Abstract
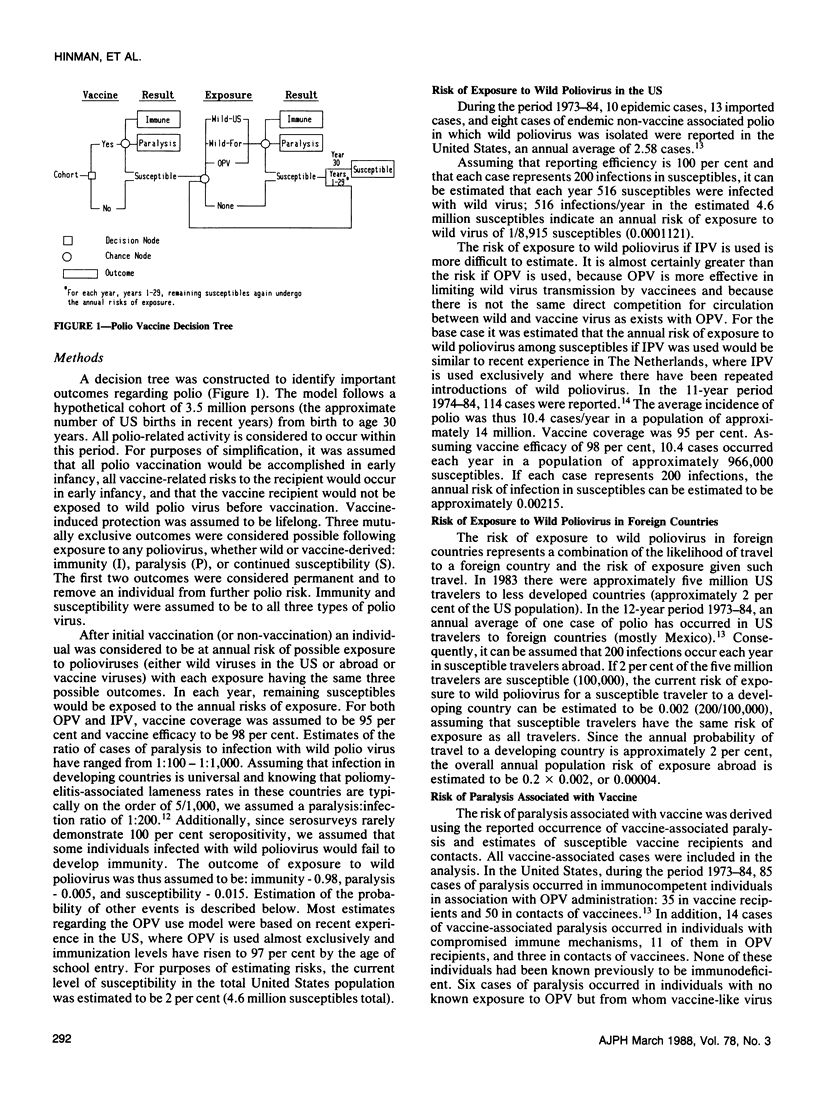
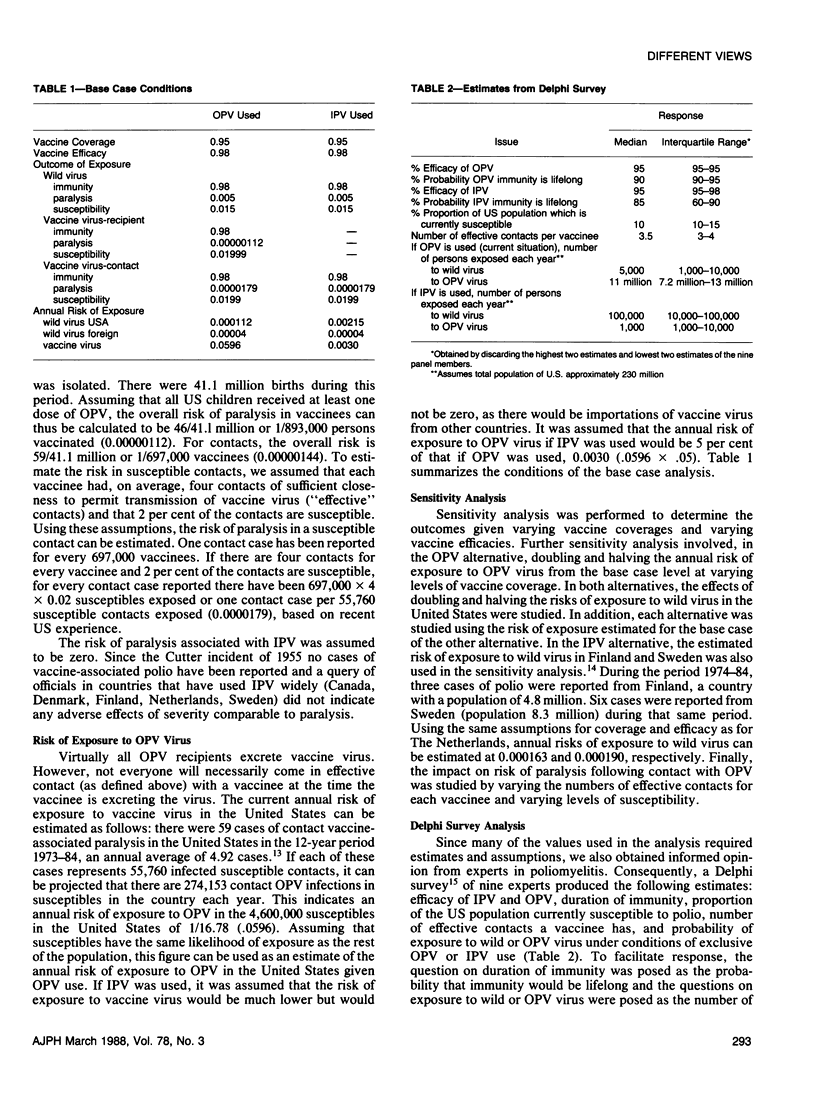
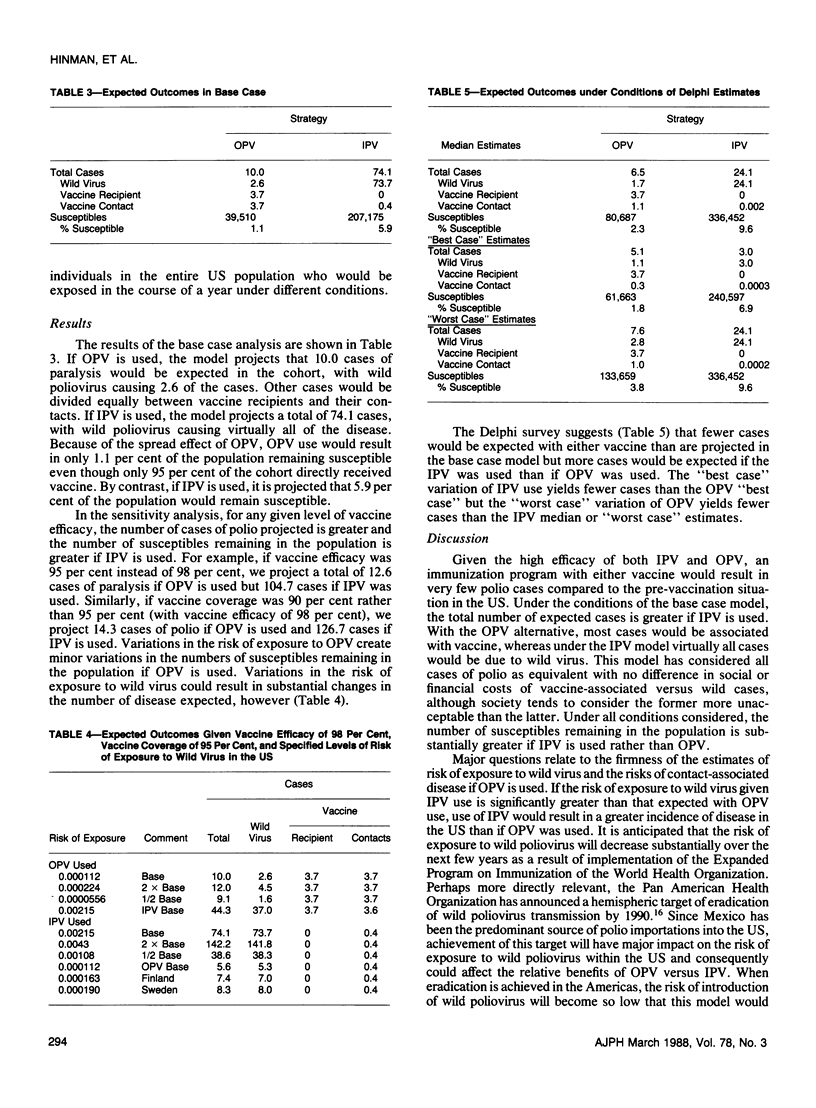
Using decision analysis we evaluated the benefits and risks of continued primary reliance on oral poliomyelitis vaccine (OPV) compared to use of inactivated poliovirus vaccine (IPV). We followed a hypothetical cohort of 3.5 million children from birth to age 30 assuming 95 per cent coverage with 98 per cent effective vaccine. Primary reliance on IPV would result in more cases of paralytic poliomyelitis as well as more susceptibles remaining in the population than would be expected with continuing OPV use (74.1 vs 10.0 cases and 5.9 per cent vs 1.1 per cent susceptibles, respectively). However, with OPV use, most cases of paralysis seen would be associated with the vaccine. Our analysis supports a continuation of current US policy placing primary reliance on OPV but the conclusion is heavily dependent on assumptions of risk of exposure to wild virus in the United States. Major declines in risk of exposure to wild virus could alter the balance significantly.