Abstract
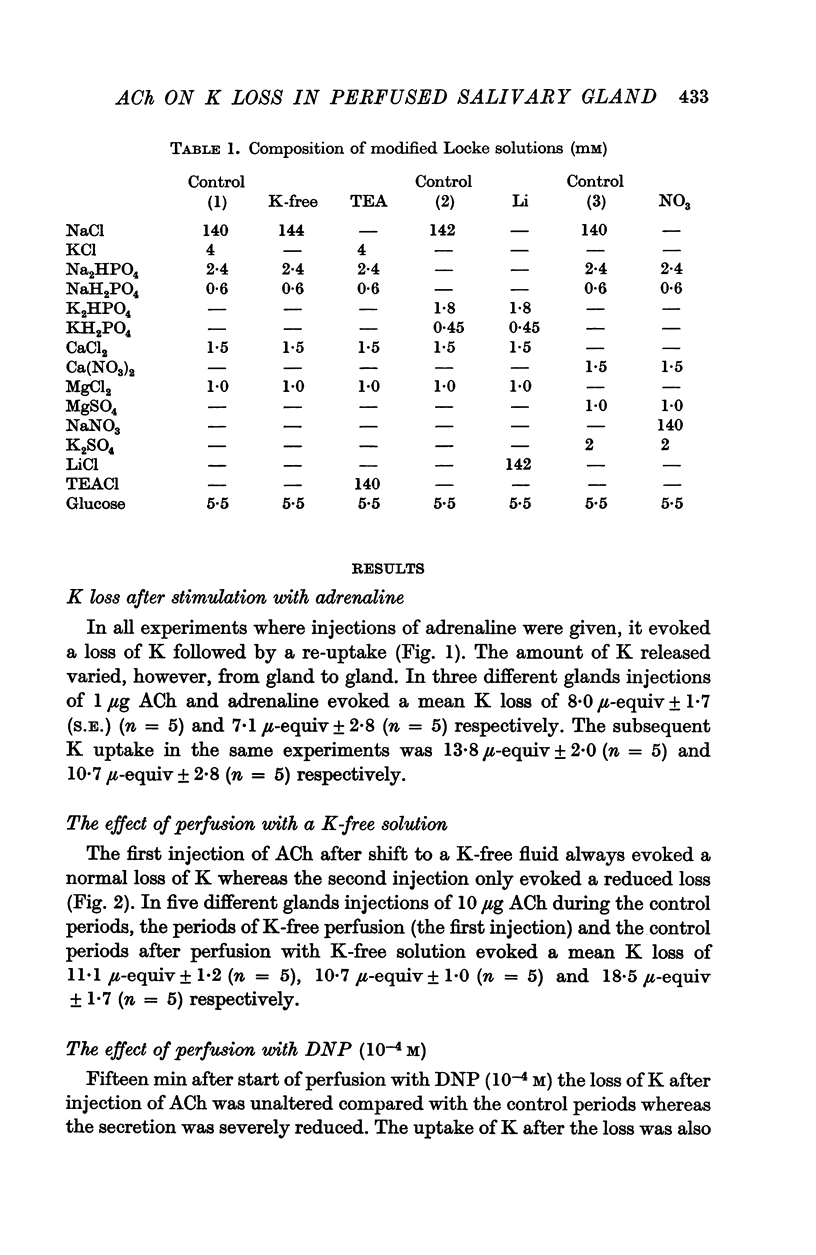
1. The release of K from the cat submandibular gland to the extracellular fluid (ECF) after stimulation with acetylcholine (ACh) and the subsequent uptake of K from the ECF was studied in glands perfused artificially with Locke solutions.
