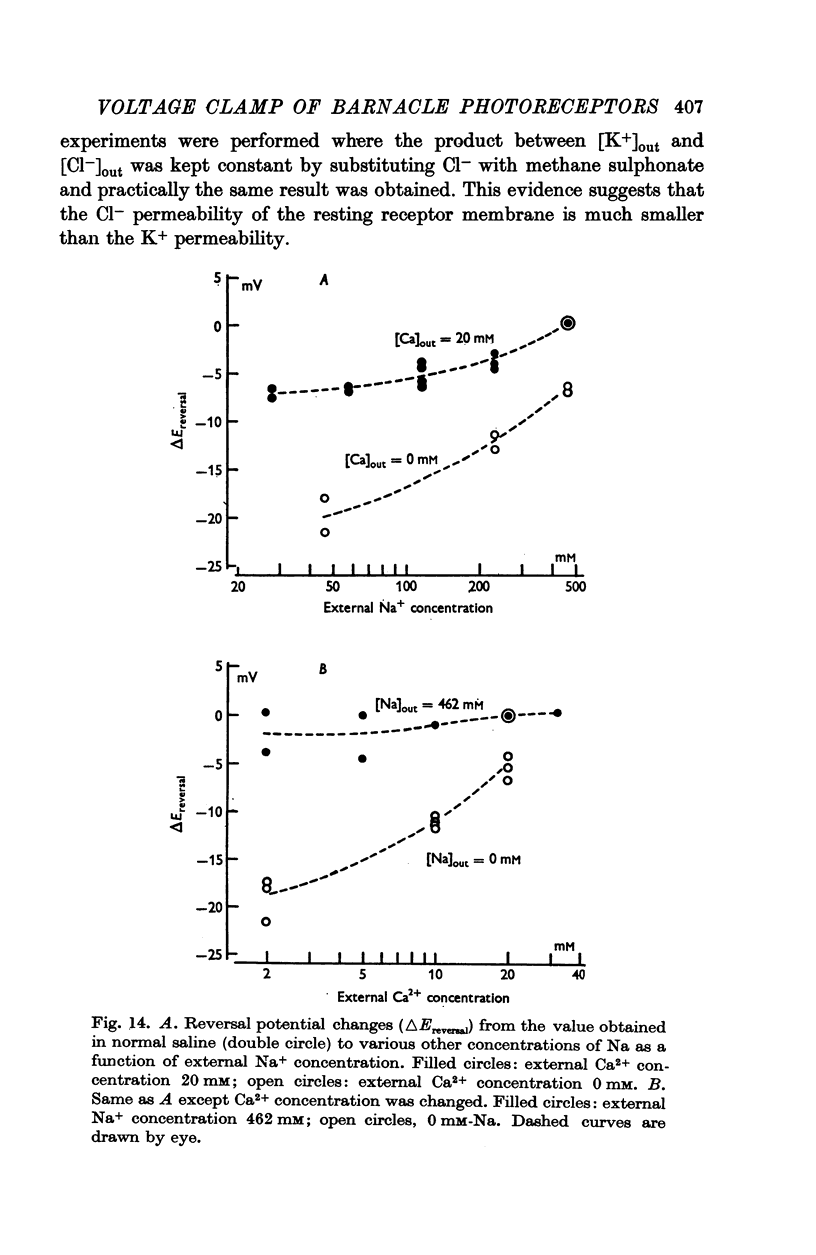
10. The reversal potential shifted in the negative direction with decreasing external Na+ concentration (the rate was 10-15 mV for a tenfold change in concentration) and the rate was augmented in the absence of Ca2+ but did not exceed 21 mV.
11. The change of reversal potential with changes of external Ca2+ concentration was negligible in normal Na+ media but was significant in the absence of Na+ (rate as high as 20 mV).
