Abstract
The S-box transcription termination control system, first identified in Bacillus subtilis, is used for regulation of gene expression in response to methionine availability. The presence of the S-box motif provided the first indication that the ykrTS and ykrWXYZ genes could play a role in recycling of 5′-methylthioadenosine, a by-product of polyamine biosynthesis that can be converted to methionine. In this study we demonstrate a role for the ykrTS and ykrWXYZ gene products in this pathway.
A common problem in the analysis of genomic sequence data is the assignment of function to newly identified open reading frames. It is often difficult to determine the functional role of the newly identified open reading frame in the absence of additional information on expression patterns or effects of gene inactivation. One useful tool can be the recognition of potential signals for regulation of gene expression. The S-box regulon in gram-positive bacteria represents an example where easily recognized regulatory signals provide a clear indication of metabolic function.
The S-box regulon encompasses approximately 60 transcriptional units in a variety of bacterial species, 11 of which are in Bacillus subtilis (7; unpublished results). Members of the family were identified on the basis of conserved leader region structural and sequence features and were proposed to be regulated in concert at the level of transcription termination in response to methionine availability (7). The conserved leader elements include a transcriptional terminator and competing antiterminator, as well as a highly conserved element that functions as an anti-antiterminator and was postulated to be a target for repression during growth in the presence of methionine. Roles in methionine biosynthesis have been demonstrated or postulated for a number of these genes (7, 8), and expression of several of the genes in this group was shown to be repressed by methionine (7; unpublished results). Conservation of the regulatory pattern suggested that other genes with these features might have unknown functions in methionine metabolism, e.g., in alternate routes to the biosynthesis of methionine. The combination of the regulatory clues and sequence similarity to proteins of known function led us to investigate a role for the ykrTS and ykrWXYZ S-box genes in recycling of 5′-methylthioadenosine (MTA).
MTA recycling.
MTA is a sulfur-containing compound that is generated as a by-product of polyamine biosynthesis and can be recycled to methionine by a number of organisms, including B. subtilis and Klebsiella pneumoniae (6, 16) (Fig. 1). Decarboxylated S-adenosylmethionine (SAM) is generated by the speD gene product; the aminopropyl moiety of decarboxylated SAM is then transferred to putrescine by spermidine synthase, the product of the speE gene (15), generating spermidine and MTA. MTA is cleaved by the mtn gene product, MTA/S-adenosylhomocysteine nucleosidase (pfs gene product in Escherichia coli), to yield adenine and 5-methylthioribose (MTR) (2, 3, 4, 16). MTR is excreted in E. coli (14, 17), whereas B. subtilis is capable of using either MTA or MTR as the sole sulfur source (16).
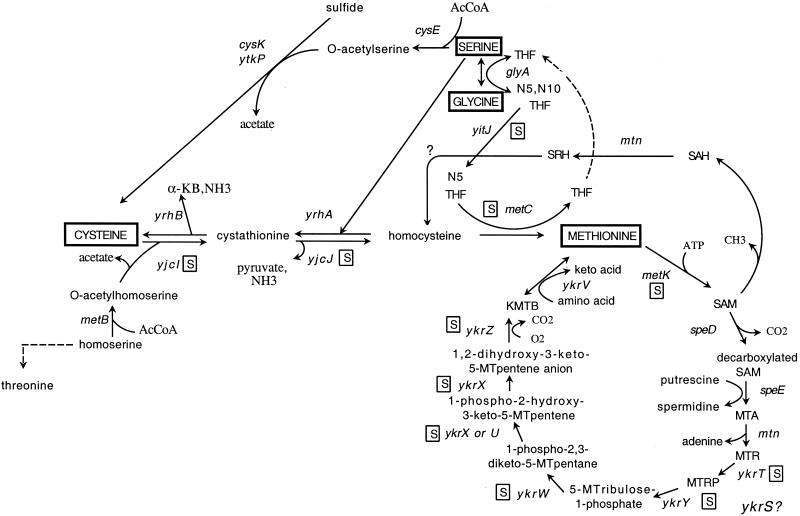
FIG. 1.
Methionine biosynthesis pathways in B. subtilis. Genes that are preceded by S-box regulatory elements are shown with a boxed “S.” The “y” designation in gene names indicates genes whose function has not been experimentally established; possible assignments to enzymatic steps demonstrated in K. pneumoniae (6, 19) are shown but have not yet been tested (8). The function of the ykrS gene product is not known. SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; SRH, S-ribosylhomocysteine; MT, methylthio; KMTB, 2-keto-4-methylthiobutyrate; THF, tetrahydrofolate.