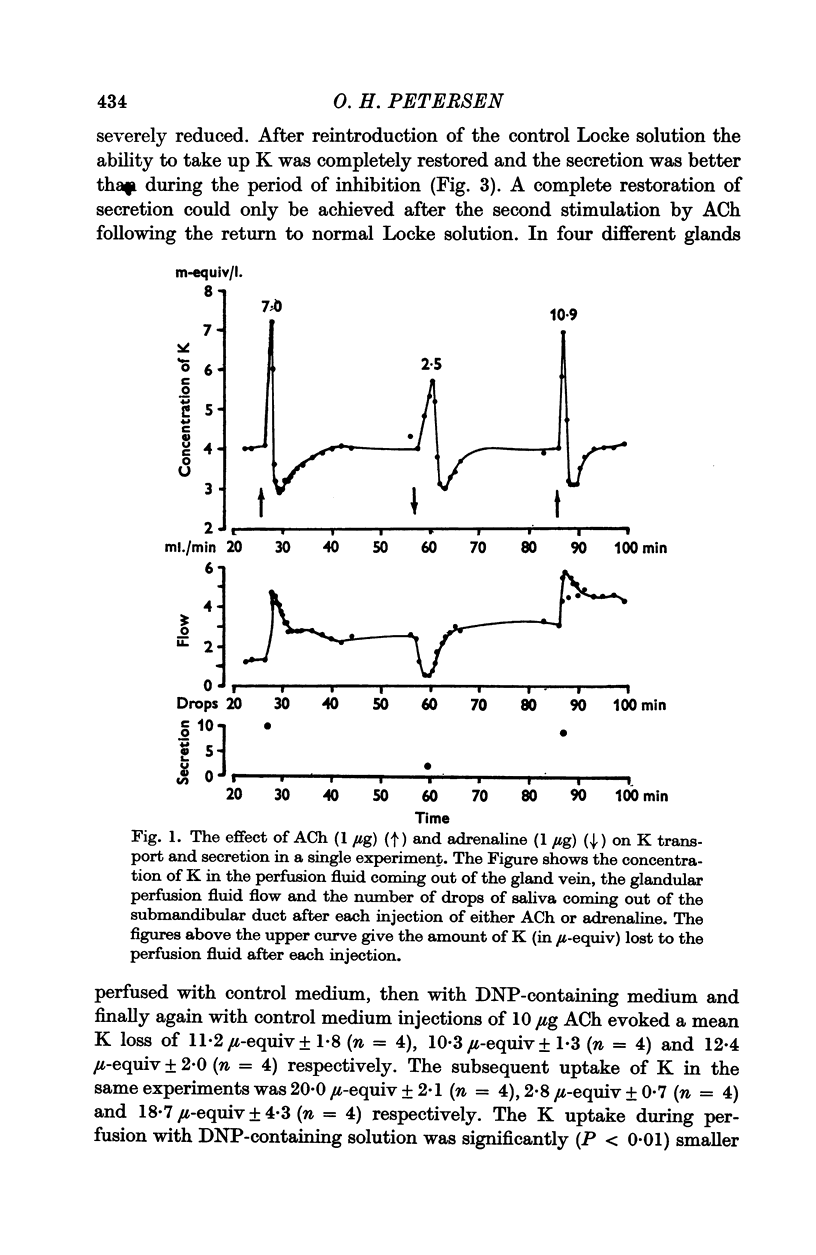
2. The first injection of ACh after shift of the perfusion fluid from control to K-free Locke solution evoked a normal loss of K and a normal secretion of saliva. The second injection only evoked a small release of K and a reduced secretion.