12. Alteration of the external K+ or Cl- concentrations did not affect the amplitude or reversal potential of L.I.C.
13. The results indicate that illumination increases the membrane permeability mainly to Na+ ions and that the primary effect of Ca2+ ions is suppression of the permeability increase; Ca2+ permeability may increase slightly during illumination.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown H. M., Meech R. W., Koike H., Hagiwara S. Current-voltage relations during illumination: photoreceptor membrane of a barnacle. Science. 1969 Oct 10;166(3902):240–243. doi: 10.1126/science.166.3902.240. [DOI] [PubMed] [Google Scholar]
- CALMA I. IONS AND THE RECEPTOR POTENTIAL IN THE MUSCLE SPINDLE OF THE FROG. J Physiol. 1965 Mar;177:31–41. doi: 10.1113/jphysiol.1965.sp007573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS C., TERZUOLO C. A., WASHIZU H. THE EFFECT OF CHANGES OF THE IONIC ENVIRONMENT UPON AN ISOLATED CRUSTACEAN SENSORY NEURON. J Neurophysiol. 1963 Nov;26:948–957. doi: 10.1152/jn.1963.26.6.948. [DOI] [PubMed] [Google Scholar]
- Eguchi E. Rhabdom structure and receptor potentials in single crayfish retinular cells. J Cell Physiol. 1965 Dec;66(3):411–429. doi: 10.1002/jcp.1030660314. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G. Initiation of impulses in visual cells of Limulus. J Physiol. 1959 Oct;148:14–28. doi: 10.1113/jphysiol.1959.sp006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G. VISUAL RESPONSES IN THE EYE OF THE DRAGON FLY. Science. 1963 Oct 4;142(3588):69–70. doi: 10.1126/science.142.3588.69. [DOI] [PubMed] [Google Scholar]
- Fulpius B., Baumann F. Effects of sodium, potassium, and calcium ions on slow and spike potentials in single photoreceptor cells. J Gen Physiol. 1969 May;53(5):541–561. doi: 10.1085/jgp.53.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D., Junge D. Sodium and calcium components of action potentials in the Aplysia giant neurone. J Physiol. 1968 Dec;199(2):347–365. doi: 10.1113/jphysiol.1968.sp008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMASAKI D. I. The effect of sodium ion concentration on the electroretinogram of the isolated retina of the frog. J Physiol. 1963 Jun;167:156–168. doi: 10.1113/jphysiol.1963.sp007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLINE H. K., WAGNER H. G., MACNICHOL E. F., Jr The peripheral origin of nervous activity in the visual system. Cold Spring Harb Symp Quant Biol. 1952;17:125–141. doi: 10.1101/sqb.1952.017.01.013. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K., Junge D. Excitation-contraction coupling in a barnacle muscle fiber as examined with voltage clamp technique. J Gen Physiol. 1968 Feb;51(2):157–175. doi: 10.1085/jgp.51.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge D. Multi-ionic action potentials in molluscan giant neurones. Nature. 1967 Jul 29;215(5100):546–548. doi: 10.1038/215546a0. [DOI] [PubMed] [Google Scholar]
- KIKUCHI R., NAITO K., TANAKA I. Effect of sodium and potassium ions on the electrical activity of single cells in the lateral eye of the horseshoe crab. J Physiol. 1962 May;161:319–343. doi: 10.1113/jphysiol.1962.sp006889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecchia R., Bradbury J., Mauro A. Simple photoreceptors in Limulus polyphemus. Science. 1966 Dec 2;154(3753):1199–1201. doi: 10.1126/science.154.3753.1199. [DOI] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. 3. A voltage-clamp study. J Gen Physiol. 1969 Sep;54(3):331–351. doi: 10.1085/jgp.54.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKA K. I., EGUCHI E. Spike potentials recorded from the insect photoreceptor. J Gen Physiol. 1962 Mar;45:663–680. doi: 10.1085/jgp.45.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKA K. I. Recording of retinal action potentials from single cells in the insect compound eye. J Gen Physiol. 1961 Jan;44:571–584. doi: 10.1085/jgp.44.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., DEGUCHI T., URAKAWA N., OHKUBO Y. Stabilization and rectification of muscle fiber membrane by tetrodotoxin. Am J Physiol. 1960 May;198:934–938. doi: 10.1152/ajplegacy.1960.198.5.934. [DOI] [PubMed] [Google Scholar]
- OTTOSON D. THE EFFECT OF SODIUM DEFICIENCY ON THE RESPONSE OF THE ISOLATED MUSCLE SPINDLE. J Physiol. 1964 May;171:109–118. doi: 10.1113/jphysiol.1964.sp007365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara S. Effects of some organic cations on generator potential of crayfish stretch receptor. J Gen Physiol. 1968 Aug;52(2):363–386. doi: 10.1085/jgp.52.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara S., Grundfest H. Effects of lithium on different membrane components of crayfish stretch receptor neurons. J Gen Physiol. 1968 May;51(5):635–654. doi: 10.1085/jgp.51.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. A theoretical treatment of Fuortes's observations upon eccentric cell activity in Limulus. J Physiol. 1959 Oct;148:29–38. doi: 10.1113/jphysiol.1959.sp006271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. G., Stell W. K., Brown J. E. Conductance changes associated with receptor potentials in Limulus photoreceptors. Science. 1968 Oct 25;162(3852):454–456. doi: 10.1126/science.162.3852.454. [DOI] [PubMed] [Google Scholar]
- Smith T. G., Stell W. K., Brown J. E., Freeman J. A., Murray G. C. A role for the sodium pump in photoreception in Limulus. Science. 1968 Oct 25;162(3852):456–458. doi: 10.1126/science.162.3852.456. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI N. Effects of calcium on the conductance change of the end-plate membrane during the action of transmitter. J Physiol. 1963 Jun;167:141–155. doi: 10.1113/jphysiol.1963.sp007137. [DOI] [PMC free article] [PubMed] [Google Scholar]