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E. R. Landmark perspective: Inactivated poliomyelitis vaccination. Issues reconsidered. JAMA. 1984 May 25;251(20):2710–2712. [PubMed] [Google Scholar]
- Bernier R. H. Some observations on poliomyelitis lameness surveys. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S371–S375. doi: 10.1093/clinids/6.supplement_2.s371. [DOI] [PubMed] [Google Scholar]
- HENDERSON D. A., WITTE J. J., MORRIS L., LANGMUIR A. D. PARALYTIC DISEASE ASSOCIATED WITH ORAL POLIO VACCINES. JAMA. 1964 Oct 5;190:41–48. doi: 10.1001/jama.1964.03070140047006. [DOI] [PubMed] [Google Scholar]
- Kassirer J. P., Moskowitz A. J., Lau J., Pauker S. G. Decision analysis: a progress report. Ann Intern Med. 1987 Feb;106(2):275–291. doi: 10.7326/0003-4819-106-2-275. [DOI] [PubMed] [Google Scholar]
- McBean A. M., Thoms M. L., Johnson R. H., Gadless B. R., MacDonald B., Nerhood L., Cummins P., Hughes J., Kinnear J., Watts C. A comparison of the serologic responses to oral and injectable trivalent poliovirus vaccines. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S552–S555. doi: 10.1093/clinids/6.supplement_2.s552. [DOI] [PubMed] [Google Scholar]
