Abstract
1. Artificial cerebrospinal fluid containing isotopically labelled sugars was perfused from the lateral cerebral ventricles to an effluent catheter inserted into the cerebral aqueduct of anaesthetized cats. This system was used for a quantitative study of the absorption of the sugars during steady state.
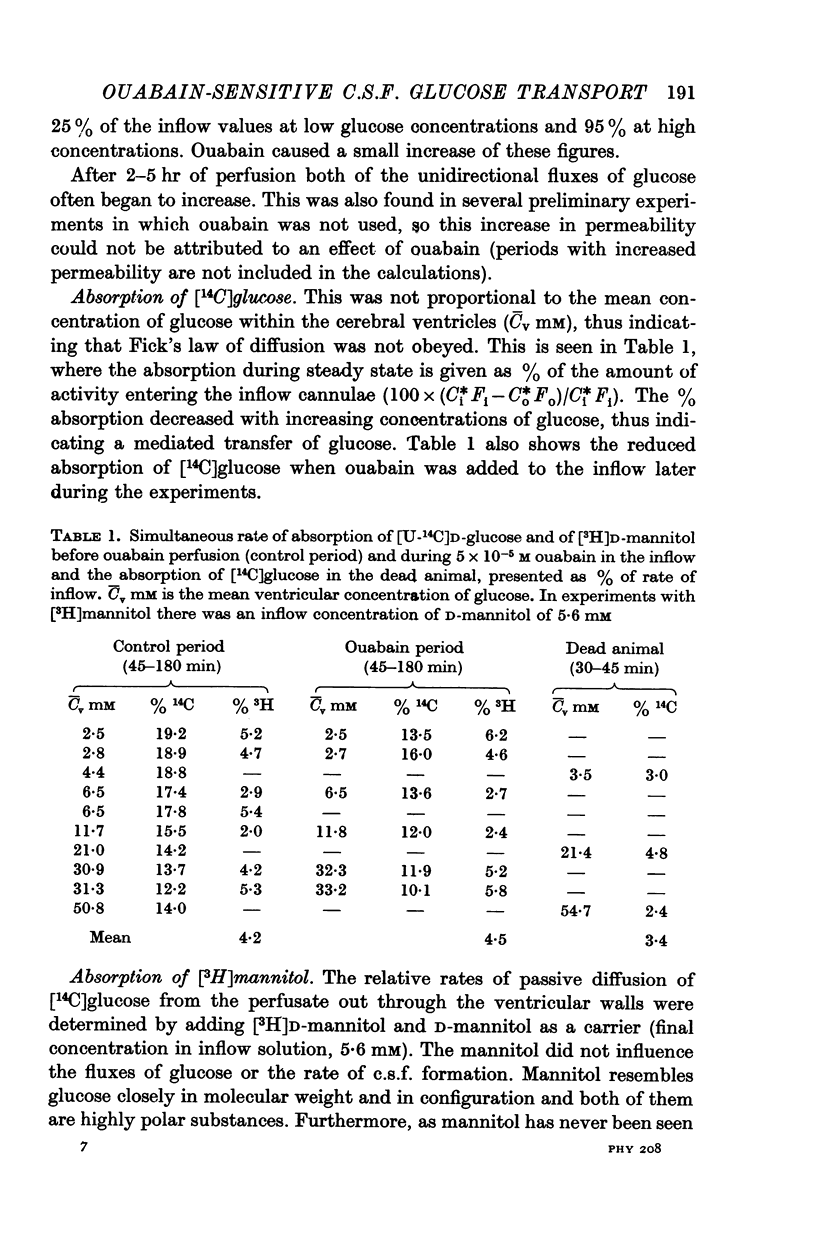
2. A saturable mechanism was involved in the absorption of [U-14C]D-glucose and [14C]D-galactose. Absorption of [U-14C]D-glucose in the dead animal was similar to that of [3H]D-mannitol.