The MTA recycling pathway is best characterized in K. pneumoniae, although few of the genes involved have been identified (6, 17). Examination of the intermediates in the pathway identified in K. pneumoniae and analysis of enzymatic steps likely to be required led us to hypothesize that the ykrTS and ykrWXYZ S-box operons, as well as the nearby ykrU and ykrV genes, were likely to be involved in this pathway in B. subtilis (8) (Fig. 1). The K. pneumoniae gene encoding MTR kinase, which converts MTR to MTR phosphate (MTRP; 5) has been identified (GenBank accession number AF212863), and levels of the enzyme were shown to be reduced during growth in the presence of methionine (19), consistent with a role in methionine production. The B. subtilis ykrT gene product is similar to K. pneumoniae MTR kinase (data not shown). MTRP is converted to 5-methylthioribulose-1-phosphate in K. pneumoniae by an isomerase (6); the ykrY gene product, encoded in the ykrWXYZ operon, is a likely candidate for this activity, based on similarity to sugar isomerases. We previously noted that YkrW is related to ribulose-1,5-bisphosphate carboxylase (RubisCO), the key enzyme in CO2 fixation (7). However, no homolog of ribulose kinase, which is required for production of ribulose-1,5-bisphosphate, the substrate for RubisCO, could be identified in the B. subtilis genome. The similarity of 5-methylthioribulose-1-phosphate to ribulose-1,5-bisphosphate and the position of ykrW in an S-box operon adjacent to genes implicated in the MTA recycling pathway led us to hypothesize a role for this gene in this pathway (8). An intriguing connection between an unusual RubisCO homolog and sulfur metabolism has also been uncovered in Chlorobium tepidum (9). The next step in the pathway requires an enolase-phosphatase, for which the product of the ykrX gene is a candidate, while the final steps could be catalyzed by YkrZ and YkrV; ykrV is located between the divergent ykrTS and ykrWXYZ operons and encodes a protein related to aminotransferases. No enzymatic function could be attributed to YkrS in our analysis. YkrS exhibits some similarity to eukaryotic translation initiation factor eIF2Bα, the significance of which is unknown; related genes are found in a variety of microorganisms, including archaea (11), but no information about its physiological role is available.
Roles of ykrTS and ykrWXYZ genes in growth on MTA or MTR.
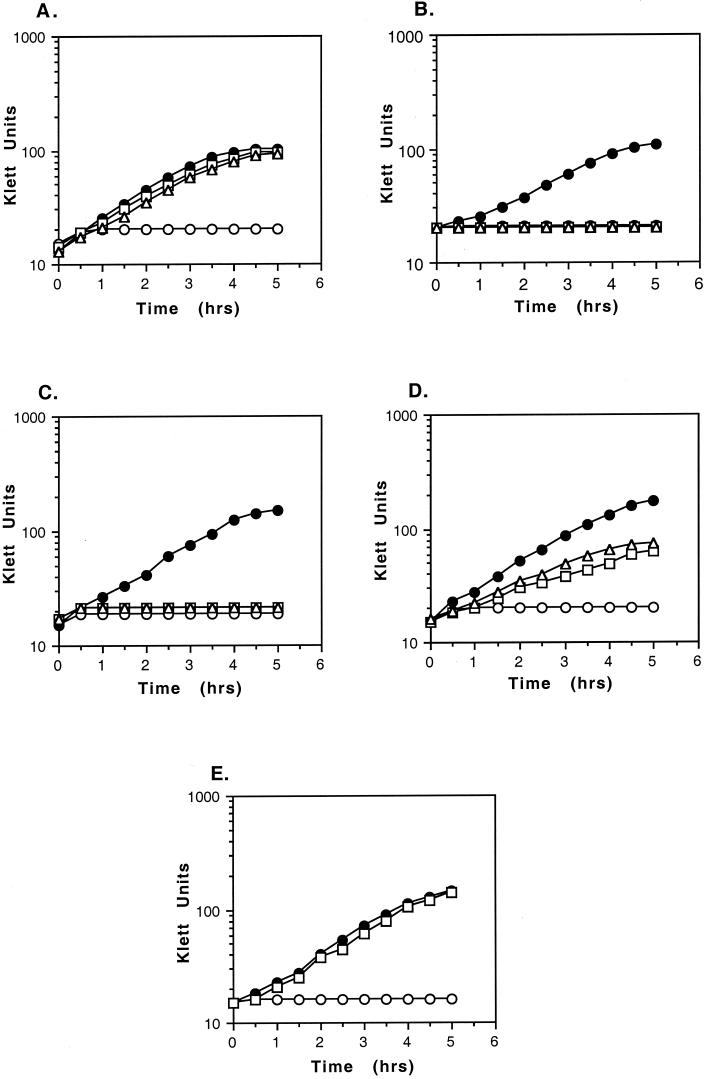
