Abstract
We previously demonstrated that Treponema pallidum TroA is a periplasmic metal-binding protein (MBP) with a distinctive alpha-helical backbone. To better understand the mechanisms of metal binding and release by TroA, we determined the crystal structure of the apoprotein at a resolution of 2.5 Å and compared it to that of the Zn(II)-bound form (Protein Data Bank accession code 1toa). apo-TroA shows a conformation even more closed than that of its Zn(II)-bound counterpart due to a 4° tilt of the C-terminal domain (residues 190 through 308) about an axis parallel to the poorly flexible backbone helix. This domain tilting pushes two loops (residues 248 through 253 and 277 through 286) towards the metal-binding site by more than 1 Å, resulting in an unfavorable interaction of I251 with D66. To avoid this contact, D66 shifts towards H68, one of the four Zn(II)-coordinating residues. The approach of this negative charge coincides with the flipping of the imidazole side chain of H68, resulting in the formation of a new hydrogen bond. The conformational change of H68, along with a slight rearrangement of D279, a C-terminal domain Zn(II)-coordinating residue, distorts the metal-binding site geometry, presumably causing the release of the bound metal ion. Ligand binding and release by TroA, and presumably by other members of the MBP cluster, differs from the “Venus flytrap” mechanism utilized by bacterial nonmetal solute-binding receptors.
Originally reported to be an outer membrane protein of the syphilis spirochete Treponema pallidum (3, 4), TroA has been shown by genetic, ultrastructural, and X-ray crystallographic studies to actually be the binding component of an ATP-binding cassette (ABC) transport system which shuttles Zn(II) and possibly other transition metals (e.g., manganese) across the T. pallidum cytoplasmic membrane (1, 11-13, 18, 21; K. R. O. Hazlett, F. Rusnak, and J. D. Radolf, unpublished observations). TroA is 1 of more than 40 members of a newly defined cluster of metal-binding proteins within the extended family of bacterial solute-binding receptors (SBRs) (8, 23).
Members of the SBR family have N- and C-terminal domains separated by a crevice which serves as the ligand-binding site (22). The domains of the receptors for nonmetal ligands such as sulfate, phosphate, amino acids, oligopeptides, monosaccharides, and oligosaccharides are hinged by two or three β strands that function according to a so-called “Venus flytrap” mechanism. According to this model, a hinge-bending motion traps the ligand within the cleft between the two domains. Conversely, upon release of the ligand, the receptor adopts an open conformation, in which the two domains of the apoprotein are further apart and the binding site is more solvent accessible (22). Unlike with the nonmetal SBRs, the two domains of the MBPs are linked by an extended and presumably poorly flexible α-helix which interacts along its entire length with both domains (17, 18). Because of this structural difference, we hypothesized that ligand binding and release by MBPs would proceed via mechanisms different from those by which non-metal-binding SBRs proceed. We have obtained evidence for this hypothesis by determining the crystal structure of apo-TroA and comparing it to that of the Zn(II)-bound form of the protein.
Preparation and crystallization of apo-TroA.
A recombinant DNA construct encoding TroA with an N-terminal His6 tag but without its leader peptide (residues 23 through 308)6 was previously described (11). Protein was overexpressed in Escherichia coli DH5α and purified from cell lysates by use of a Ni-nitrilotriacetic acid agarose affinity matrix (Qiagen, Santa Clarita, Calif.). The Ni-nitrilotriacetic acid column eluate was dialyzed and further purified with a Mono-Q HR 5/5 column (Amersham Pharmacia Biotech, Piscataway, N.J.). The purified protein was eluted with a linear gradient of NaCl (20 to 800 mM) and diluted to a concentration of 2 to 3 mg/ml. The diluted sample was dialyzed overnight against a buffer of 0.1 M Na acetate (pH 4.6) and 10 mM 1,10-phenanthroline (Sigma Chemical Co., St. Louis, Mo.); nonglass containers were used to prevent inadvertent metal contamination. Removal of Zn(II) from TroA was confirmed by electrospray mass spectrometry and inductively coupled plasma emission spectrometry. The functionality of the apoprotein was confirmed in binding studies which revealed a dissociation constant for Zn(II) in the midnanomolar range (Hazlett at el., unpublished). The apoprotein was reduced to a concentration of 10 to 20 mg per ml in 10 mM HEPES-HCl (pH 7.4)-10 mM NaCl-10 mM 1,10-phenanthroline with a Centricon-10 device (Amicon, Beverly, Mass.) with a molecular weight exclusion of 10,000 and stored at 4°C.