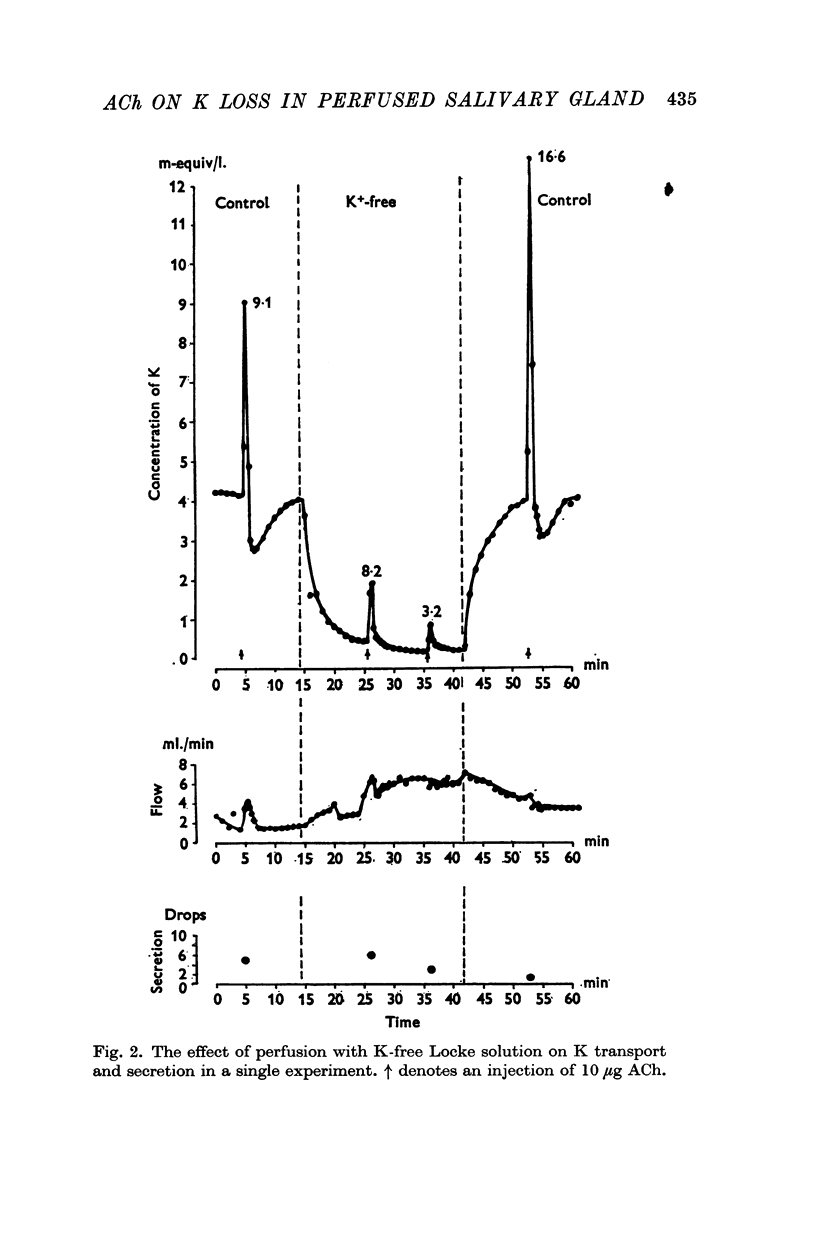
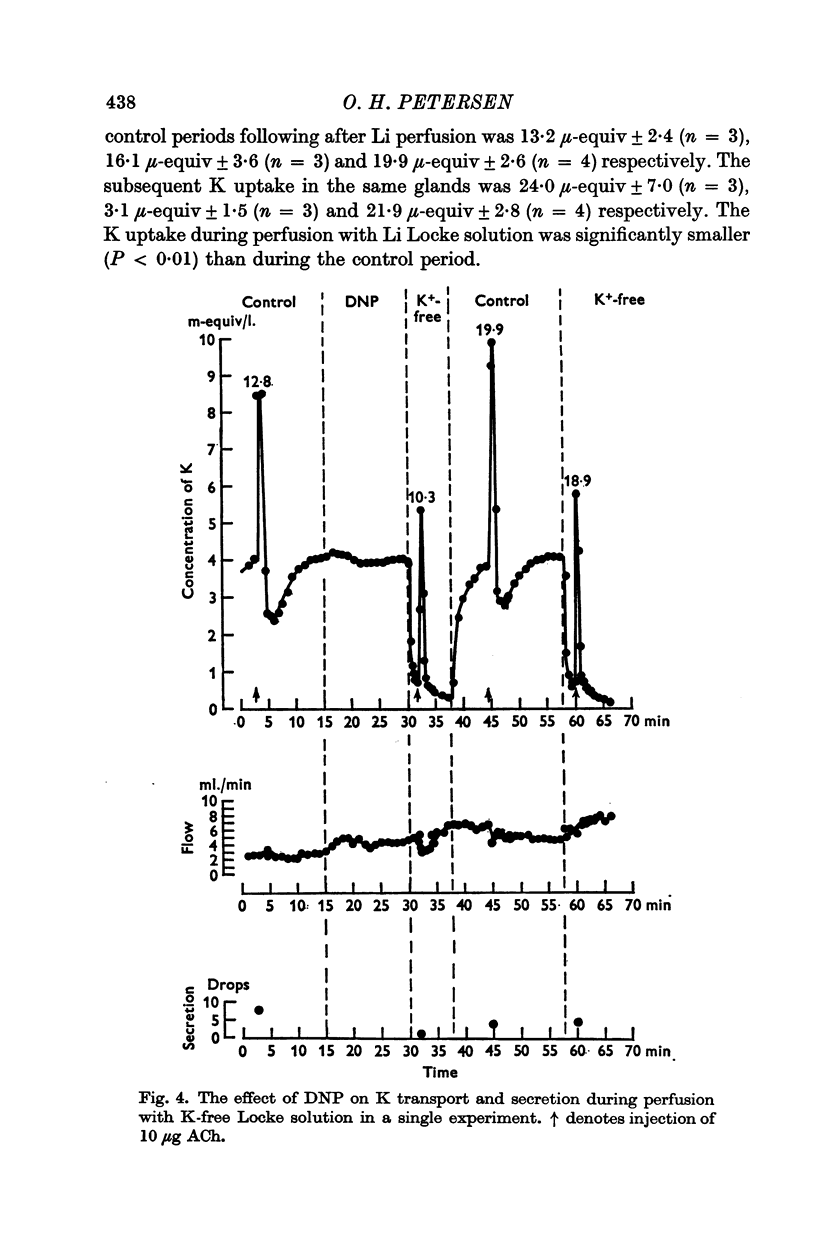
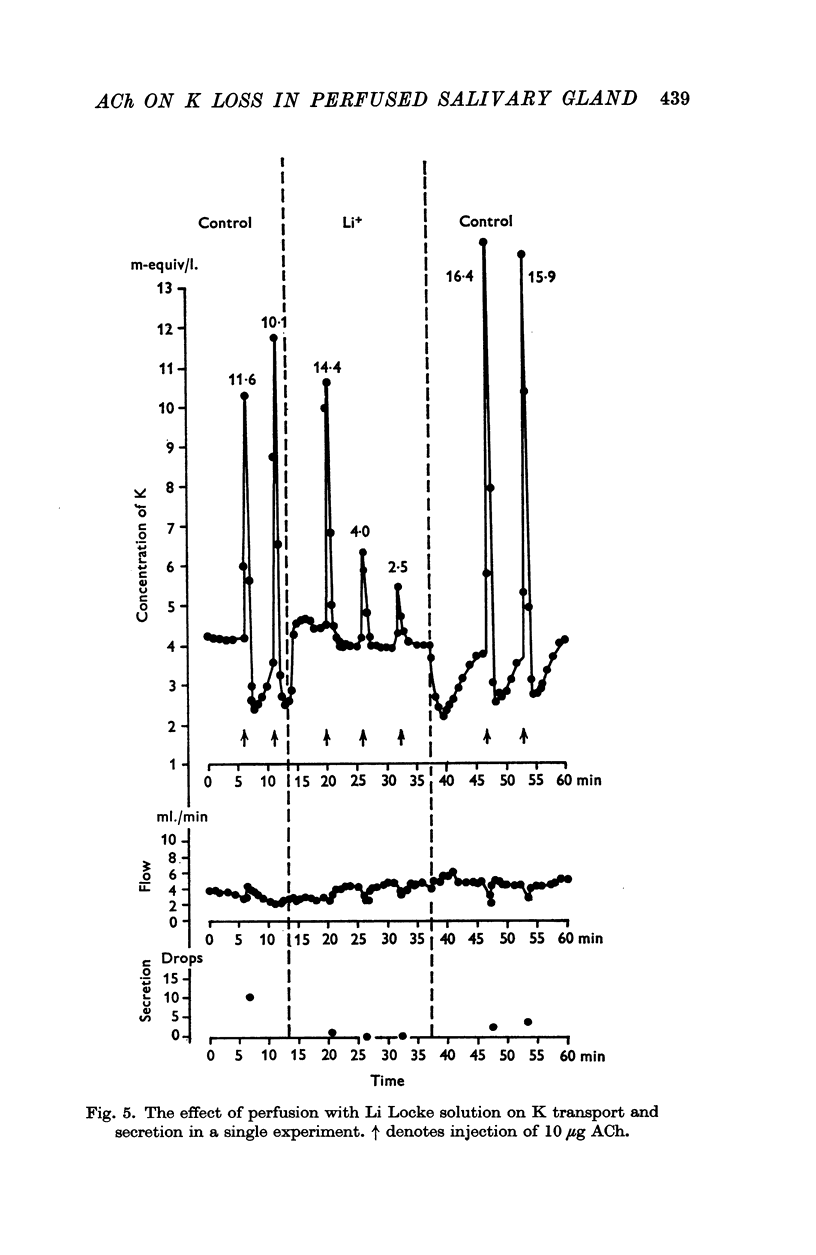
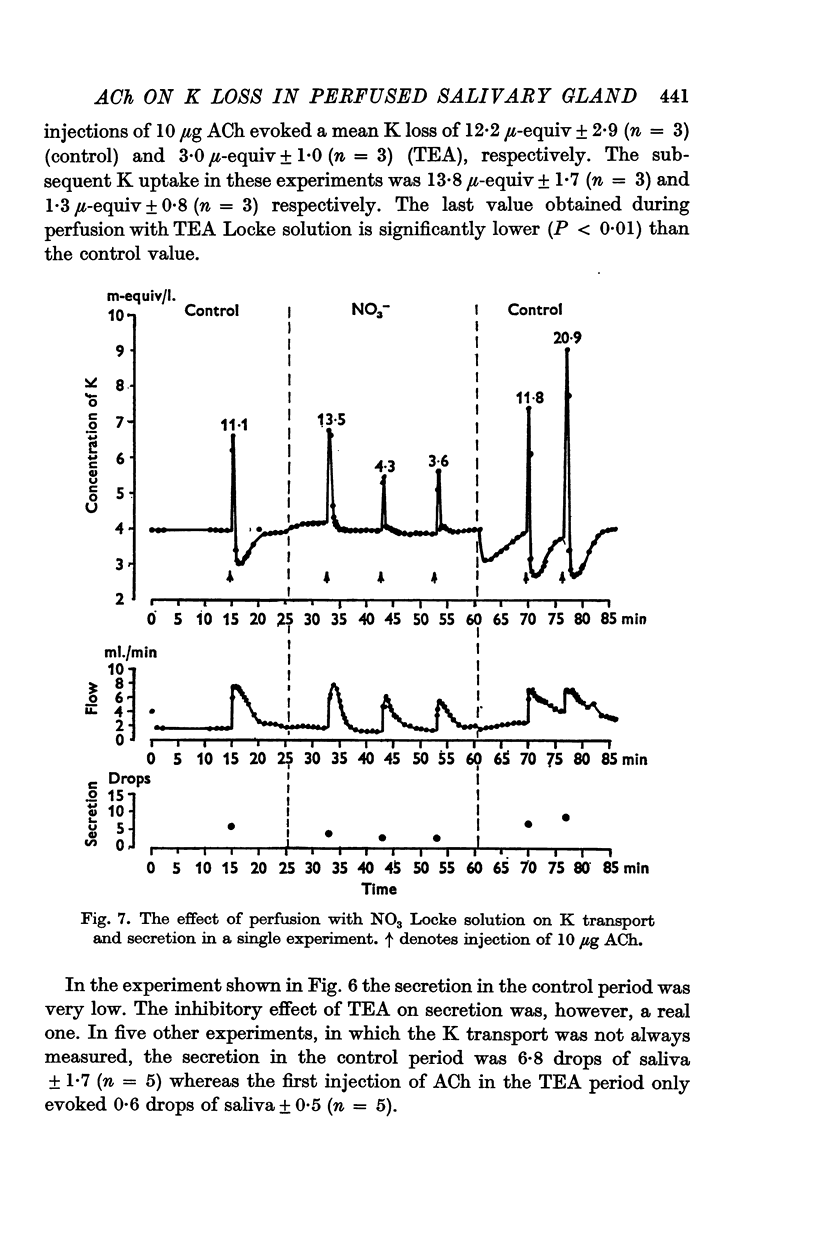
3. Perfusion with dinitrophenol (DNP) (10-4 M) containing solutions, Na-free Li Locke solutions and chloride-free nitrate Locke solutions inhibited salivary secretion and the uptake of K. The first injection of ACh after shift of the perfusion fluid from control to test solution gave a normal K loss, but thereafter the ACh-induced K-loss declined.
