Abstract
Using the nitroimidazopyran-based antituberculosis drug PA-824 as a selective agent, transposon-generated Mycobacterium bovis strain BCG (M. bovis) mutants that could not make coenzyme F420 were identified. Four independent mutants that could not make F420 or the biosynthesis intermediate FO were examined more closely. These mutants contained transposons inserted in the M. bovis homologue of the Mycobacterium tuberculosis gene Rv1173, which we have named fbiC. Complementation of an M. bovis FbiC− mutant with fbiC restored the F420 phenotype. These data demonstrate that fbiC is essential for F420 production and that FbiC participates in a portion of the F420 biosynthetic pathway between pyrimidinedione and FO. Homologues of fbiC were found in all 11 microorganisms that have been fully sequenced and that are known to make F420. Four of these homologues (all from members of the aerobic actinomycetes) coded for proteins homologous over the entire length of the M. bovis FbiC, but in seven microorganisms two separate genes were found to code for proteins homologous with either the N-terminal or C-terminal portions of the M. bovis FbiC. Histidine-tagged FbiC overexpressed in Escherichia coli produced a fusion protein of the molecular mass predicted from the M. bovis BCG sequence (∼95,000 Da), as well as three other histidine-tagged proteins of significantly smaller size, which are thought to be proteolysis products of the FbiC fusion protein.
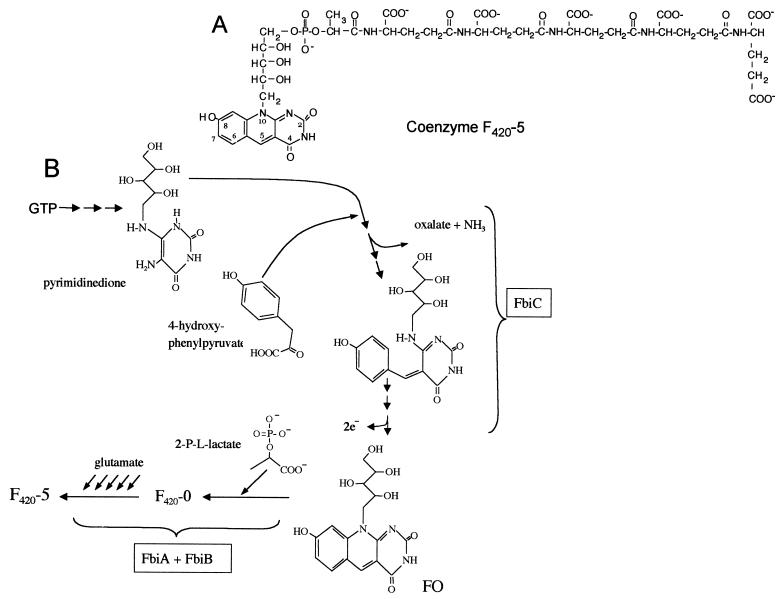
The structure of coenzyme F420 (a 7,8-didemethyl-8-hydroxy 5-deazaflavin electron transfer agent that is present in few microorganisms) was first determined in studies with the methanogenic Archaea (13, 14). This coenzyme had been previously purified from a methanogen, and its spectral and fluorescence properties were described in detail by Cheeseman et al. (6), who gave it its name (for a factor that absorbed maximally in visible wavelengths at 420 nm). However, an earlier report by Cousins described a yellow compound of unknown structure from Mycobacterium smegmatis that, based on its UV-visible spectrum, was almost certainly F420 (11). Naraoka et al. reported that F420 was present in Mycobacterium avium (37), and it was subsequently discovered that F420 was present in Mycobacterium tuberculosis (12) and all other mycobacteria examined (2, 43). Methanobacterium thermoautotrophicum F420 contains two glutamyl residues (14), but Mycobacterium species contain primarily five- and six-glutamyl forms of F420 (F420-5 and F420-6) (2). The structure of F420-5 is shown in Fig. 1A.
FIG. 1.
Structure of F420-5 and an overview of the hypothetical pathway for F420 biosynthesis. (A) F420-5 (2); (B) overview of the hypothetical pathway for F420 biosynthesis (2, 7, 15, 19, 23, 45). FbiA and FbiB are clearly involved in the conversion of FO into F420; evidence supports FbiA acting earlier in the path than FbiB, but the specific reactions catalyzed by these enzymes are not known.