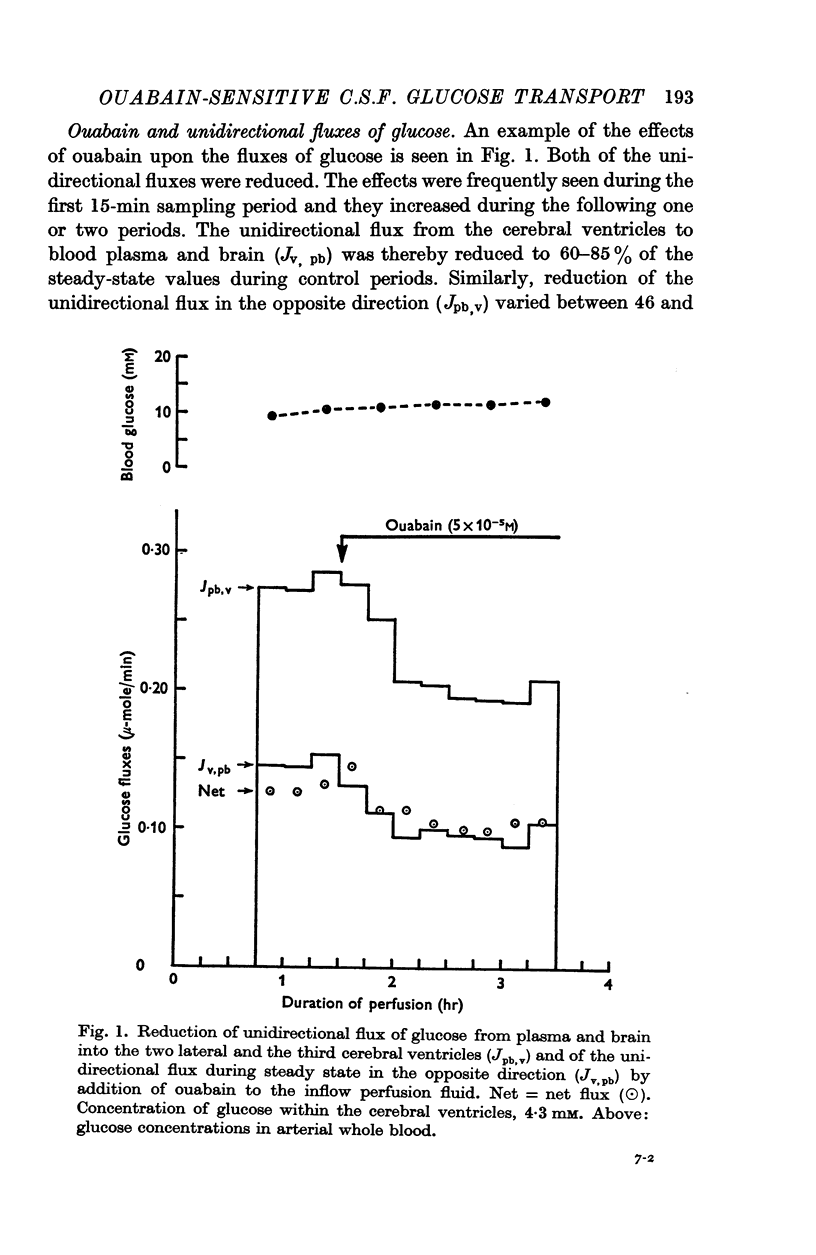
3. 5 × 10-5 M ouabain in the inflow reduced cerebrospinal fluid formation and the unidirectional fluxes of glucose from the ventricles into brain tissue and plasma. Ouabain did not alter the absorption of [3H]D-mannitol.