- NATHANSON N., LANGMUIR A. D. THE CUTTER INCIDENT. POLIOMYELITIS FOLLOWING FORMALDEHYDE- INACTIVATED POLIOVIRUS VACCINATION IN THE UNITED STATES DURING THE SPRING OF 1955. I. BACKGROUND. Am J Hyg. 1963 Jul;78:16–28. doi: 10.1093/oxfordjournals.aje.a120327. [DOI] [PubMed] [Google Scholar]
- Nightingale E. O. Recommendations for a national policy on poliomyelitis vaccination. N Engl J Med. 1977 Aug 4;297(5):249–253. doi: 10.1056/NEJM197708042970505. [DOI] [PubMed] [Google Scholar]
- Nkowane B. M., Wassilak S. G., Orenstein W. A., Bart K. J., Schonberger L. B., Hinman A. R., Kew O. M. Vaccine-associated paralytic poliomyelitis. United States: 1973 through 1984. JAMA. 1987 Mar 13;257(10):1335–1340. [PubMed] [Google Scholar]
- Pauker S. G., Kassirer J. P. Decision analysis. N Engl J Med. 1987 Jan 29;316(5):250–258. doi: 10.1056/NEJM198701293160505. [DOI] [PubMed] [Google Scholar]
- Salk D. Eradication of poliomyelitis in the United States. II. Experience with killed poliovirus vaccine. Rev Infect Dis. 1980 Mar-Apr;2(2):243–257. doi: 10.1093/clinids/2.2.243. [DOI] [PubMed] [Google Scholar]