Hanging-drop vapor diffusion experiments were performed using 24-well Linbro plates (Hampton Research) at 20°C. Hanging drops (5 to 10 μl) were composed of equal volumes of protein solution and the reservoir solution [0.1 M Na acetate (pH 4.6), 0.2 M (NH4)2SO4, 10 mM 1,10-phenanthroline, 22.5% polyethylene glycol 2000]. After incubation for 1 week at 20°C, crystals grew to a size of 0.1 by 0.05 by 0.2 mm. Prior to data collection, crystals were soaked for 15 min in 15% glycerol-enriched mother liquor for cryogenic conditioning.
Data collection and processing.
The reflection data were collected using a Rigaku RU-200 rotating copper anode X-ray generator (Rigaku Corporation, Tokyo, Japan). The crystals were maintained at 100 K by using an MSC X-Stream cooling device (Molecular Structure Corporation, The Woodlands, Tex.). Diffraction images were recorded and scanned with an R-axis IV image plate system, and 1° oscillations (with 15 min per oscillation) were collected over a 180° sweep to guarantee completeness of the data. The reflection data were processed and merged with DENZO/SCALEPACK (20) after we collected a total of 48,569 reflections, producing 14,480 unique reflections with a merging R factor of 0.078 in a resolution range of 30.0 to 2.5 (Table 1). The apo-TroA crystals were found to belong to space group C2, with unit cell dimensions a, b, c, and β of 117.36, 38.35, and 104.47 Å, and 104.11°, respectively, and one molecule of apo-TroA per asymmetric unit (19).
TABLE 1.
Statistics of reflection data and structure refinement
| Characteristic | Value(s) |
|---|---|
| Space group and unit cell dimensions (Å) | C2, 117.36 by 38.35 by 104.47 with β = 104.11° |
| Resolution range (Å) | 30.0-2.5 |
| Number of reflections [F ≥ σ(F)] | 13,646 |
| Completeness (%) | 90.3 (92.1) |
| Redundancy | 3.4 (3.4)a |
| I/σ(I) | 17.4 (2.5)a |
| Rsymb | 0.078 |
| Rcrys | 0.208 |
| Rfrce | 0.253 |
| No. of amino acids | 276 |
| No. of protein atoms | 2,138 |
| No. of water atoms | 28 |
| No. of observations/no. of parameters | 1.58 |
| R.m.s. deviationc of: | |
| bond lengths (Å) | 0.017 |
| angles (°) | 1.831 |
| dihedral angles (°) | 24.05 |
| Mean B factor of: | |
| protein atoms (Å2) | 36.7 |
| water atoms (Å2) | 59.4 |
| Overall B factor (from Wilson plot) (Å2) | 56.5 |
The number in parentheses is the value in the highest-resolution shell.
Rsym = Σh(Σj|Ih,j − 〈Ih〉|/ΣIh,j), where h is the set of Miller indices and j is the set of observations of reflection h.
R.m.s. values are deviations from ideal values.
Molecular replacement and model refinement.
The structure of apo-TroA was determined by the molecular replacement method with X-PLOR (6). A single-polyalanine form of TroA (18) (Protein Data Bank accession code 1toa) was used as a search model. An X-PLOR rotation and subsequent translation search was performed using a data subset between 15.0 and 4.0 Å. Assignment of the side chains was made with O (14). Refinement of the structure was performed with X-PLOR with an initial protocol of rigid-body refinement, positional refinement, simulated annealing, and temperature factor refinement by using all data from 30.0 to 2.5 Å. Correction of the model was guided by SIGMAA-corrected 2Fo-Fc electron density maps. During the entire rebuilding and refinement procedure, adjustments were made only to the original model such that early refinement errors would not be propagated in subsequent cycles. Further rounds of refinement followed a protocol of iterative positional (20- to 40-cycle) and B factor (20-cycle) refinement, which was continued until Rfree values converged (5). The refinement R factors (Rcrys and Rfree) dropped to 0.219 and 0.274, respectively, with only the protein atoms being used. After the addition of 28 water molecules to the structure, the R values dropped to 0.208 and 0.253, respectively, with all 13,646 reflections (I ≥ σI) in a resolution range of 30.0 to 2.5 Å being used (Table 1). The final structure has a total of 2,166 scatterers, providing a datum/scatterer ratio of 1 to 58. Geometric analysis with PROCHECK (9) showed that no residue is within “disallowed” regions in a phi and psi angle distribution. Key statistics of the quality of the model, reflection data, and structure refinement are presented in Table 1.