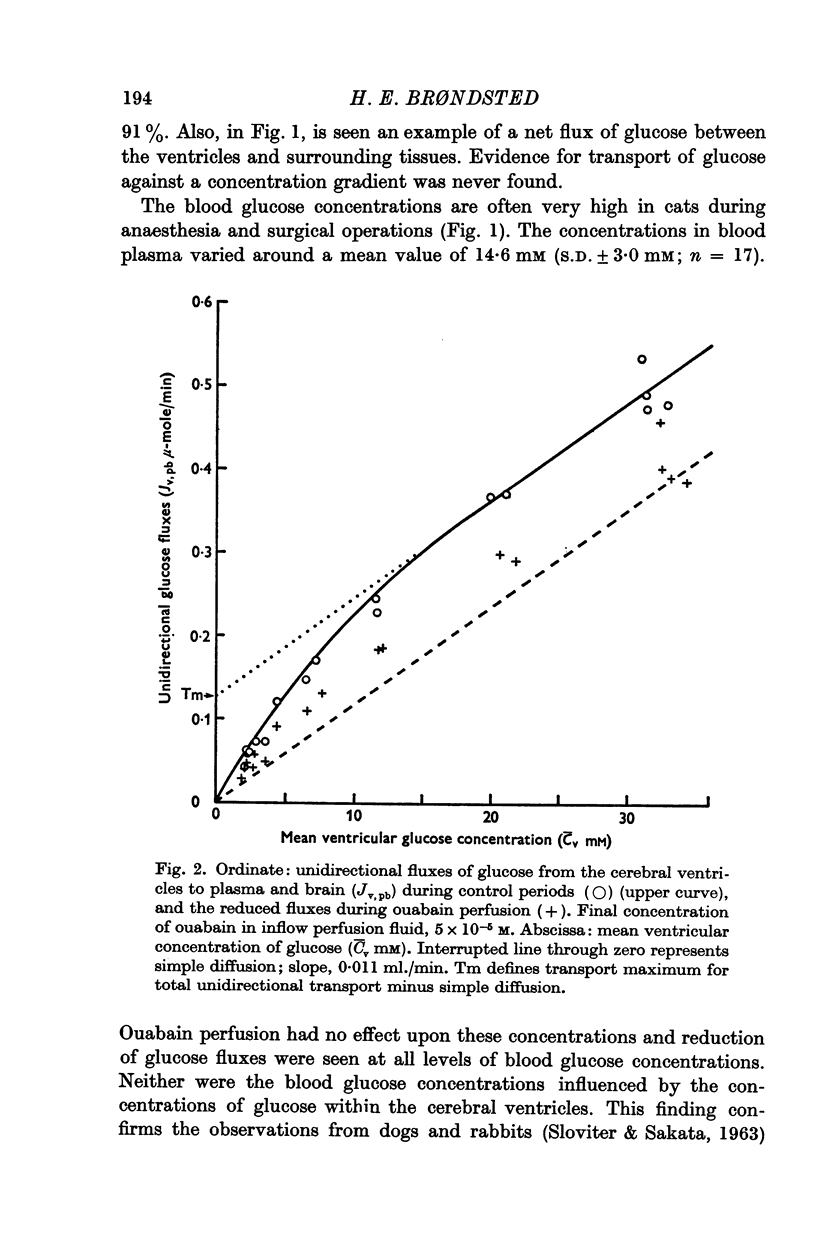
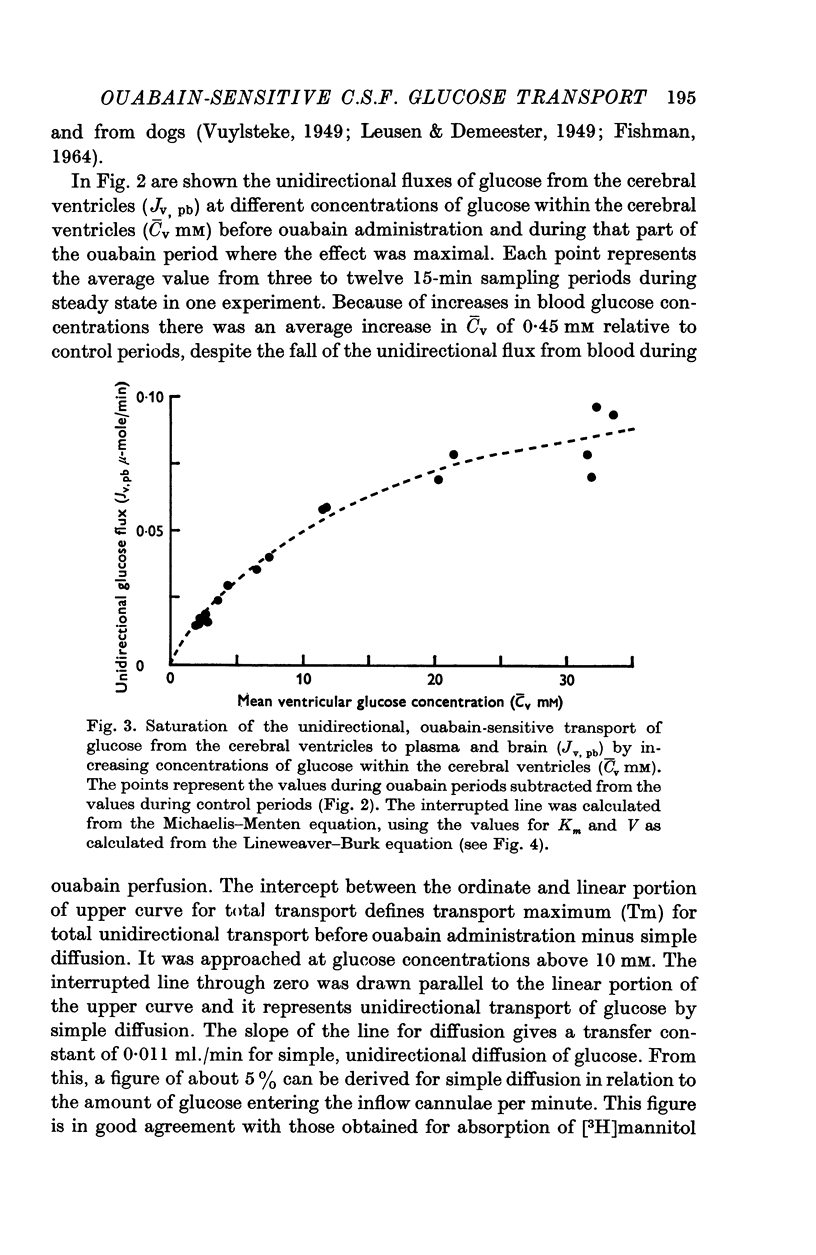
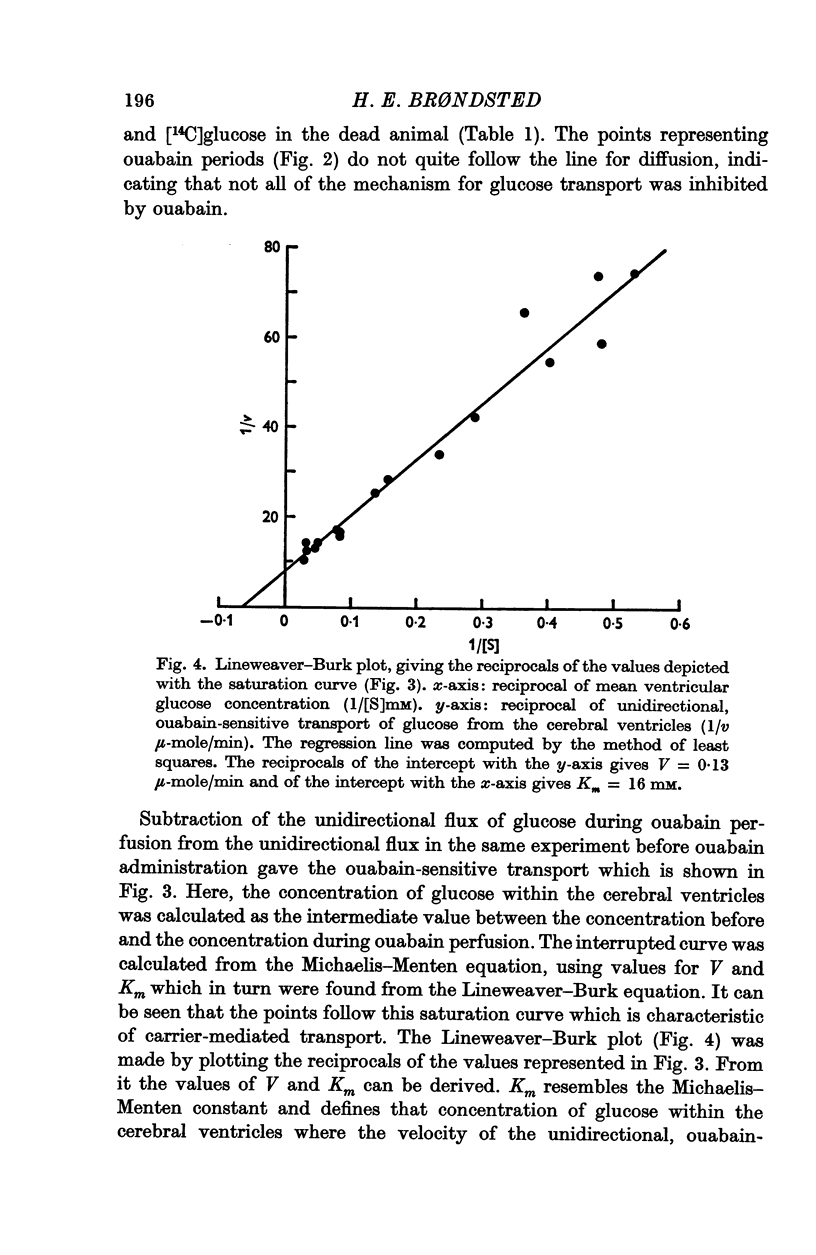
4. Three types of unidirectional fluxes of glucose from the cerebral ventricles were separated. One was ouabain-sensitive and followed Michaelis—Menten kinetics. The second was insensitive to ouabain and the third occurred by simple diffusion.
