Abstract
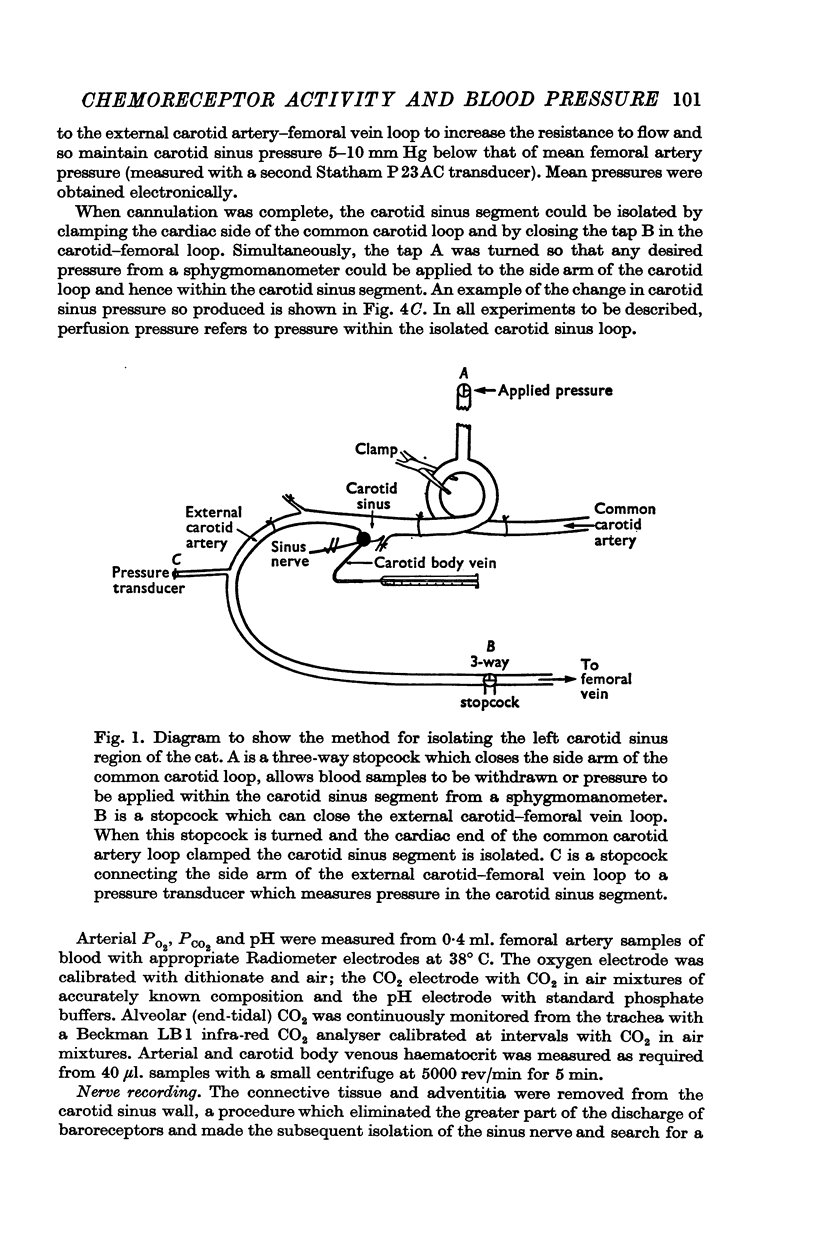
1. Activity in forty-two single chemoreceptor afferent fibres from the carotid body in thirty-nine cats was measured when the carotid body was naturally and artificially perfused. In nine of these cats, carotid body venous flow was also measured.
