Abstract
All of the transmembrane glutamates of Tet(L) are essential for tetracycline (TET) resistance, and E397 has been shown to be essential for all catalytic modes, i.e., TET-Me2+ and Na+ efflux and K+ uptake. Loop residues D74 and G70 are essential for TET flux but not for Na+ or K+ flux. A cysteineless Tet(L) protein exhibits all activities.
Tet(L) and Tet(K), sharing about 80% sequence similarity, are group II tetracycline (TET) efflux proteins, which have 14 transmembrane segments (TMS) and are found predominantly in gram-positive bacteria (23). In contrast, group I TET efflux proteins, such as Tet(B), have 12 TMS and are found in gram-negative bacteria. Both groups belong to the major facilitator superfamily (MFS) (22) and confer TET resistance by translocating a TET-Me2+ complex in exchange for protons (11, 33, 35). Extensive mutagenesis studies on Tet(B) have been reported, especially by Yamaguchi and colleagues. Several residues essential for TET translocation were identified, such as three aspartates in TMS (30) and one aspartate in a cytoplasmic loop (31, 32). Recently finished cysteine scanning of the whole Tet(B) molecule revealed that four amphiphilic helices (II, V, VIII, and XI) and four partly amphiphilic helices (I, IV, VII, and X) contribute to form a putative water-filled translocation pathway, within which a permeability barrier was defined (13, 15, 16, 17, 18, 19, 27). By contrast, limited mutagenesis and structure-function studies have been conducted on 14-TMS TET efflux proteins. It was shown that three glutamates in TMS (E30, E152, and E397) and one aspartate in a cytoplasmic loop (D318) are essential for Tet(K)-dependent TET efflux (6, 7). That 12-TMS Tet(B) and 14-TMS Tet(K) both require three carboxylates in equivalent TMS regions for TET efflux led to the hypothesis that both groups of TET efflux proteins may possess similar three-dimensional structures in the core catalytic region (12). This interesting possibility was one impetus for initiating a broad site-directed mutagenesis screen of Tet(L). Moreover, the multifunctional nature of Tet(L) increases the interest in such a screen inasmuch as it may reveal residues or regions that are important for particular catalytic modes of Tet(L). In addition to the TET-Me2+/H+ antiport mode, Tet(L) and Tet(K) have been shown to have an Na+(K+)/H+ antiport mode; i.e., they utilize Na+ or K+ as alternate cytoplasmic substrates to TET (1, 2, 3, 4, 11). In the third catalytic mode, a net K+ uptake mode, Tet(L) and Tet(K) use K+ instead of H+ as the coupling ion for antiport of the cytoplasmic substrates (10). These additional catalytic modes are of physiological significance, since they contribute to Na+ resistance (2, 3), K+ acquisition (20, 29), and pH homeostasis (3).
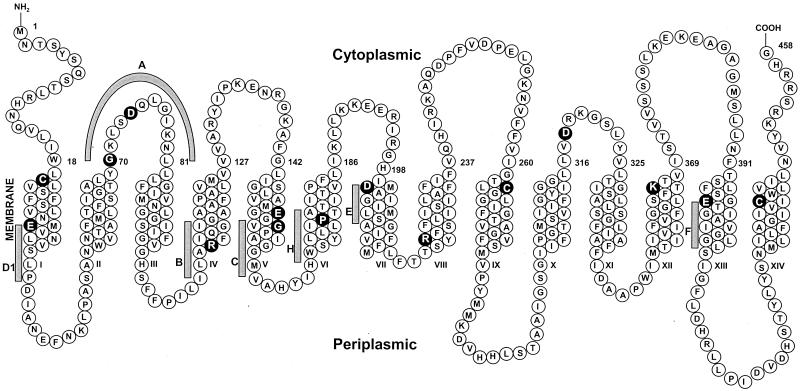
In this screen, we focused on the seven motifs that are conserved in the 14-TMS MFS subfamily (25) and some additional charged residues of particular interest (Fig. 1). At least one residue was selected from each motif as a representative. Several charged residues, such as E30, E152, E397, D200, R100 (all in TMS), and D74 (in the CII-III loop, motif A), were replaced individually with a neutral residue. G70 (motif A) was targeted since the corresponding glycine in Tet(B) was shown to be critical for TET efflux (33). G155 (motif C) was selected since conserved glycines within this motif were shown to be important for TET efflux mediated by Tet(K) (9) and Tet(B) (26). P175 (motif H), a proline located within TMS VI, was replaced with cysteine. D318, which has been shown to be essential for Tet(K)-dependent TET efflux (7), was replaced with asparagine in Tet(L). Although they are not in any motifs, R222 and K366, two positively charged residues within TMS regions, were each replaced with a cysteine. In addition, three cysteines of Tet(L) were replaced with alanines to evaluate the usefulness of a cysteineless Tet(L) protein for further studies.