5. At normal ventricular glucose concentrations (3·5 mM) the three fluxes comprised (roughly): 25% (ouabain-sensitive), 35% (ouabain-insensitive) and 40% (simple diffusion) of total, unidirectional transport.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BHATTACHARYA B. K., FELDBERG W. Perfusion of cerebral ventricles: effects of drugs on outflow from the cisterna and the aqueduct. Br J Pharmacol Chemother. 1958 Jun;13(2):156–162. doi: 10.1111/j.1476-5381.1958.tb00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADBURY M. W., DAVSON H. THE TRANSPORT OF UREA, CREATININE AND CERTAIN MONOSACCHARIDES BETWEEN BLOOD AND FLUID PERFUSING THE CEREBRAL VENTRICULAR SYSTEM OF RABBITS. J Physiol. 1964 Jan;170:195–211. doi: 10.1113/jphysiol.1964.sp007323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M. W., Davson H. The transport of potassium between blood, cerebrospinal fluid and brain. J Physiol. 1965 Nov;181(1):151–174. doi: 10.1113/jphysiol.1965.sp007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSAKY T. Z., RIGOR B. M., Sr A CONCENTRATIVE MECHANISM FOR SUGARS IN THE CHOROID PLEXUS. Life Sci. 1964 Sep;3:931–936. doi: 10.1016/0024-3205(64)90101-8. [DOI] [PubMed] [Google Scholar]
- Clausen T. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. II. Effects of K+-free medium, ouabain and insulin upon the fate of glucose in rat diaphragm. Biochim Biophys Acta. 1966 Jul 13;120(3):361–368. doi: 10.1016/0926-6585(66)90303-7. [DOI] [PubMed] [Google Scholar]
- Cserr H. Potassium exchange between cerebrospinal fluid, plasma, and brain. Am J Physiol. 1965 Dec;209(6):1219–1226. doi: 10.1152/ajplegacy.1965.209.6.1219. [DOI] [PubMed] [Google Scholar]
- DAVSON H., KLEEMAN C. R., LEVIN E. Quantitative studies of the passage of different substances out of the cerebrospinal fluid. J Physiol. 1962 Apr;161:126–142. doi: 10.1113/jphysiol.1962.sp006877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDBERG W., FLEISCHHAUER K. Penetration of bromophenol blue from the perfused cerebral ventricles into the brain tissue. J Physiol. 1960 Feb;150:451–462. doi: 10.1113/jphysiol.1960.sp006397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHMAN R. A. CARRIER TRANSPORT OF GLUCOSE BETWEEN BLOOD AND CEREBROSPINAL FLUID. Am J Physiol. 1964 Apr;206:836–844. doi: 10.1152/ajplegacy.1964.206.4.836. [DOI] [PubMed] [Google Scholar]
- GOODNER C. J. STUDIES OF ANTERIOR PITUITARY TISSUE IN VITRO: COMPARISON OF CARBOHYDRATE METABOLISM IN GLANDS FROM MALE AND FEMALE RATS. Endocrinology. 1964 Dec;75:846–856. doi: 10.1210/endo-75-6-846. [DOI] [PubMed] [Google Scholar]
- Graziani L. J., Kaplan R. K., Escriva A., Katzman R. Calcium flux into CSF during ventricular and ventriculocisternal perfusion. Am J Physiol. 1967 Sep;213(3):629–636. doi: 10.1152/ajplegacy.1967.213.3.629. [DOI] [PubMed] [Google Scholar]
- HEISEY S. R., HELD D., PAPPENHEIMER J. R. Bulk flow and diffusion in the cerebrospinal fluid system of the goat. Am J Physiol. 1962 Nov;203:775–781. doi: 10.1152/ajplegacy.1962.203.5.775. [DOI] [PubMed] [Google Scholar]