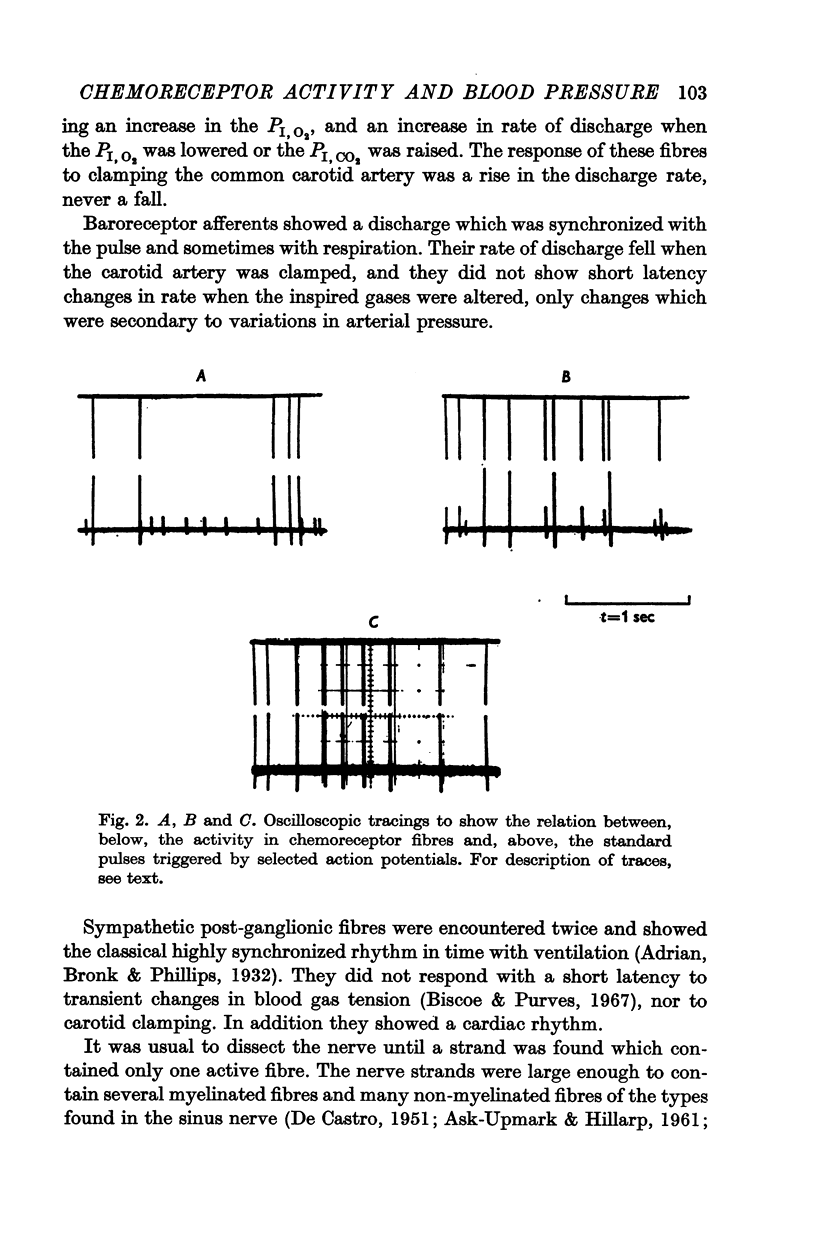
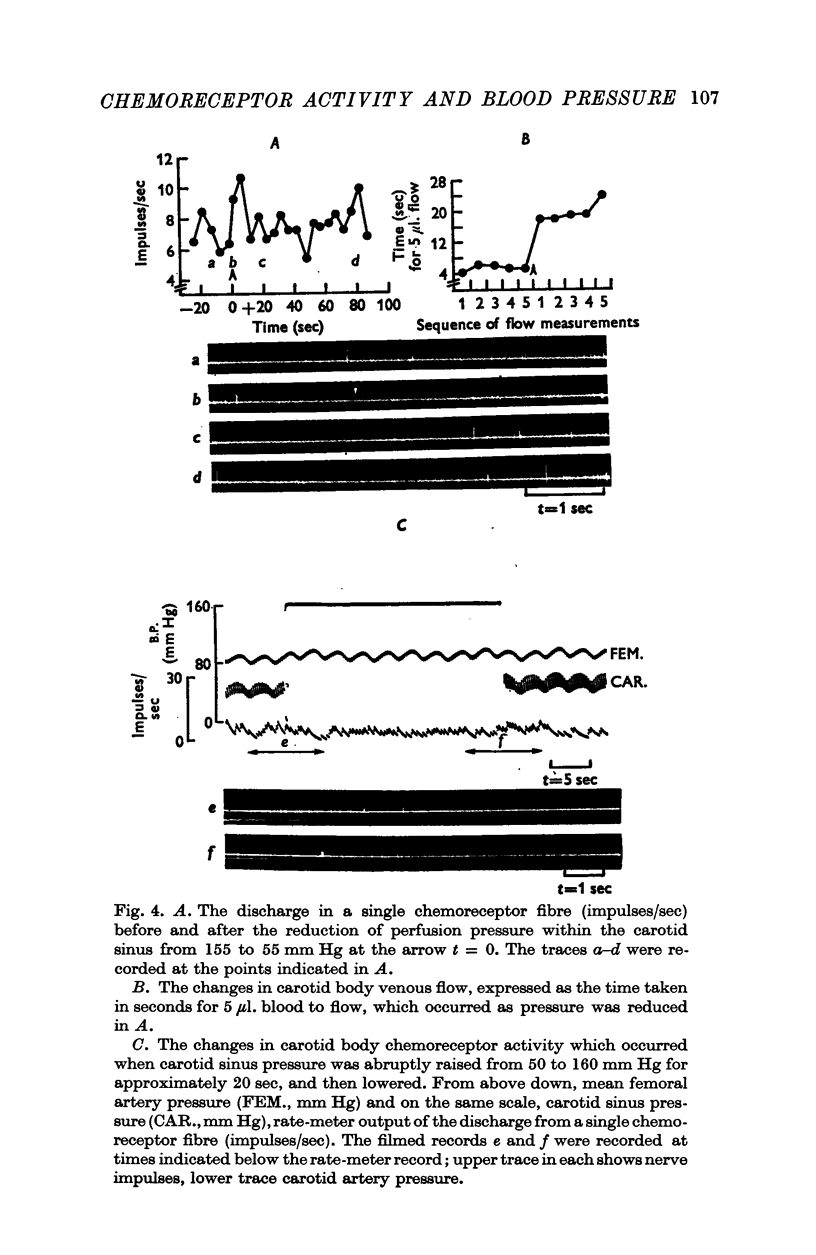
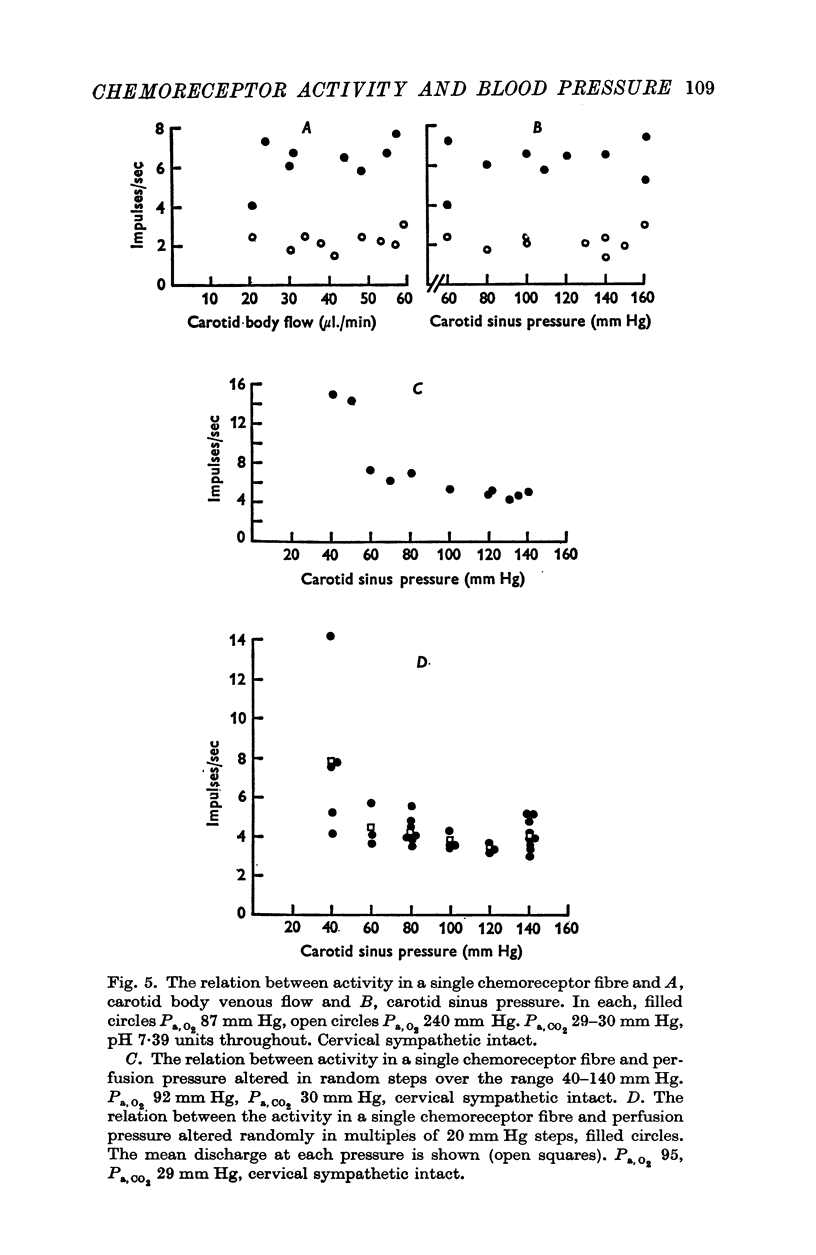
2. When pressure within the carotid sinus segment was suddenly raised or lowered, chemoreceptor activity changed in the opposite direction within the first 5-10 sec by an amount which was significantly greater than the variation of activity in the control period. Thereafter activity stabilized at a level which was not different from control.