In Mycobacterium and Nocardia species, F420 is used by F420-dependent glucose-6-phosphate dehydrogenase (42, 43) and is required for activation of the experimental antituberculosis drug PA-824 by M. tuberculosis and Mycobacterium bovis strain BCG (hereafter referred to as M. bovis) (48). It is likely that other F420-dependent reactions will be discovered in mycobacteria, since genes corresponding to several proteins with homology to F420-dependent enzymes from other organisms are present in the M. tuberculosis genome (41). In Archaea, F420 is required for hydrogenase, formate, methylene-tetrahydromethanopterin, and alcohol dehydrogenases, methylene-tetrahydromethanopterin reductase, and quinone oxidoreductase (20, 22, 24, 28, 31, 50). F420 is used by Streptomyces for lincomycin and tetracycline biosynthesis (8, 29, 34, 46), and possibly in mitomycin C biosynthesis (32). The Archaea M. thermoautotrophicum and Halobacterium sp., the green alga Scenedesmus acutus, and the cyanobacterium Synechocystis sp. use F420 in photolyase (16, 17, 26, 38).
In M. thermoautotrophicum, the pathway by which F420 is made has been studied with labeling (15, 23, 45) and enzymatic approaches (19), which have allowed development of a hypothetical pathway, an overview of which is shown in Fig. 1B. Due to our interest in F420 biosynthesis and in the metabolism of pathogenic mycobacteria, we have begun a study to identify the genes involved in F420 biosynthesis in Mycobacterium. The only description of genes that are required for F420 biosynthesis has been a recent report that homologues of the M. tuberculosis genes Rv3261 and Rv3262 are required for F420 biosynthesis in M. bovis (7). We named these genes fbiA and fbiB, respectively. Here we report that transposon Tn5367 insertion into the M. bovis homologue of the Rv1173 gene from M. tuberculosis creates mutants that cannot produce F420 or the biosynthetic intermediate FO. We name this gene fbiC (for F420 biosynthesis) and conclude that it is required for a step in the pathway prior to FO, before the deazaflavin ring is formed.
MATERIALS AND METHODS
Bacteria, plasmids, and culture conditions.
The plasmids and bacteria used in this study are given in Table 1. M. bovis was grown in Middlebrook 7H9 medium supplemented with 0.2% (vol/vol) glycerol, 10% (vol/vol) Bacto Middlebrook ADC Enrichment, 0.05% Tween 80 (vol/vol; liquid medium only), and 1.5% agar (plate medium only). With Escherichia coli JM109 as the recombinant host, pGEM, pSMT3, and pHAT-12 were the vectors. E. coli was grown at 37°C on Luria-Bertani medium; ampicillin (100 μg/ml for work with pGEM and 200 μg/ml with pHAT), isopropyl-β-d-thiogalactopyranoside (IPTG; 0.5 mM), and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 80 μg/ml) were included for recombinant selection and identification.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description or DNA sequence | Source or reference |
|---|---|---|
| Strains | ||
| M. bovis BCG | Montreal M. bovis BCG strain | C. Kendall Stover, PathoGenesis |
| E. coli JM109 | RecA−, recombinant vector host strain | Promega |
| M. bovis FbiC−-1 | BCG strain with Tn5367 inserted in fbiC | This study |
| M. bovis FbiC−-2 | BCG strain with Tn5367 inserted in fbiC | This study |
| M. bovis FbiC−-3 | BCG strain with Tn5367 inserted in fbiC | This study |
| M. bovis FbiC−-4 | BCG strain with Tn5367 inserted in fbiC | This study |
| Plasmids | ||
| pGEM | T/A PCR cloning vector | Promega |
| pSMT3 | Expression vector for complementation in mycobacteria; carries hygromycin resistance marker | Larry Schlesinger (39) |
| pPR29 | ts-sacB delivery plasmid pPR27 with a Tn5367 insert (oriM cannot function at 39°C) | Brigitte Gicquel, Institut Pasteur (33, 40) |
| pFbiC | pGEM containing fbiC as 2,976-bp insert | This study |
| p1173 | pSMT3 containing fbiC as 2,750-bp insert | This study |
| pHAT-12 | Cloning vector for addition of HAT tag to the N terminus; Ampr | Clontech |
| pHAT DHFR | Positive control expression vector with murine dihydrofolate reductase gene tagged with HAT at the N terminus | Clontech |
| pHAT1173 | pHAT-12 with fbiC insert as SacI fragment | This study |
| Transposon | ||
| Tn5367 | IS1096 with kanamycin resistance cassette | Brigitte Gicquel, Institut Pasteur (3, 33, 40) |
HPLC.