FIG. 1.
Topological diagram of Tet(L). The topology shown is based on experimental findings for the closely related protein Tet(K) (8, 12). Seven conserved motifs of 14-TMS members of the MFS (reviewed by Paulsen et al. [25]), A, B, C, D1, E, F, and H, are highlighted with gray bars. The residues mutated in this study are shaded in black.
This battery of Tet(L) mutant genes was constructed by the method of Kunkel et al. (21) with a bacteriophage M13mp19 template containing a 1.4-kb EcoRI/BamHI DNA fragment of wild-type tet(L). All of the mutant genes were confirmed by sequencing of the entire Tet(L) coding sequence. The EcoRI/BamHI cassettes of the wild-type and mutant genes were cloned individually into pGEM3zf(+) (Promega) under control of the T7 promoter (giving a low level of expression that is useful in Na+-related assays) and into shuttle vector pBK15 (3) under control of the ermC promoter (giving a higher level of expression that is useful in TET- and K+-related assays and Western analyses). The pGEM3zf(+) constructs were transformed into E. coli NM81 (with the nhaA antiporter deleted [24]) to test the effects of diverse mutations on the Na+ resistance and Na+/H+ antiport of the Na+-sensitive strain. The attempt to use the complementation screen was unsuccessful because the data were not consistent enough, so only transport data on the Na+ flux capacity of selected strains are presented. The pBK15 constructs were transformed into E. coli DH5α to test the TET resistance and TET transport of selected strains and into E. coli TK2420 (deficient in three K+ transporters [5]) to test the capacity to complement the K+ uptake deficiency and to assay 86Rb+ transport of selected strains. Membrane incorporation and transport activities were assayed in membrane vesicles as described previously (14).
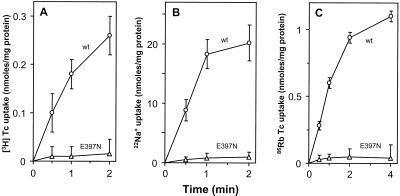
A mutant with a change in one of three glutamates in TMS, E30C, E152Q, or E397N, showed significant membrane assembly (more than 30% of the wild-type level) but conferred no TET resistance (Table 1), consistent with the idea that these three glutamates of Tet(K) are essential for TET efflux (6). Interestingly, all three mutants also conferred a reduced ability to complement the growth of E. coli TK2420 at low K+ concentrations (Table 1), suggesting that these glutamates are required for more than one catalytic mode. This notion was supported by the transport analyses of the Tet(L) mutant, E397N, which conferred no [3H]TET uptake in everted membrane vesicles (Fig. 2A), no 22Na+ uptake in everted vesicles (Fig. 2B), and no 86Rb+(K+) uptake in right-side-out vesicles (Fig. 2C). It is possible that while the E397N mutation abolished transport, the mutant protein introduced a small K+ leak pathway that accounts for the residual complementation of E. coli TK2420. Although the cytoplasmic substrates of different modes range from a TET-Me2+ complex (that includes hydrophobic ring structures) to much smaller monovalent cations, all catalytic modes have in common the use of cationic substrates with a single net charge, which may relate to the importance of a negatively charged transmembrane carboxylate(s).
TABLE 1.