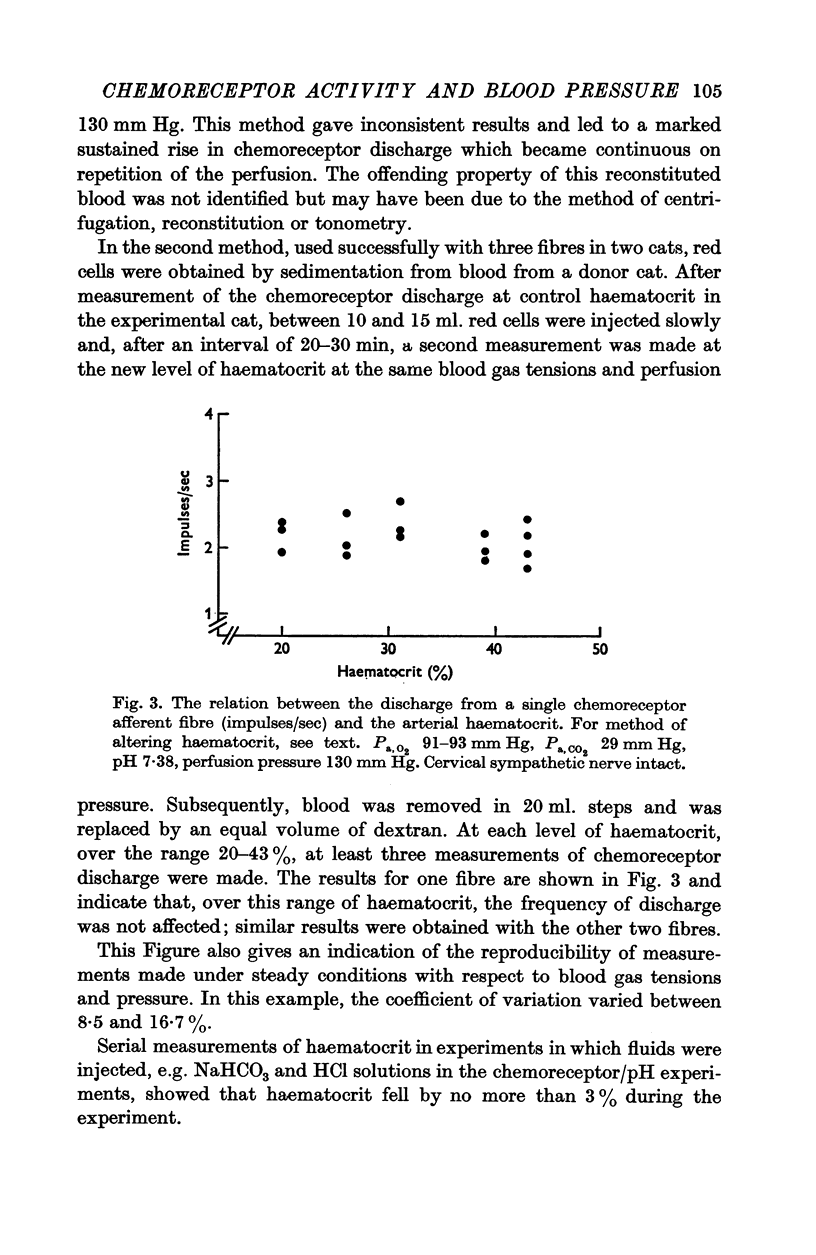
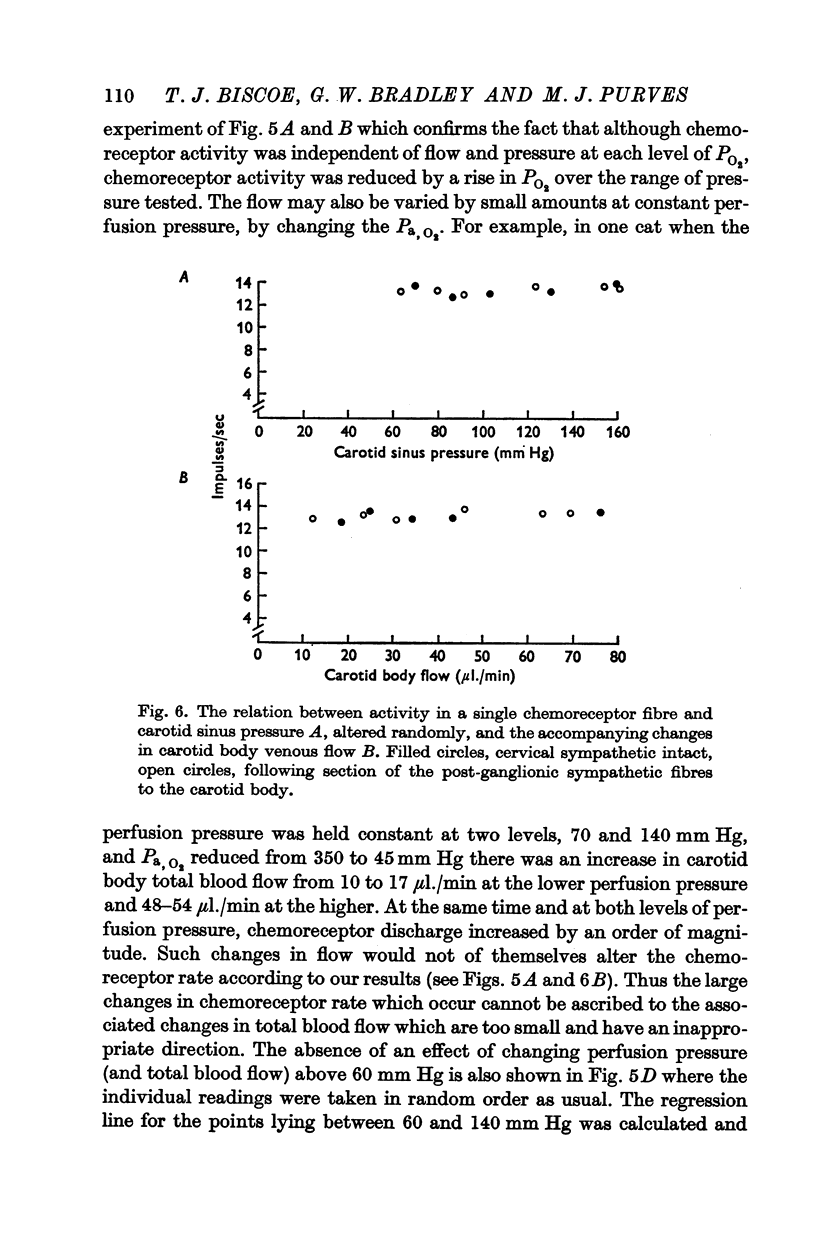
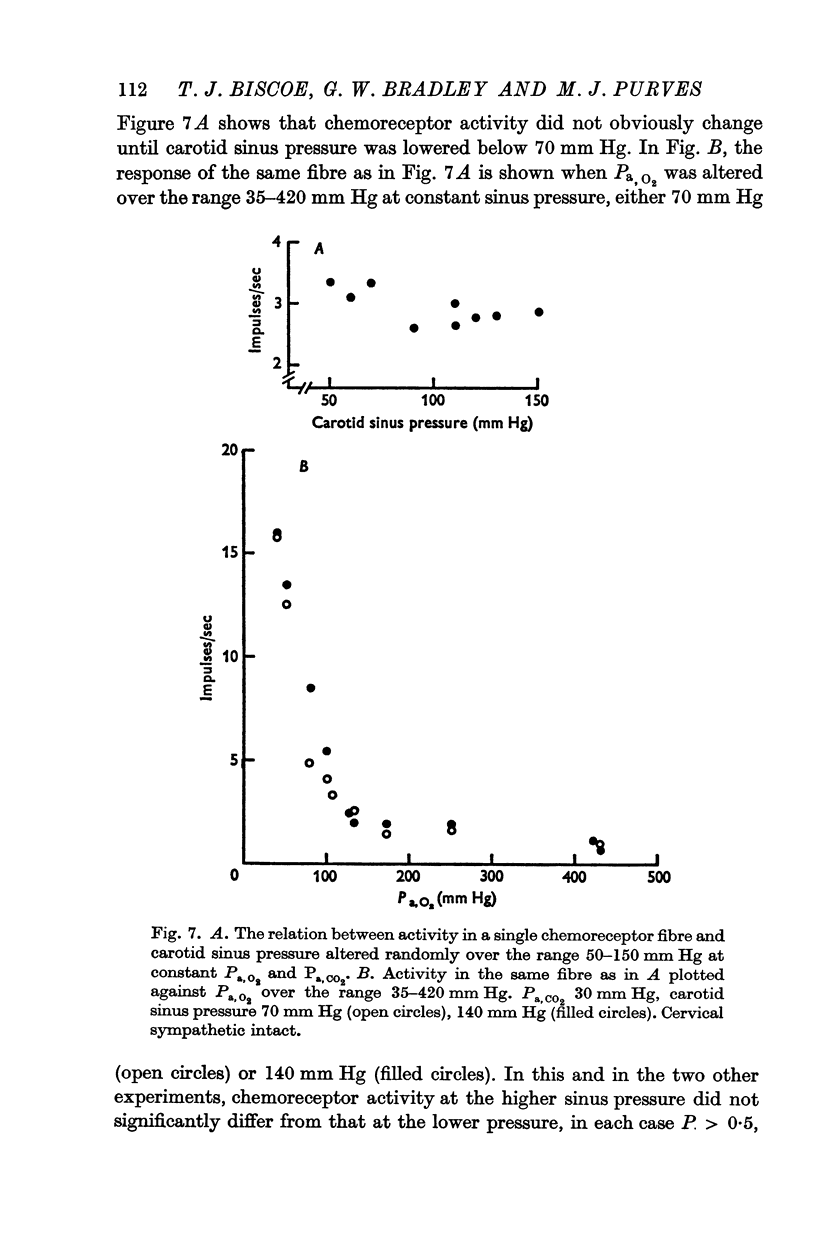
3. Whether the carotid body was naturally or artificially perfused, carotid body chemoreceptor activity, following this initial transient change, was unaffected in eight out of twelve fibres by alterations in carotid sinus pressure within the range 60-160 mm Hg and carotid body venous flow 10-60 μl./min, blood gas tensions and pH being maintained constant. In the four remaining fibres, chemoreceptor activity increased slightly but significantly as pressure was lowered in this range. Chemoreceptor activity increased in all fibres tested when pressure was lowered below 50-60 mm Hg.