Cell extracts were prepared for high-performance liquid chromatography (HPLC) by resuspending wet cell pellets in approximately the same volume of 25 mM sodium acetate (pH 4.7) as the pellet volume, followed by heating at >90°C for 15 min and centrifugation in a microcentrifuge. F420 and FO were separated by HPLC by methods similar to that of Gorris and van der Drift (18), with modifications, as recently described (7).
General molecular biology techniques.
Chromosomal DNA was purified according to the method of Husson et al. (21). Wizard minicolumns from Promega (Madison, Wis.) were used for pGEM- and pSMT3-based plasmid purification, and QIAquick kits (Qiagen) were used for PCR cleanup and gel extraction with pHAT constructs. All PCR cloning amplification was done with Pfu polymerase, except for the addition of an A overhang with Taq polymerase in pGEM cloning. Sequencing was conducted at the University of Iowa DNA Facility using an Applied Biosystems 373A DNA sequencer.
Transposon mutagenesis and gene identification.
Transposon insertion mutants in M. bovis were created with the plasmid pPR29 as described by Pelicic et al. (40), but with the incorporation of PA-824 selection (7). The nitroimidazopyran PA-824 (used to select for mutants unable to make F420 [F420− mutants]) was a gift of the PathoGenesis Corporation (48). Colonies that survived this selection were grown and analyzed by HPLC for the presence of 5-deazaflavins. Transposon insertion sites were found as recently described (7).
Complementation of the fbiC mutant.
PCR to amplify fbiC and the surrounding region was performed, the products were analyzed by agarose gel electrophoresis, and the expected 2,976-bp band was cloned into pGEM, resulting in pFbiC. One round of sequencing confirmed that fbiC had been cloned. pFbiC was amplified in E. coli and used as the template for PCR amplification of a 2,750-bp segment using primers which contained PstI and HindIII sites. This PCR product was cut by PstI and HindIII, and the product was inserted into PstI- and HindIII-digested pSMT3 to make p1173. p1173 was used as an expression vector to complement insertion mutants. Following electroporation of 1 or 2 μg of p1173 into the mutants, transformants were selected for using hygromycin-supplemented liquid or agar media (50 μg/ml for M. bovis). Electroduction and/or electroporation to confirm that pSMT3 containing an insert was present in complemented cells was conducted using a modification of the method described by O Gaora (39), as recently described (7).
Expression of histidine-tagged FbiC.
To create a vector to overproduce FbiC, fbiC was amplified from p1173 by using primers which contained SacI sites near their 5′ end, and this product was inserted into SacI-digested pHAT-12 such that the histidine affinity tag (HAT) was in frame with FbiC. Plasmids derived from E. coli transformants were examined with one round of sequencing to confirm correct insertion, and one such fbiC clone (pHAT1173) was then completely sequenced.
Western blotting to identify proteins containing the HAT tag was conducted according to the manufacturer's instructions by using a polyclonal anti-HAT antibody (Clontech, Palo Alto, Calif.) that recognizes epitopes throughout the HAT tag. The secondary antibody was a polyclonal goat anti-rabbit immunoglobulin G conjugated to alkaline phosphatase, which was used with the Immun-Star Chemoluminescent substrate (Bio-Rad). Protein bands from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of protein fractions which contained HAT-tagged proteins were identified with Kodak BioMax MR photographic film after 10- to 30-s exposures. E. coli JM109 carrying an expression vector coding for HAT-tagged dihydrofolate reductase (DHFR; Clontech) was used as a positive control, and E. coli JM109 with no expression vector was used as a negative control.