Effects of Tet(L) mutations on protein assembly into the membrane, TET resistance in E. coli DH5α, and K+ complementation in E. coli TK2420
| Location of mutation | Protein | % Membrane assemblya | MIC of TETb (μg/ml) | [KCl]c (mM) |
|---|---|---|---|---|
| Vector | 0 | 2 | 24 | |
| Wild-type Tet(L) | 100 | 30 | 13 | |
| Transmembrane regions | ||||
| Charged residues | ||||
| Motif D1 | E30C | 108 | 2.5 | 22 |
| Motif C | E152Q | 98 | 2.5 | 18 |
| Motif F | E397N | 32 | 2.5 | 18 |
| Motif E | D200C | 15 | 10 | 13 |
| Motif B | R110C | 15 | 2.5 | 14 |
| R222C | 90 | 28 | 16 | |
| K366C | —d | 2.5 | 20 | |
| Motif C | G155S | 152 | 8 | 22 |
| Motif H | P175C | 88 | 16 | 15 |
| Cytoplasmic loops Motif A | G70S | 112 | 18 | 14 |
| G70R | 93 | 2.5 | 13 | |
| D74C | 84 | 13 | 13 | |
| D318N | 70 | 14 | 13 | |
| Cysteines | C20A | 64 | 32 | 10 |
| C264A | 70 | 26 | 13 | |
| C443A | 60 | 33 | 14 | |
| Cysteineless | 70 | 33 | 12 |
The membrane assembly of each mutant protein was evaluated by Western analysis, and results are shown as relative percentages of the wild-type protein. ImageQuant was used for quantification.
The MIC is the minimal tetracycline concentration at which there was no growth of E. coli DH5α after a 15-h incubation in rich medium (14).
The minimal concentration of KCl in a defined medium permitting E. coli TK2420 to grow to an A600 of 1.0 after a 15-h incubation is shown. A range of added K+ concentration of 0 to 25 mM was examined. Values for wild-type Tet(L) were reproducibly <15 mM K+ for an A600 of 1.0 (14).
—, The membrane assembly of the K366C mutant was too low for detection by Western analysis.
FIG. 2.
Transport activities of the transmembrane E397N mutant form of Tet(L). (A) [3H]TET uptake assays were conducted with everted membrane vesicles prepared from E. coli DH5α cells harboring the pBK15 constructs. (B) 22Na+ uptake assays were conducted with everted membrane vesicles prepared from E. coli NM81 cells harboring pGEM3zf(+) constructs. (C) 86Rb+(K+) transport assays were conducted with right-side-out membrane vesicles prepared from E. coli TK2420. The net transport activities for both TET and Na+ uptake, presented with standard deviations, were each corrected for the background derived with a vector control, and the net Rb+ uptake was corrected for the choline control, in which there is no active Rb+ transport. Circles represent the wild type (wt), and triangles represent the E397N mutant.
Mutation of other charged transmembrane residues produced a variety of transformant phenotypes. K366C, of all of the mutants analyzed, is the only one that showed extremely defective membrane incorporation (Table 1). All of the other mutants, as shown by Western analyses, had significant membrane assembly (more than 30% of the wild-type level), except R110C (15%) and D200C (15%). The R110C mutant conferred no TET resistance but did support normal K+ complementation. The mutant with a change in another charged transmembrane residue, D200C, was earlier shown to be active in Na+ and K+ transport but defective in TET efflux mode, although it conferred some TET resistance (14). However, not every charged transmembrane residue is critical for at least one catalytic activity, since transformants of the R222C mutant were not affected in any of the catalytic modes (14).