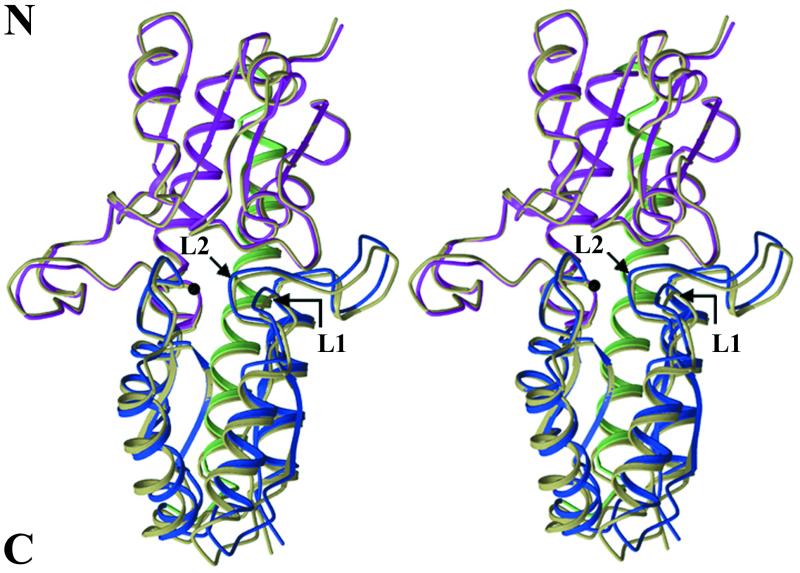
C-terminal domain tilting of apo-TroA.
The current structure of apo-TroA consists of 276 amino acid residues (residues 33 through 308). Figure 1 demonstrates that the folding pattern and secondary structural elements of Zn(II)-bound TroA were retained by the apoprotein. A significant difference in overall conformation, however, was found in residues 221 through 291 of the C-terminal domain, which were tilted approximately 4° from their position in the Zn(II)-bound form about an axis parallel to the backbone helix. It has been shown that Zn(II) significantly contributes to the interactions between the N- and C-terminal domains of TroA by coordinating with two residues from each domain (H68 and H133 from the N-terminal domain and H199 and D279 from the C-terminal domain) (18). The loss of Zn(II) from this central location causes an effect analogous to the removal of a prop from two objects leaning on each other. The change in the C-terminal domain is mainly the result of a partial collapse into the empty Zn(II)-binding pocket by loops L1 (residues 248 through 253) and L2 (residues 277 through 286) from the C-terminal domain (Fig. 1). If the helix served as a hinge for the two domains, some conformational changes such as unwinding or bending of this helix would accompany the C-terminal domain tilting. Interestingly, as shown in Fig. 1, the C-terminal domain tilting is not accompanied by any conformational change in the backbone helix. The structures interacting with the helix also do not appear to follow the domain tilting. Except for the Zn(II)-binding site, the domain-domain interface is highly hydrophobic and the result of its exposure to solvent is highly unfavorable (18). Furthermore, while the crystal contact of apo-TroA in the C2 space group is formed mainly through the C-terminal domain, exposure of the N-terminal domain to the solvent allows conformational flexibility due to the lack of restraining protein-protein interactions; thus, the limited conformational changes shown by the C-terminal domain are not due to crystal packing. Rather, the inflexible nature of the helix and its surrounding core and the hydrophobic nature of the domain interface act in concert to limit the changes in conformation associated with Zn(II) binding.
FIG.1.
Stereoscopic views of the superimposed ribbon diagrams of apoprotein and Zn(II)-bound TroA. The structures of Zn(II)-free and Zn(II)-bound TroA were superimposed by a least-squares method fit of the N-terminal domain of the Zn(II)-bound TroA onto that of the Zn(II)-free TroA using O (14). The N- and C-terminal domains of the Zn(II)-free form are colored lavender and blue, respectively, while the backbone helix is green. The superimposed structure of Zn(II)-bound TroA is grayish green. The dark ball represents Zn(II) in metal-bound TroA. The two C-terminal domain loops related to the collapse of the binding site in the apoprotein are labeled L1 (residues 248 through 253) and L2 (residues 277 through 286). Note that the major part of the C-terminal domain of the apoprotein is tilted approximately 4° about an axis parallel to the backbone helix. Structural illustrations were made with MOLSCRIPT (16).