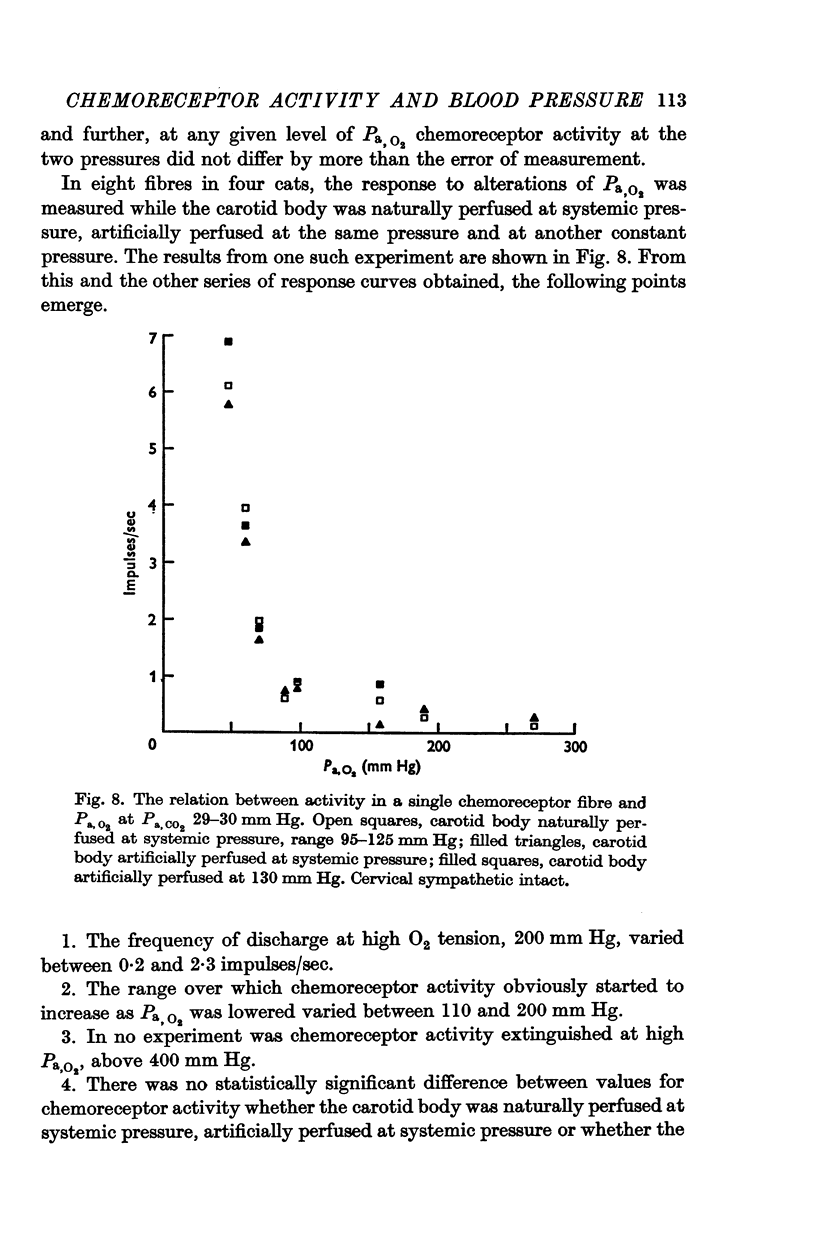
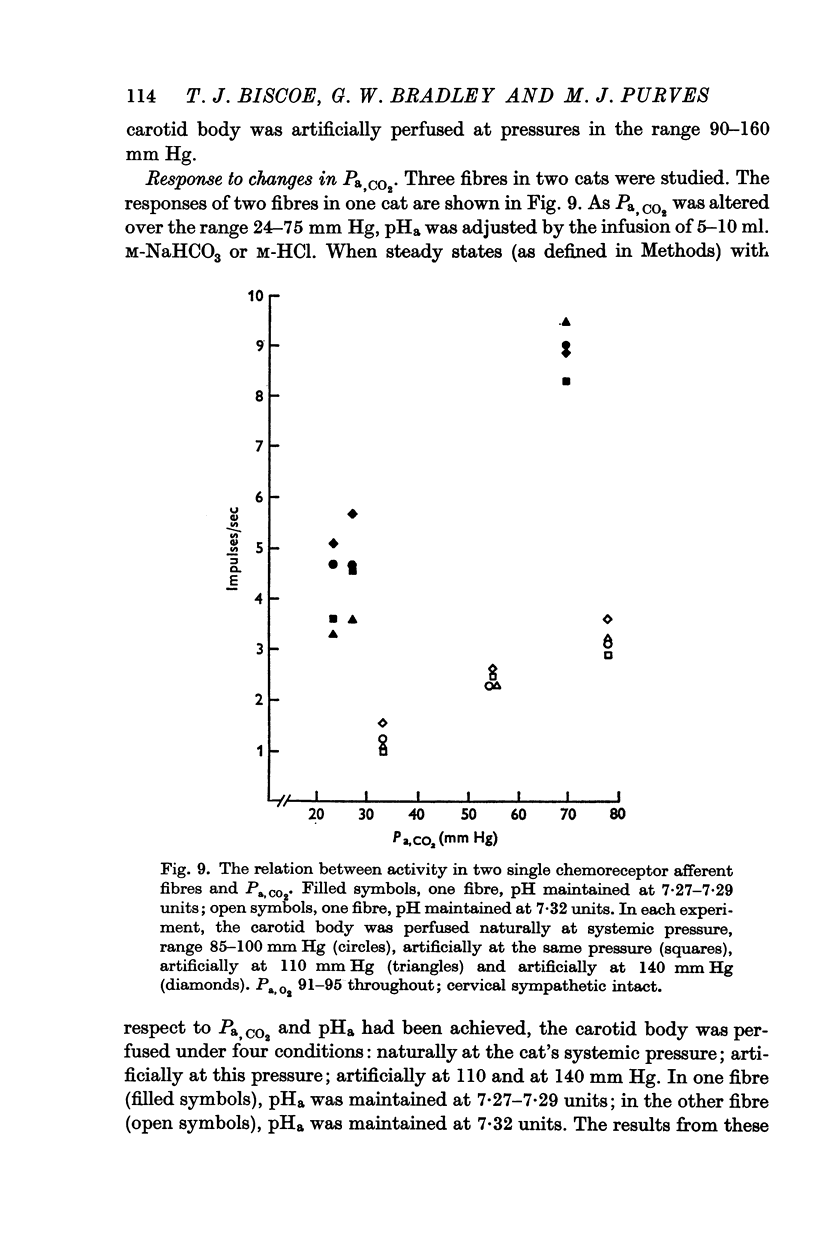
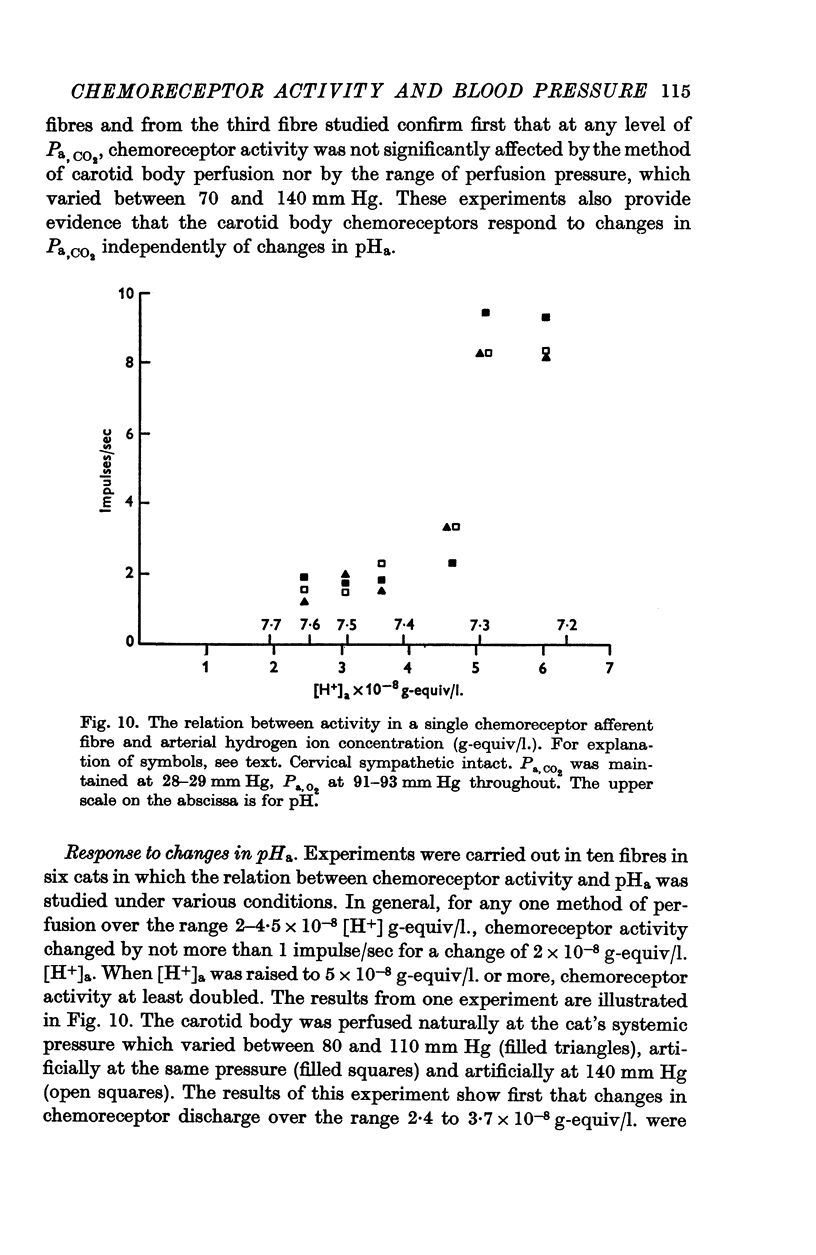
4. Chemoreceptor response curves to changes in Pa,O2 (30-450 mm Hg), Pa,CO2 (27-62 mm Hg) or [H+]a (3-7 × 10-5 m-equiv/l.) were not significantly different whether the carotid body was perfused (a) naturally at the prevalent systemic pressure, (b) artificially at the same pressure, or (c) artificially at one higher pressure, 130 or 140 mm Hg.