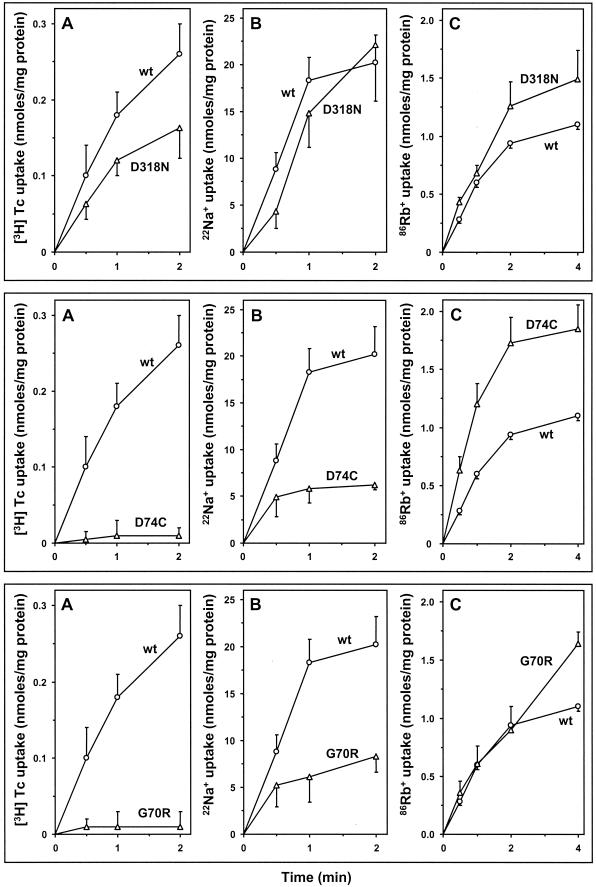
It was reported that D318, in the cytoplasmic loop between TMS X and XI of Tet(K), is essential for TET efflux (7). However, the D318N mutant form of Tet(L), surprisingly, conferred significant TET resistance and K+ complementation (Table 1). Results of assays of transport in membrane vesicles confirmed that D318 of Tet(L) is not essential for TET efflux or other modes: D318N conferred about 60% of the wild-type level of TET uptake and wild-type levels of Na+ uptake and Rb+ transport (Fig. 3, top panel).
FIG. 3.
Transport activities of Tet(L) mutant proteins with changes in cytoplasmic loop residues, D318N, D74C, and G70R. Results of [3H]TET uptake in everted vesicles (A), 22Na+ uptake in everted vesicles (B), and 86Rb+(K+) transport in right-side-out vesicles (C) are presented for D318N (top panel), D74C (middle panel), and G70R (bottom panel). Shown in each graph are the net activities corrected for controls as described in the legend to Fig. 2. Circles represent the wild type (wt), and triangles represent the mutants.
D74C, the Tet(L) mutant with a change in an aspartate located in the CII-III loop (motif A) confers K+ complementation and some TET resistance, albeit lower than that of the wild type (Table 1). As with the D200C mutant (14), D74C conferred no TET uptake in transport assays (Fig. 3, middle panel), suggesting that D74 is essential for active TET efflux, even though residual TET resistance was observed. A discrepancy between antibiotic resistance level and transport activity observed in some mutants (this study and reference 14) has also been observed by others in several Tet(K) (7) and Tet(B) (13) mutants. The bases for this kind of discrepancy are incompletely understood but may relate to effects of TET-Me2+ trapping at the surface and/or effects of certain mutant proteins on cell surface properties beyond the protein itself.
The importance of the D74 residue is consistent with the findings that a corresponding aspartate in Tet(B) is essential for TET efflux (31) and D74 of Tet(K) is important for TET efflux (a mutant retains only 5% of wild-type transport activity [7]). Aspartates in cytoplasmic loops play a role in TET efflux in both Tet(L) and Tet(K), but the different aspartate residues may differ in relative importance. While D74 of Tet(L) is essential for TET transport, it is not essential for other modes. The D74C mutant conferred about 30% of the wild-type level Na+ transport and conferred higher activity of K+ transport than the wild type (Fig. 3, middle panel). The different effects of the D74C mutation on TET, Na+, and K+ transport may indicate that the role of this cytoplasmic loop (motif A) is to interact with cytoplasmic substrates such as TET and Na+ but not with an outside substrate such as K+, which had the role of a coupling ion in these assays. This is supported by the analysis of a glycine mutant with a change in the same motif, G70R, which behaved almost exactly the same as D74C in three transport assays, conferring no TET transport, reduced but significant Na+ transport, and a wild-type level of inward K+ transport (Fig. 3, bottom panel). The TET efflux data for the D74C and G70R mutant Tet(L) transformants are consistent with the finding that motif A of Tet(B) is critical for TET efflux (34). Since G70S does confer low but significant TET resistance while G70R does not (Table 1), replacing G70 with a small uncharged residue, such as serine, may still allow maintenance of the local structure of motif A required for TET-Me2+ binding or flux. This structure of motif A is apparently not essential for Na+ or K+ transport. Since TET and Na+ are both cytoplasmic substrates [while Rb+(K+) is an alternate coupling ion to H+ in the assays conducted here], these transport data suggest that motif A, located in an intracellular loop, may be especially critical for the most complicated cytoplasmic substrate, the TET-Me2+ complex, but not absolutely essential for monovalent cation cytoplasmic substrates.