To study HAT-tagged FbiC production by cells carrying pHAT1173, a 1-liter culture was grown in a rotary shaker at 37°C overnight to an optical density at 600 nm of ∼1, followed by addition of IPTG to 1 mM and further incubation for 2 h. The entire culture was then frozen at −65°C overnight, thawed in warm water, and centrifuged to separate cells from the medium. Cell pellets were resuspended in 4 ml of cold extraction buffer (50 mM sodium phosphate, 300 mM NaCl, pH 7.0) per 50 ml of original culture volume, vortexed for 2 to 3 min, and then incubated with lysozyme (0.75 mg/ml) for 45 min at room temperature with gentle agitation. Crude lysate (2 ml) was transferred to 2-ml screw-cap conical tubes to which zirconia and silica beads (0.1-mm diameter; Biospec Products) had been added to a height of 1 to 2 mm. The cells were then disrupted in a Mini-Beadbeater (Biospec Products) by shaking at 5,000 rpm for 30 s; tubes were kept on ice except when they were in the Beadbeater. This lysate was centrifuged at 10,000 × g for 20 min at 4°C to separate soluble and insoluble material. The resulting supernatant (cleared lysate) was stored on ice, and the pellet was resuspended in 1.5 ml of cold extraction buffer by shaking for 3 s at 5,000 rpm in the Beadbeater; the beads were allowed to settle, and the resuspended material was removed and placed on ice. Talon resin (a Cobalt-complexed resin made by Clontech that specifically binds the HAT tag) was used to purify HAT-tagged FbiC. Samples were bound to the resin, which was then washed with buffer and eluted with 100 and 200 mM imidazole, according to the manufacturer's instructions. In some cases, a protease inhibitor cocktail suitable for use with polyhistidine-tagged protein (P8849; Sigma) was used in cell breakage and all subsequent Talon steps. Samples of the culture supernatant, the cleared lysate, the resuspended lysed cell pellet, and fractions from Talon purification steps were mixed with an equal volume of 2× SDS-PAGE sample buffer (90 mM Tris HCl [pH 6.8], 20% glycerol, 2% SDS, 0.02% bromphenol blue), heated at 95°C for 5 to 10 min, and then analyzed by SDS-PAGE followed by Coomassie blue staining or Western blot analysis.
Computer analysis of sequences.
Comparison of derived amino acid sequences to the protein database sequences was performed by the National Center for Biotechnology Information (NCBI) BlastP program (1). FbiC homologue sequences were compared by using the ClustalW (49) multiple-alignment program with default settings, available at the Baylor College of Medicine Search Launcher (http://dot.imgen.bcm.tmc.edu). Relationships of protein sequences were examined by the NCBI COGs and CD programs. The Sanger Centre TBLASTN program was used to locate and analyze the M. bovis, M. leprae, and Streptomyces coelicolor sequences that were homologous with Rv1173 and the surrounding regions (http://www.sanger.ac.uk). Preliminary sequence data from the Department of Energy Joint Genome Institute (JGI; at http://www.jgi.doe.gov/tempweb/JGI_microbial/html/index.html) were used to locate fbiC homologues in Nostoc punctiforme, Synechococcus sp., Thermobifida fusca, and Methanosarcina barkeri by conducting a TBLASTN analysis of M. bovis fbiC at the corresponding organism-specific JGI server.
Nucleotide sequence accession number.
The nucleotide sequence data for fbiC from M. bovis BCG have been deposited in GenBank under accession number AF479769.