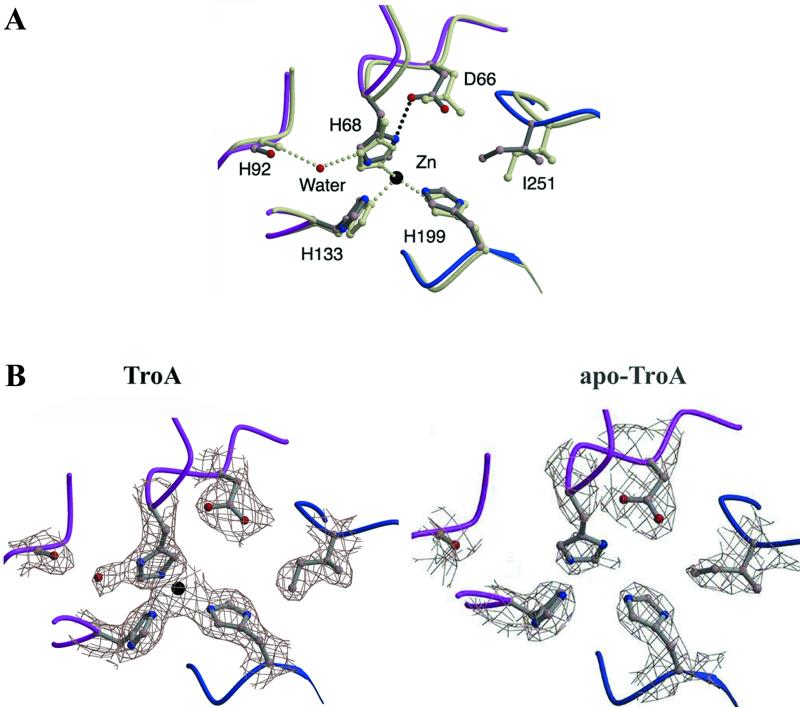
Changes in the Zn(II)-binding pocket.
As mentioned above, C-terminal domain tilting results in two C-terminal domain loops collapsing into the Zn(II)-binding cavity, thereby reducing its volume almost twofold, from 97.2 to 59.3 Å3, as calculated by using “voidoo” (15). As a consequence of this collapse, I251 is shifted towards the active site by more than 1.0 Å, which results in a 0.8-Å displacement of D66 towards H68 (Fig. 2A). The imidazole ring of H68 has flipped in the apo-TroA structure, positioning Nδ1 to form a hydrogen bond with Oδ1 and Oδ2 of D66 and D279, respectively, thus moving Nɛ2 out of position to coordinate a Zn(II) atom. This is in contrast to the Zn(II)-bound form, in which the Nδ1 of H68 is hydrogen bonded to water molecule 434, thereby orienting Nɛ2 for Zn(II) binding (18). Upon Zn(II) release from the active site, the side chains of the other Zn-coordinating residues, H133 and H199, do not undergo significant changes in their conformations or hydrogen-bonding patterns and no additional water molecules replace the Zn(II) atom in the binding site.
FIG. 2.
Comparison of the Zn-binding site in apoprotein- and Zn(II)-bound TroA. (A) Ball and stick representation. The backbones of the N- and C-terminal domains of the apoprotein are shown in lavender and blue, respectively, while the side chains are dark gray. The superimposed structure of Zn(II)-bound TroA is grayish green. The Zn(II) ion is depicted by a dark ball. Note that the imidazole ring of H68 has flipped to form a new hydrogen bond to D66, indicated by the dark broken line. (B) 2Fo-Fc electron density maps drawn at a resolution of 2.5 Å and a 1.3 σ level. As in panel A, lavender and blue are used to represent the N- and C-terminal domains, respectively. Note that the electron densities for the Zn(II) ion and water molecules are not observed in the apoprotein. In both panels, the Zn(II)-coordinating residue D279 is not shown in order to highlight the interactions between I251, D66, and H68 in the apoprotein.
Proposed mechanisms for binding and release of metal ligands.