- HJELM M., DE VERDIERCH C. H. A METHODOLOGICAL STUDY OF THE ENZYMATIC DETERMINATION OF GLUCOSE IN BLOOD. Scand J Clin Lab Invest. 1963;15:415–428. doi: 10.3109/00365516309079764. [DOI] [PubMed] [Google Scholar]
- Hochwald G. M., Wallenstein M. Exchange of albumin between blood, cerebrospinal fluid, and brain in the cat. Am J Physiol. 1967 May;212(5):1199–1204. doi: 10.1152/ajplegacy.1967.212.5.1199. [DOI] [PubMed] [Google Scholar]
- Katzman R., Graziani L., Kaplan R., Escriva A. Exchange of cerebrospinal fluid potassium with blood and brain. Study in normal and Ouabain perfused cats. Arch Neurol. 1965 Nov;13(5):513–524. doi: 10.1001/archneur.1965.00470050061007. [DOI] [PubMed] [Google Scholar]
- OCHS S., VAN HARREVELD A. Cerebral impedance changes after circulatory arrest. Am J Physiol. 1956 Sep;187(1):180–192. doi: 10.1152/ajplegacy.1956.187.1.180. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H., Davson H. Brain extracellular space and the sink action of cerebrospinal fluid. Measurement of rabbit brain extracellular space using sucrose labeled with carbon 14. Arch Neurol. 1967 Aug;17(2):196–205. doi: 10.1001/archneur.1967.00470260086010. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., FENCL V., HEISEY S. R., HELD D. ROLE OF CEREBRAL FLUIDS IN CONTROL OF RESPIRATION AS STUDIED IN UNANESTHETIZED GOATS. Am J Physiol. 1965 Mar;208:436–450. doi: 10.1152/ajplegacy.1965.208.3.436. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., HEISEY S. R., JORDAN E. F. Active transport of Diodrast and phenolsulfonphthalein from cerebrospinal fluid to blood. Am J Physiol. 1961 Jan;200:1–10. doi: 10.1152/ajplegacy.1961.200.1.1. [DOI] [PubMed] [Google Scholar]
- POLLAY M., DAVSON H. The passage of certain substances out of the cerebrosphinal fluid. Brain. 1963 Mar;86:137–150. doi: 10.1093/brain/86.1.137. [DOI] [PubMed] [Google Scholar]
- Ruscák M., Whittam R. The metabolic response of brain slices to agents affecting the sodium pump. J Physiol. 1967 Jun;190(3):595–610. doi: 10.1113/jphysiol.1967.sp008230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLOVITER H. A., SAKATA K. Inactivity of cerebrospinal fluid in regulation of blood glucose concentration. Am J Physiol. 1963 Jan;204:153–156. doi: 10.1152/ajplegacy.1963.204.1.153. [DOI] [PubMed] [Google Scholar]
- VANHARREVELD A., CROWELL J., MALHOTRA S. K. A STUDY OF EXTRACELLULAR SPACE IN CENTRAL NERVOUS TISSUE BY FREEZE-SUBSTITUTION. J Cell Biol. 1965 Apr;25:117–137. doi: 10.1083/jcb.25.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VATES T. S., Jr, BONTING S. L., OPPELT W. W. NA-K ACTIVATED ADENOSINE TRIPHOSPHATASE FORMATION OF CEREBROSPINAL FLUID IN THE CAT. Am J Physiol. 1964 May;206:1165–1172. doi: 10.1152/ajplegacy.1964.206.5.1165. [DOI] [PubMed] [Google Scholar]
- Welch K., Sadler K. Permeability of the choroid plexus of the rabbit to several solutes. Am J Physiol. 1966 Mar;210(3):652–660. doi: 10.1152/ajplegacy.1966.210.3.652. [DOI] [PubMed] [Google Scholar]
- Zadunaisky J. A., Wald F., De Robertis E. D. Osmotic behavior and glial changes in isolated frog brains. Prog Brain Res. 1965;15:196–218. doi: 10.1016/s0079-6123(08)60947-4. [DOI] [PubMed] [Google Scholar]