Since B. subtilis efficiently scavenges sulfur-containing compounds, we used a genetic background in which the metB gene, encoding homoserine O-acetyltransferase, is defective, so that the normal pathway of methionine biosynthesis is blocked. Strains in which ykrT (BR151-YkrTKO) or ykrS (BR151-YkrSKO) were inactivated were generated and compared to an isogenic control strain (BR151-ZKO). For the ykrT mutant, expression of the downstream ykrS gene was left under the control of the normal ykrTS promoter and leader region, so that normal expression levels were maintained. This was important since inactivation of ykrS was previously shown to result in reduced expression of S-box genes (7). No growth was observed for any strain containing the metB allele in media containing sulfate alone, and all strains grew equally well in the presence of methionine (Fig. 2). MTA or MTR could replace methionine for growth of the control strain, but mutation of either ykrT or ykrS resulted in total loss of growth on MTA or MTR, indicating that both genes are required for utilization of these compounds. The defect in utilization of MTA and MTR confirms a role for ykrT and ykrS in this pathway. Similar results were obtained by Sekowska et al. (18), who also demonstrated that the ykrT gene product exhibits MTR kinase activity in vitro.
FIG.2.
Growth of ykrTS and ykrWXYZ mutants. ykrS, ykrW, and yitJ mutants were generated by insertional inactivation by Campbell recombination of a derivative of plasmid pBEST 501 (10) containing an internal fragment of the target gene into the chromosome of strain BR151MA (lys-3 trpC2). The ykrT mutant was generated by homologous recombination, using a plasmid in which the ykrT coding region was deleted and the ykrS coding region was placed immediately downstream of the ykrT leader region. Strain constructions were verified by PCR and DNA sequencing. Cultures were grown overnight in Spizizen minimal medium (1) containing methionine (0.34 mM), and cells were collected by centrifugation and resuspended in medium containing sulfate alone (open circles), methionine (filled circles), MTA (Sigma) at 0.4 mM (squares), or MTR at 0.4 mM (triangles). MTR was prepared by acid hydrolysis of MTA as described by Schlenk et al. (13). (A) BR151-ZKO (metB10); (B) BR151-YkrTKO (metB10 ΔykrT); (C) BR151-YkrSKO (metB10 ykrS::neo); (D) BR151-YkrWKO (metB10 ykrW::neo); (E) BR151MA-YitJKO (yitJ::neo).
Insertional inactivation of the ykrWXYZ operon resulted in a partial defect in growth on MTA (Fig. 2D), suggesting that these genes are also involved in MTA recycling and that there may be an alternate pathway for generation of methionine from MTRP in B. subtilis. This is supported by the apparent absence of genes related to ykrWXYZ in organisms in which ykrT is found (data not shown). Inactivation of the yitJ gene, which is in the primary pathway of methionine biosynthesis (7, 8) (Fig. 1), resulted in loss of growth in the absence of methionine, but growth on MTA was unaffected (Fig. 2E), indicating that the pathway for methionine generation from MTA is independent of the normal biosynthetic pathway.
Regulation of ykrTS and ykrWXYZ expression.