While the structural changes shown inside the Zn(II)-binding pocket appear to be microscopic in scale compared with those observed in nonmetal SBRs, they are significant enough to distort the geometry for Zn(II) coordination and disallow metal binding. Interestingly, the geometry for Zn(II) coordination appears to be restorable by the incorporation of two water molecules into the pocket, one near D66 to reduce the approach of I251 and the other near H92. Considering the hydrophilic nature of the TroA Zn(II)-binding pocket and the conformational flexibility of globular proteins in general, incorporation of water into the site seems highly feasible. Thus, as summarized in the scheme
 |
we propose that apo-TroA exists in a state of equilibrium between two conformational states, Tn, the nonpermissive state, which is represented by the current structure and prohibits Zn(II) binding, and Tp, the hydrated form which restores the geometry for Zn(II) coordination. In the permissive state, the negative charge of the orphaned D279 probably serves as a driving force to attract positively charged Zn(II) ions to the binding site.
Structural evidence for the existence of a conformational equilibrium between the two forms of apo-TroA includes a Wilson B factor much higher than that of the Zn(II)-bound TroA (31 and 56 Å2, respectively [Table 1]) and a much weaker electron density of H68 (Fig. 2B). Meanwhile, Zn(II)-bound TroA assumes only one conformational state, Tz, represented by the previously described structure (18). A simple transition between the two proposed states of apo-TroA would allow binding of Zn(II) and possibly other metal ligands with an affinity (i.e., midnanomolar range) comparable to that of the nonmetal SBRs (Hazlett at el., unpublished).
Biological implications.
It has long been accepted that the ligand-free forms of nonmetal SBRs exist in an equilibrium between the open and the closed forms and that the binding of ligands shifts the equilibrium toward the closed conformation (2). Furthermore, proteoliposome reconstitution experiments have demonstrated that the ligand-bound form of a prototypical SBR, maltose-binding protein, is selectively recognized by the transporter complex (7, 10). The comparatively minor conformational difference associated with C-terminal domain tilting is presumably large enough for the permease to distinguish between the metal-loaded and -unloaded forms of TroA. Furthermore, by extension from findings with the maltose ABC transporter (7), conformational changes induced in metal-loaded TroA upon interaction with the permease would cause a controlled release of the metal ligand, enabling ATP-driven vectorial transport across the cytoplasmic membrane and regeneration of the apoprotein.
Nucleotide sequence accession number.
The refined coordinates of apo-TroA have been deposited in the Protein Data Bank (accession code 1k0f).
Acknowledgments
This research was supported in part by U.S. Public Health Service R01 grants AI-26756 (J.D.R.), DK-52089 (C.A.H.), and AI-16692 (M.V.N.); by National Research Service Award AI-10573 (K.R.O.H.); by training grant GM-08203 (M.R.D.); and by Robert A. Welch Foundation grants I-0940 and I-1318 to M.V.N. and C.A.H., respectively.
We are grateful to Frank Rusnak (Mayo Clinic, Rochester, Minn.) for performing inductively coupled emission spectrometry of apo-TroA.
REFERENCES
- 1.Akins, D. R., E. Robinson, D. V. Shevchenko, C. Elkins, D. L. Cox, and J. D. Radolf. 1997. Tromp1, a putative rare outer membrane protein, is anchored by an uncleaved leader peptide to the Treponema pallidum cytoplasmic membrane. J. Bacteriol. 179:5076-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjorkman, A. J., and S. L. Mowbray. 1998. Multiple open forms of ribose-binding protein trace the path of its conformational change. J. Mol. Biol. 279:651-664. [DOI] [PubMed] [Google Scholar]
- 3.Blanco, D. R., C. I. Champion, M. M. Exner, H. Erdjument-Bromage, R. E. Hancock, P. Tempst, J. N. Miller, and M. A. Lovett. 1995. Porin activity and sequence analysis of a 31-kilodalton Treponema pallidum subsp. pallidum rare outer membrane protein (Tromp1). J. Bacteriol. 177:3556-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco, D. R., C. I. Champion, M. M. Exner, E. S. Shang, J. T. Skare, R. E. W. Hancock, J. N. Miller, and M. A. Lovett. 1996. Recombinant Treponema pallidum rare outer membrane protein 1 (Tromp1) expressed in Escherichia coli has porin activity and surface antigenic exposure. J. Bacteriol. 178:6685-6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brünger, A. T. 1992. Free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature 355:472-475. [DOI] [PubMed] [Google Scholar]
- 6.Brünger, A. T. 1993. X-PLOR version 3.1: a system for crystallography and NMR. Yale University Press, New Haven, Conn.