5. These results indicate that the carotid body chemoreceptors are relatively unaffected by sustained changes in arterial pressure or in total carotid body flow within the physiological range.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASK-UPMARK E., HILLARP N. A. The fibre size in the carotid sinus nerve of the cat. Acta Anat (Basel) 1961;46:25–29. doi: 10.1159/000141766. [DOI] [PubMed] [Google Scholar]
- Adrian E. D., Bronk D. W., Phillips G. Discharges in mammalian sympathetic nerves. J Physiol. 1932 Feb 8;74(2):115–133. doi: 10.1113/jphysiol.1932.sp002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Millar R. A. Effects of inhalation anaesthetics on carotid body chemoreceptor activity. Br J Anaesth. 1968 Jan;40(1):2–12. doi: 10.1093/bja/40.1.2. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J. Observations on the rhythmic variation in the cat carotid body chemoreceptor activity which has the same period as respiration. J Physiol. 1967 Jun;190(3):389–412. doi: 10.1113/jphysiol.1967.sp008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J., Sampson S. R. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol. 1970 May;208(1):121–131. doi: 10.1113/jphysiol.1970.sp009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMROE J. H., Jr, MORTIMER L. THE RESPIRATORY AND CARDIOVASCULAR RESPONSES OF TEMPORALLY SEPARATED AORTIC AND CAROTID BODIES TO CYANIDE, NICOTINE, PHENYLDIGUANIDE AND SEROTONIN. J Pharmacol Exp Ther. 1964 Oct;146:33–41. [PubMed] [Google Scholar]
- DE BURGH DALY M., LAMBERTSEN C. J., SCHWEITZER A. Observations on the volume of blood flow and oxygen utilization of the carotid body in the cat. J Physiol. 1954 Jul 28;125(1):67–89. doi: 10.1113/jphysiol.1954.sp005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE CASTRO F. Sur la structure de la synapse dans les chemocepteurs; leur mécanisme d'excitation et rôle dans la circulation sanguine locale. Acta Physiol Scand. 1951 Feb 21;22(1):14–43. doi: 10.1111/j.1748-1716.1951.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Daly M., Ungar A. Comparison of the reflex responses elicited by stimulation of the separately perfused carotid and aortic body chemoreceptors in the dog. J Physiol. 1966 Jan;182(2):379–403. doi: 10.1113/jphysiol.1966.sp007828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYZAGUIRRE C., LEWIN J. Effect of different oxygen tensions on the carotid body in vitro. J Physiol. 1961 Dec;159:238–250. doi: 10.1113/jphysiol.1961.sp006805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYZAGUIRRE C., UCHIZONO K. Observations on the fibre content of nerves reaching the carotid body of the cat. J Physiol. 1961 Dec;159:268–281. doi: 10.1113/jphysiol.1961.sp006807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORNBEIN T. F., GRIFFO Z. J., ROOS A. Quantitation of chemoreceptor activity: interrelation of hypoxia and hypercapnia. J Neurophysiol. 1961 Nov;24:561–568. doi: 10.1152/jn.1961.24.6.561. [DOI] [PubMed] [Google Scholar]
- LANDGREN S., NEIL E. Chemoreceptor impulse activity following haemorrhage. Acta Physiol Scand. 1951 Aug 25;23(2-3):158–167. doi: 10.1111/j.1748-1716.1951.tb00805.x. [DOI] [PubMed] [Google Scholar]
- LEE K. D., MAYOU R. A., TORRANCE R. W. THE EFFECT OF BLOOD PRESSURE UPON CHEMORECEPTOR DISCHARGE TO HYPOXIA, AND THE MODIFICATION OF THIS EFFECT BY THE SYMPATHETIC-ADRENAL SYSTEM. Q J Exp Physiol Cogn Med Sci. 1964 Apr;49:171–183. doi: 10.1113/expphysiol.1964.sp001717. [DOI] [PubMed] [Google Scholar]
- Purves M. J. Changes in oxygen consumption of the carotid body of the cat. J Physiol. 1969 Feb;200(2):132P–133P. [PubMed] [Google Scholar]
- WITZLEB E. Uber die Wirkung des Veratrins auf die chemo- und pressoreceptorischen Aktionspotentiale im Carotissinus-nerven. Pflugers Arch. 1953;256(3):234–241. doi: 10.1007/BF00364329. [DOI] [PubMed] [Google Scholar]