G155S, a mutant with a change in motif C of Tet(L), conferred reduced TET resistance and reduced K+ complementation. Residual TET resistance had also been observed when the corresponding glycine was mutated in Tet(B) (13) and Tet(K) (9), although G155 is part of a GP dipeptide that has been proposed to be an important element of this transporter family (13, 28). A different transmembrane proline of Tet(L), P175 of motif H, is unlikely to be important for transport since P175C conferred significant TET resistance and K+ complementation (Table 1).
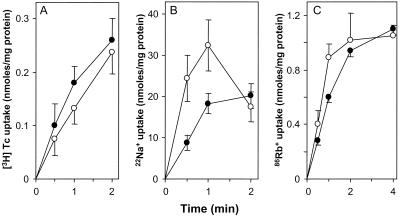
Finally, the cysteineless mutant form of Tet(L) conferred TET resistance and K+ complementation (Table 1). It was shown to be active in all transport modes (Fig. 4). Relative to the wild type, the cysteineless mutant showed faster initial Na+ uptake but the uptake was not sustained after 1 min (Fig. 4B). The cysteineless mutant may also have conferred higher activities than the wild type in TET (Fig. 4A) and Rb+ (Fig. 4C) transport, if activities are normalized to membrane assembly, which is lower for the mutant (Table 1). These data suggest that loss of Tet(L) cysteines may cause some subtle change in folding and/or membrane incorporation that modestly affects the pattern of membrane permeability to monovalent cations, while the protein retains all of its catalytic activities. The retention of activity by the cysteineless mutant makes it likely to be a useful template for cysteine scanning and targeted cross-linking experiments intended to further investigate the structure-function relationships in this multifunctional TET efflux protein.
FIG. 4.
Transport activities of a cysteineless Tet(L) mutant. [3H]TET transport assays (A), 22Na+ uptake assays (B), and 86Rb+(K+) transport assays (C) were conducted and results are presented as described in the legend to Fig. 2. Closed circles represent the wild type, and open circles represent the cysteineless mutant.
Acknowledgments
This work was supported by research grant GM52837 from the National Institute of General Medical Sciences.
We thank Arthur Guffanti for invaluable advice and Shankar Iyer for help with the phenotypic screen experiments.
REFERENCES
- 1.Cheng, J., K. Baldwin, A. A. Guffanti, and T. A. Krulwich. 1996. Na+/H+ antiport activity conferred by Bacillus subtilis tetA(L), a 5′ truncation product of tetA(L), and related plasmid genes upon Escherichia coli. Antimicrob. Agents Chemother. 40:852-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng, J., A. A. Guffanti, and T. A. Krulwich. 1994. The chromosomal tetracycline resistance locus of Bacillus subtilis encodes a Na+/H+ antiporter that is physiologically important at elevated pH. J. Biol. Chem. 269:27365-27371. [PubMed] [Google Scholar]
- 3.Cheng, J., A. A. Guffanti, W. Wang, T. A. Krulwich, and D. H. Bechhofer. 1996. Chromosomal tetA(L) gene of Bacillus subtilis: regulation of expression and physiology of a tetA(L) deletion strain. J. Bacteriol. 178:2853-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, J., D. B. Hicks, and T. A. Krulwich. 1996. The purified Bacillus subtilis tetracycline efflux protein TetA(L) reconstitutes both tetracycline-cobalt/H+ and Na+/H+ exchange. Proc. Natl. Acad. Sci. USA 93:14446-14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein, W., E. Buurman, D. McLaggan, and J. Naprstek. 1993. Multiple mechanisms, roles and controls of K+ transport in Escherichia coli. Biochem. Soc. Trans. 21:1006-1010. [DOI] [PubMed] [Google Scholar]
- 6.Fujihira, E., T. Kimura, Y. Shiina, and A. Yamaguchi. 1996. Transmembrane glutamic acid residues play essential roles in the metal-tetracycline/H+ antiporter of Staphylococcus aureus. FEBS Lett. 391:243-246. [DOI] [PubMed] [Google Scholar]