Previous studies indicated that expression of ykrTS responds to methionine availability (7). Identification of a role in the MTA recycling pathway led us to test expression during growth in the presence of MTA. A ykrT-lacZ transcriptional fusion, containing the promoter, leader, and leader region terminator fused to a lacZ reporter gene, was integrated in single copy into the B. subtilis chromosome using an SPβ prophage. Strains were grown to early exponential phase in the presence of methionine, the cultures were split, and growth was continued in the presence or absence of methionine or MTA. In a metB strain (BR151-ZKO), expression was very low during growth in the presence of exogenous methionine and was induced 1,300-fold when cells were starved for methionine (Table 1), as previously reported (7). Replacement of methionine by MTA resulted in moderate expression of the ykrT-lacZ fusion, consistent with the ability of the cells to utilize this compound to generate intracellular methionine. Growth in the presence of both methionine and MTA resulted in nearly complete repression of ykrT-lacZ expression, indicating that utilization of MTA is repressed during growth in the presence of exogenous methionine. Similar results were obtained with MTR in place of MTA (data not shown).
TABLE 1.
Regulation of lacZ fusions in response to methionine and MTA
| Fusion | Strain | Relevant genotype | β-Galactosidase activitya
|
|||
|---|---|---|---|---|---|---|
| −Met | +Met | +MTA | +Met, +MTA | |||
| ykrT-lacZ | BR151-ZKO | metB tyrZ::neo | 130 | 0.10 | 7.9 | 0.18 |
| ykrT-lacZ | BR151-YkrTKO | metB ΔykrT tyrZ::neo | 70 | 0.14 | 77 | 0.23 |
| ykrT-lacZ | BR151-YkrSKO | metB ykrS::neo | 50 | 0.11 | 61 | 0.11 |
| ykrW-lacZ | BR151-ZKO | metB tyrZ::neo | 38 | 0.63 | 8.0 | 0.92 |
| yitJ-lacZ | BR151 | metB | 340 | 0.17 | 10 | 0.32 |
Cultures were grown in Spizizen minimal medium (1) containing methionine (0.34 mM), split, and grown in the presence or absence of methionine and/or MTA (0.4 mM). Samples were taken 4 h after the cultures were split. β-Galactosidase activities are in Miller units (12).
Expression of the ykrT-lacZ fusion responded normally to methionine availability in strain BR151-YkrTKO or BR151-YkrSKO, in which the ykrT or ykrS gene was inactivated (Table 1), although expression under inducing conditions was reduced somewhat, as previously reported for a ykrS mutant (7). Since inactivation of ykrT and ykrS resulted in a similar decrease in ykrT-lacZ expression, this effect may in some way be due to disruption of the MTA recycling pathway. Addition of MTA (or MTR) had no repressive effect on expression, in contrast to the results for the parent strain. This is consistent with the inability of the ykrT and ykrS mutant strains to convert MTA or MTR to methionine, so that expression of ykrTS remained fully derepressed during growth in the presence of MTA or MTR.
Our results on regulation of ykrTS expression in response to methionine differ from those of Sekowska et al. (18), who found little effect of addition of exogenous methionine. However, those studies were performed using a methionine prototroph, so that intracellular methionine pools were high during growth in sulfate, masking the substantial effect of methionine deprivation in cells unable to generate endogenous methionine.
Expression of a ykrW-lacZ fusion was also induced by methionine starvation (Table 1), consistent with the presence of an S-box leader and a role in methionine biosynthesis. Expression during growth in the presence of methionine was not as tightly repressed by methionine availability as was expression of ykrT. The expression pattern was similar to that of ykrT during growth in MTA.
The effect of MTA on expression of the yitJ gene, which is in the primary pathway of methionine biosynthesis (Fig. 1), was also tested to determine if this compound generally affects expression of S-box genes. Expression of a yitJ-lacZ fusion was induced by starvation for methionine, as previously reported (7), and was moderate during growth in the presence of MTA (Table 1), as was observed for the ykrT-lacZ fusion. This indicates that the partial repression of ykrTS expression observed during growth on MTA and MTR is likely to be a consequence of the intracellular methionine generated by the recycling pathway and reflects the general pattern of regulation of genes in the S-box family rather than a specific regulatory effect for genes directly involved in this pathway.
Acknowledgments
This work was supported by grant GM47823 from the National Institute of General Medical Sciences, National Institutes of Health (NIH), and by NIH Predoctoral Fellowship F31 GM20923 to B.A.M.
We thank C. Bobst for assistance in preparation of MTR, F. R. Tabita for discussion of RubisCO structure and function, J. Collins for construction of strain BR151MA-YitJKO, and E. Sirohi for construction of strain BR151-YkrWKO and the ykrW-lacZ fusion.