4. Perfusion with g-strophanthin (10-5-10-4 M) always inhibited K uptake whereas K release was not affected primarily. The sensitivity of the secretory mechanism of different glands to strophanthin varied considerably.
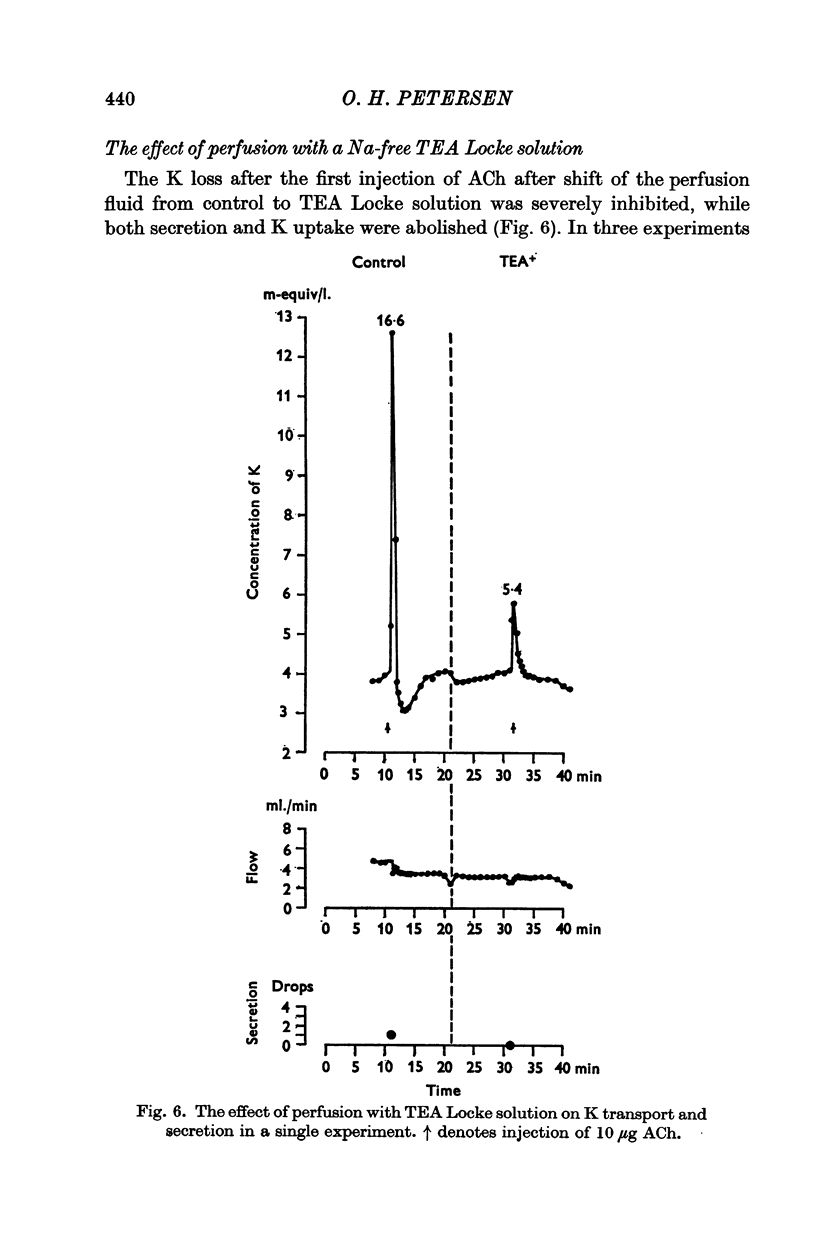
5. Perfusion with tetraethylammonium Locke solution inhibited secretion, K uptake and release of K.
6. It is suggested that the release of K from salivary glands to the ECF after stimulation with ACh can be explained by diffusion as a consequence of an enhanced permeability of the cell membranes to K. Concomitantly with the release of K, Na is taken up. It is suggested that the subsequent uptake of K and extrusion of Na is due to active transport processes probably involving a Na—K activated ATP-ase.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGEN A. S. The secretion of lithium in saliva. Can J Biochem Physiol. 1958 Apr;36(4):409–411. [PubMed] [Google Scholar]
- BURGEN A. S. The secretion of potassium in saliva. J Physiol. 1956 Apr 27;132(1):20–39. doi: 10.1113/jphysiol.1956.sp005500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHNEYER L. H., SCHNEYER C. A. SALIVARY SECRETION IN THE RAT AFTER OUABAIN. Am J Physiol. 1965 Jul;209:111–118. doi: 10.1152/ajplegacy.1965.209.1.111. [DOI] [PubMed] [Google Scholar]
- Frederiksen O., Leyssac P. P. Transcellular transport of isosmotic volumes by the rabbit gall-bladder in vitro. J Physiol. 1969 Mar;201(1):201–224. doi: 10.1113/jphysiol.1969.sp008751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M. E., Botelho S. Y. Membrane potentials in unstimulated parotid gland of the cat. Am J Physiol. 1969 May;216(5):1180–1183. doi: 10.1152/ajplegacy.1969.216.5.1180. [DOI] [PubMed] [Google Scholar]