- 7.Chen, J., S. Sharma, F. A. Quiocho, and A. L. Davidson. 2001. Trapping the transition state of an ATP-binding cassette transporter: evidence for a concerted mechanism of maltose transport. Proc. Natl. Acad. Sci. USA 98:1525-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claverys, J.-P. 2001. A new family of high-affinity ABC manganese and zinc permeases. Res. Microbiol. 152:231-243. [DOI] [PubMed] [Google Scholar]
- 9.Collaborative Computational Project 4. 1994. The CCP4 Suite: programs for protein crystallography. Acta Crystallogr. Sect. D 50:760-763. [DOI] [PubMed] [Google Scholar]
- 10.Davidson, A. L., H. A. Shuman, and H. Nikaido. 1992. Mechanism of maltose transport in Escherichia coli: transmembrane signaling by periplasmic binding proteins. Proc. Natl. Acad. Sci. USA 89:2360-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deka, R. K., Y.-H. Lee, K. E. Hagman, D. V. Shevchenko, C. A. Lingwood, C. A. Hasemann, M. V. Norgard, and J. D. Radolf. 1998. Physicochemical evidence that Treponema pallidum TroA is a zinc-containing metalloprotein that lacks porin-like structure. J. Bacteriol. 181:4420-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser, C. M., S. J. Norris, G. M. Weinstock, O. White, G. C. Sutton, R. Dodson, M. Gwinn, E. K. Hickey, R. Clayton, K. A. Ketchum, E. Sodergren, J. M. Hardham, M. P. McLeod, S. Salzberg, J. Peterson, H. Khalak, D. Richardson, J. K. Howell, M. Chidambaram, T. Utterback, L. McDonald, P. Artiach, C. Bowman, M. D. Cotton, C. Fujii, S. Garland, B. Hatch, K. Horst, K. Roberts, M. Sandusky, J. Weidman, H. O. Smith, and J. C. Venter. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375-388. [DOI] [PubMed] [Google Scholar]
- 13.Hardham, J. M., L. V. Stamm, S. F. Porcella, J. G. Frye, N. Y. Barnes, J. K. Howell, S. L. Mueller, J. D. Radolf, G. M. Weinstock, and S. J. Norris. 1997. Identification and transcriptional analysis of a Treponema pallidum operon encoding a putative ABC transporter system, an iron-activated repressor protein homolog, and a glycolytic pathway enzyme homolog. Gene 197:47-64. [DOI] [PubMed] [Google Scholar]
- 14.Jones, T. A., M. Bergdoll, and M. O. Kjeldgaard. 1990. A macromolecule modeling environment, p. 189-199. In C. E. Bugg and S. E. Ealick (ed.), Crystallographic and modeling methods in molecular design. Springer-Verlag, New York, N.Y.
- 15.Kleywick, G. J., and T. A. Jones. 1994. Detection, delineation, measurement and display of cavities in macromolecular structures. Acta Crystallogr. Sect. D 50:178-185. [DOI] [PubMed] [Google Scholar]
- 16.Kraulis, P. J. 1991. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24:946-950. [Google Scholar]
- 17.Lawrence, M. C., P. A. Pilling, V. C. Epa, A. M. Berry, A. D. Ogunniyi, and J. C. Paton. 1999. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type protein. Structure 6:1553-1561. [DOI] [PubMed] [Google Scholar]
- 18.Lee, Y.-H., R. K. Deka, M. V. Norgard, J. D. Radolf, and C. A. Hasemann. 1999. Treponema pallidum TroA is a periplasmic zinc-binding protein with a helical backbone. Nat. Struct. Biol. 6:628-633. [DOI] [PubMed] [Google Scholar]
- 19.Matthews, B. W. 1968. Solvent content of protein crystals. J. Mol. Biol. 33:491-497. [DOI] [PubMed] [Google Scholar]
- 20.Otowinski, Z., and W. Minor. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276:307-326. [DOI] [PubMed] [Google Scholar]
- 21.Posey, J. E., J. M. Hardham, S. J. Norris, and F. C. Gherardini. 1999. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc. Natl. Acad. Sci. USA 96:10887-10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quiocho, F., and P. S. Ledvina. 1996. Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: variation of common themes. Mol. Microbiol. 20:17-25. [DOI] [PubMed] [Google Scholar]
- 23.Tam, R., and M. H. Saier, Jr. 1993. Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol. Rev. 57:320-346. [DOI] [PMC free article] [PubMed] [Google Scholar]