REFERENCES
- 1.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornell, K. A., and M. K. Riscoe. 1998. Cloning and expression of Escherichia coli 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase: identification of the pfs gene product. Biochim. Biophys. Acta 1396:8-14. [DOI] [PubMed] [Google Scholar]
- 3.Cornell, K. A., R. W. Winter, P. A. Tower, and M. K. Riscoe. 1996. Affinity purification of 5-methylthioribose kinase and 5-methylthioadenosine/S-adenosylhomocysteine nucleosidase from Klebsiella pneumoniae. Biochem. J. 317:285-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duerre, J. A. 1962. A hydrolytic nucleosidase acting on S-adenosylhomocysteine and on 5′-methylthioadenosine. J. Biol. Chem. 237:3737-3741. [Google Scholar]
- 5.Ferro, A. J., A. Barrett, and S. K. Shapiro. 1978. 5-methylthioribose kinase. A new enzyme involved in the formation of methionine from 5-methylthioribose. J. Biol. Chem. 253:6021-6025. [PubMed] [Google Scholar]
- 6.Furfine, E. S., and R. H. Abeles. 1988. Intermediates in the conversion of 5′-S-methylthioadenosine to methionine in Klebsiella pneumoniae. J. Biol. Chem. 263:9598-9606. [PubMed] [Google Scholar]
- 7.Grundy, F. J., and T. M. Henkin. 1998. The S box regulon: a new global transcription termination control system for methionine and cysteine biosynthesis genes in Gram-positive bacteria. Mol. Microbiol. 30:737-749. [DOI] [PubMed] [Google Scholar]
- 8.Grundy, F. J., and T. M. Henkin. 2002. Biosynthesis of serine, glycine, cysteine, and methionine, p. 245-254. In A. L. Sonenshein, R. M. Losick, and J. A. Hoch (ed.), Bacillus subtilis and its relatives: from genes to cells . American Society for Microbiology, Washington, D.C.
- 9.Hanson, T. E., and F. R. Tabita. 2001. A ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO)-like protein from Chlorobium tepidum that is involved with sulfur metabolism and the response to oxidative stress. Proc. Natl. Acad. Sci. USA 98:4397-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itaya, M., K. Kondo, and T. Tanaka. 1989. A neomycin resistance gene cassette selectable in a single copy in the Bacillus subtilis chromosome. Nucleic Acids Res. 17:4410.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyrpides, N. C., and C. R. Woese. 1998. Archael translation revisited: the initiation factor 2 and eukaryotic initiation factor 2B α−β−γ families. Proc. Natl. Acad. Sci. USA 95:3726-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 13.Schlenk, F., C. R. Zydek-Cwick, and J. L. Dainko. 1973. 5′-Methylthioadenosine and related compounds as precursors of S-adenosylmethionine in yeast. Biochim. Biophys. Acta 320:357-362. [DOI] [PubMed] [Google Scholar]
- 14.Schroeder, H. R., C. J. Barnes, R. C. Bohinski, and M. F. Mallette. 1973. Biological production of 5-methylthioribose. Can. J. Microbiol. 19:1347-1354. [DOI] [PubMed] [Google Scholar]
- 15.Sekowska, A., P. Bertin, and A. Danchin. 1998. Characterization of polyamine synthesis in Bacillus subtilis 168. Mol. Microbiol. 29:851-858. [DOI] [PubMed] [Google Scholar]
- 16.Sekowska, A., and A. Danchin. 1999. Identification of yrrU as the methylthioadenosine nucleosidase gene in Bacillus subtilis. DNA Res. 6:255-264. [DOI] [PubMed] [Google Scholar]
- 17.Sekowska, A., H. Kung, and A. Danchin. 2000. Sulfur metabolism in Escherichia coli and related bacteria: facts and fiction. J. Mol. Microbiol. Biotechnol. 2:145-177. [PubMed] [Google Scholar]
- 18.Sekowska, A., L. Mulard, S. Krogh, J. K. S. Tse, and A. Danchin. 2001. MtnK, methylthioribose kinase, is a starvation-induced protein in Bacillus subtilis. BMC Microbiol. 1:15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tower, P. A., D. B. Alexander, L. L. Johnson, and M. K. Riscoe. 1993. Regulation of methylthioribose kinase by methionine in Klebsiella pneumoniae. J. Gen. Microbiol. 139:1027-1031. [DOI] [PubMed] [Google Scholar]