- 7.Fujihira, E., T. Kimura, and A. Yamaguchi. 1997. Roles of acidic residues in the hydrophilic loop regions of metal-tetracycline/H+ antiporter Tet(K) of Staphylococcus aureus. FEBS Lett. 419:211-214. [DOI] [PubMed] [Google Scholar]
- 8.Ginn, S. L., M. H. Brown, and R. A. Skurray. 1997. Membrane topology of the metal-tetracycline/H+ antiporter TetA(K) from Staphylococcus aureus. J. Bacteriol. 179:3786-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginn, S. L., M. H. Brown, and R. A. Skurray. 2000. The TetA(K) tetracycline/H+ antiporter from Staphylococcus aureus: mutagenesis and functional analysis of motif C. J. Bacteriol. 182:1492-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guffanti, A. A., J. Cheng, and T. A. Krulwich. 1998. Electrogenic antiport activities of the gram-positive Tet proteins include a Na+(K+)/K+ mode that mediates net K+ uptake. J. Biol. Chem. 273:26447-26454. [DOI] [PubMed] [Google Scholar]
- 11.Guffanti, A. A., and T. A. Krulwich. 1995. Tetracycline/H+ antiport and Na+/H+ antiport catalyzed by the Bacillus subtilis TetA(L) transporter expressed in Escherichia coli. J. Bacteriol. 177:4557-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirata, T., E. Fujihira, T. Kimura-Someya, and A. Yamaguchi. 1998. Membrane topology of the staphylococcal tetracycline efflux protein Tet(K) determined by antibacterial resistance gene fusions. J. Biochem. 124:1206-1211. [DOI] [PubMed] [Google Scholar]
- 13.Iwaki, S., N. Tamura, T. Kimura-Someya, S. Nada, and A. Yamaguchi. 2000. Cysteine-scanning mutagenesis of transmembrane segments 4 and 5 of the Tn10-encoded metal-tetracycline/H+ antiporter reveals a permeability barrier in the middle of a transmembrane water-filled channel. J. Biol. Chem. 275:22704-22712. [DOI] [PubMed] [Google Scholar]
- 14.Jin, J., A. A. Guffanti, C. Beck, and T. A. Krulwich. 2001. Twelve-transmembrane-segment (TMS) version (ΔTMS VII-VIII) of the 14-TMS Tet(L) antibiotic resistance protein retains monovalent cation transport modes but lacks tetracycline efflux capacity. J. Bacteriol. 183:2667-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura, T., Y. Shiina, T. Sawai, and A. Yamaguchi. 1998. Cysteine-scanning mutagenesis around transmembrane segment III of Tn10-encoded metal-tetracycline/H+ antiporter. J. Biol. Chem. 273:5243-5247. [DOI] [PubMed] [Google Scholar]
- 16.Kimura, T., M. Suzuki, T. Sawai, and A. Yamaguchi. 1996. Determination of a transmembrane segment using cysteine-scanning mutants of transposon Tn10-encoded metal-tetracycline/H+ antiporter. Biochemistry 35:15896-15899. [DOI] [PubMed] [Google Scholar]
- 17.Kimura-Someya, T., S. Iwaki, S. Konishi, N. Tamura, Y. Kubo, and A. Yamaguchi. 2000. Cysteine-scanning mutagenesis around transmembrane segments 1 and 11 and their flanking loop regions of Tn10-encoded metal-tetracycline/H+ antiporter. J. Biol. Chem. 275:18692-18697. [DOI] [PubMed] [Google Scholar]
- 18.Kimura-Someya, T., S. Iwaki, and A. Yamaguchi. 1998. Site-directed chemical modification of cysteine-scanning mutants as to transmembrane segment II and its flanking regions of the Tn10-encoded metal-tetracycline/H+ antiporter reveals a transmembrane water-filled channel. J. Biol. Chem. 273:32806-32811. [DOI] [PubMed] [Google Scholar]
- 19.Konishi, S., S. Iwaki, T. Kimura-Someya, and A. Yamaguchi. 1999. Cysteine-scanning mutagenesis around transmembrane segment VI of Tn10-encoded metal-tetracycline/H+ antiporter. FEBS Lett. 461:315-318. [DOI] [PubMed] [Google Scholar]
- 20.Krulwich, T. A., J. Jin, A. A. Guffanti, and D. H. Bechhofer. 2001. Functions of tetracycline efflux proteins that do not involve tetracycline. J. Mol. Microbiol. Biotechnol. 3:237-246. [PubMed] [Google Scholar]