- Henriques B. L., Sperling A. L. Marking of sited cells after electrophysiologic study. J Appl Physiol. 1966 Jul;21(4):1247–1250. doi: 10.1152/jappl.1966.21.4.1247. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. Electrophysiology of salivary glands. Physiol Rev. 1958 Jan;38(1):21–40. doi: 10.1152/physrev.1958.38.1.21. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. Secretory potentials in the sublingual gland of the cat. Acta Physiol Scand. 1957 Sep 17;40(1):21–34. doi: 10.1111/j.1748-1716.1957.tb01475.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. The electrophysiology of the submaxillary gland of the cat. Acta Physiol Scand. 1955 Dec 22;35(1):1–25. doi: 10.1111/j.1748-1716.1955.tb01258.x. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Poulsen J. H. The effects of varying the extracellular potassium concentration on the secretory rate and on resting and secretory potentials in the perfused cat submandibular gland. Acta Physiol Scand. 1967 Jul-Aug;70(3):293–298. doi: 10.1111/j.1748-1716.1967.tb03629.x. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ A., LASETER A. H., KRAINTZ L. AN ENZYMATIC BASIS FOR ACTIVE CATION TRANSPORT IN THE PAROTID GLAND. J Cell Physiol. 1963 Oct;62:193–205. doi: 10.1002/jcp.1030620208. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Moore C. A. Highly active Na+, K+-ATPase in rat submaxillary gland bearing on salivary secretion. Am J Physiol. 1968 May;214(5):1163–1167. doi: 10.1152/ajplegacy.1968.214.5.1163. [DOI] [PubMed] [Google Scholar]
- Siegel I. A. Effects of metabolic inhibitors on potassium transport in submaxillary glands. Am J Physiol. 1969 Apr;216(4):728–733. doi: 10.1152/ajplegacy.1969.216.4.728. [DOI] [PubMed] [Google Scholar]
- ZERAHN K. Studies on the active transport of lithium in the isolated frog skin. Acta Physiol Scand. 1955 Aug 19;33(4):347–358. doi: 10.1111/j.1748-1716.1955.tb01214.x. [DOI] [PubMed] [Google Scholar]