- 21.Kunkel, T. A., K. Bebenek, and J. McClary. 1991. Efficient site-directed mutagenesis using uracil-containing DNA. Methods Enzymol. 204:125-139. [DOI] [PubMed] [Google Scholar]
- 22.Marger, M. D., and M. H. Saier, Jr. 1993. A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem. Sci. 18:13-20. [DOI] [PubMed] [Google Scholar]
- 23.McMurry, L. M., and S. B. Levy. 2000. Tetracycline resistance in gram-positive bacteria, p. 660-677. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 24.Padan, E., N. Maisler, D. Taglicht, R. Karpel, and S. Schuldiner. 1989. Deletion of ant in Escherichia coli reveals its function in adaptation to high salinity and an alternative Na+/H+ antiporter system(s). J. Biol. Chem. 264:20297-20302. [PubMed] [Google Scholar]
- 25.Paulsen, I. T., M. H. Brown, and R. A. Skurray. 1996. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60:575-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saraceni-Richards, C. A., and S. B. Levy. 2000. Evidence for interactions between helices 5 and 8 and a role for the interdomain loop in tetracycline resistance mediated by hybrid Tet proteins. J. Biol. Chem. 275:6101-6106. [DOI] [PubMed] [Google Scholar]
- 27.Tamura, N., S. Konishi, S. Iwaki, T. Kimura-Someya, S. Nada, and A. Yamaguchi. 2001. Complete cysteine-scanning mutagenesis and site-directed chemical modification of the Tn10-encoded metal-tetracycline/H+ antiporter. J. Biol. Chem. 276:20330-20339. [DOI] [PubMed] [Google Scholar]
- 28.Varela, M. F., C. E. Sansom, and J. K. Griffith. 1995. Mutational analysis and molecular modelling of an amino acid sequence motif conserved in antiporters but not symporters in a transporter superfamily. Mol. Membr. Biol. 12:313-319. [DOI] [PubMed] [Google Scholar]
- 29.Wang, W., A. A. Guffanti, Y. Wei, M. Ito, and T. A. Krulwich. 2000. Two types of Bacillus subtilis tetA(L) deletion strains reveal the physiological importance of TetA(L) in K+ acquisition as well as in Na+, alkali, and tetracycline resistance. J. Bacteriol. 182:2088-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi, A., T. Akasaka, N. Ono, Y. Someya, M. Nakatani, and T. Sawai. 1992. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by transposon Tn10. Roles of the aspartyl residues located in the putative transmembrane helices. J. Biol. Chem. 267:7490-7498. [PubMed] [Google Scholar]
- 31.Yamaguchi, A., M. Nakatani, and T. Sawai. 1992. Aspartic acid-66 is the only essential negatively charged residue in the putative hydrophilic loop region of the metal-tetracycline/H+ antiporter encoded by transposon Tn10 of Escherichia coli. Biochemistry 31:8344-8348. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi, A., N. Ono, T. Akasaka, T. Noumi, and T. Sawai. 1990. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by a transposon, Tn10. The role of the conserved dipeptide, Ser65-Asp66, in tetracycline transport. J. Biol. Chem. 265:15525-15530. [PubMed] [Google Scholar]
- 33.Yamaguchi, A., Y. Shiina, E. Fujihira, T. Sawai, N. Noguchi, and M. Sasatsu. 1995. The tetracycline efflux protein encoded by the tet(K) gene from Staphylococcus aureus is a metal-tetracycline/H+ antiporter. FEBS Lett. 365:193-197. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi, A., Y. Someya, and T. Sawai. 1992. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by transposon Tn10. The role of a conserved sequence motif, GXXXXRXGRR, in a putative cytoplasmic loop between helices 2 and 3. J. Biol. Chem. 267:19155-19162. [PubMed] [Google Scholar]
- 35.Yamaguchi, A., T. Udagawa, and T. Sawai. 1990. Transport of divalent cations with tetracycline as mediated by the transposon Tn10-encoded tetracycline resistance protein. J. Biol. Chem. 265:4809-4813. [PubMed] [Google Scholar]