RESULTS AND DISCUSSION
M. bovis mutants with an insertion in the Rv1173 homologue make neither FO nor F420.
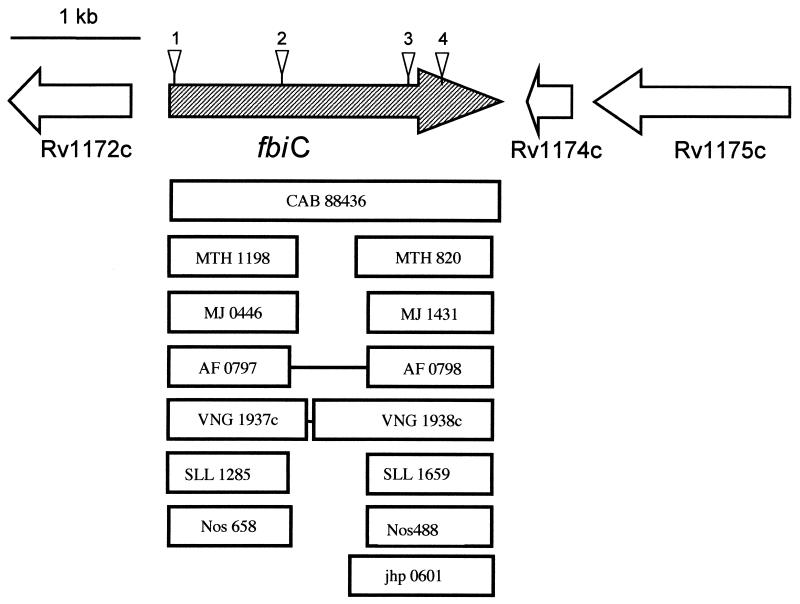
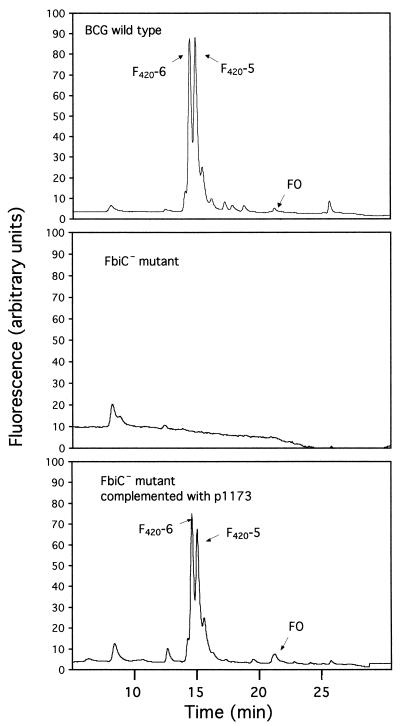
Following transformation with pPR29, M. bovis transformants were grown for 4 to 6 weeks at 32°C in medium containing gentamicin and kanamycin to select for plasmid-containing cells. Cells from the liquid medium were then spread onto agar medium containing kanamycin, sucrose, and PA-824, and the plates were incubated at 39°C. Kanamycin selected for those bacteria containing the kanamycin marker in the transposon. Sucrose selected for transposons that had inserted into the chromosome and for cells that did not contain either the plasmid-borne sacB gene or this gene inserted into the chromosome; the temperature of 39°C also restricted plasmid replication, due to its temperature-sensitive origin of replication. PA-824 selected for cells that had lost the ability to activate PA-824 (7, 48). As shown previously (7), a significant subset of PA-824-resistant mutants was expected to be defective in F420 biosynthesis. Four such F420− mutants had independent insertions in the M. bovis homologue of the M. tuberculosis gene Rv1173. The insertion locations were throughout the gene, as shown in Fig. 2, from very near the N terminus to the C-terminal portion. None of the four mutants made FO or F420. Figure 3 illustrates the HPLC profile of the wild-type M. bovis and one of the F420− and FO− mutants. We have named this Rv1173 homologue gene fbiC (for F420 biosynthesis).
FIG. 2.
Gene arrangement of the fbiC region in M. bovis, transposon insertion sites, and alignment of this gene with selected homologues from other microorganisms. Triangles indicate transposon insertion sites. Abbreviations: CAB, S. coelicolor; MTH, M. thermoautotrophicum; MJ, M. jannaschii; AF, A. fulgidus; Vng, Halobacterium sp.; SLL, Synechocystis sp.; Nos, N. punctiforme; jhp, H. pylori. Numbers refer to gene designations in the corresponding genome sequences, or contig number in the case of Nostoc. The figure is approximately to scale, with boxes indicating the sizes of homologous genes.
FIG. 3.
HPLC profiles of M. bovis wild type, an F420− mutant (FbiC−), and an FbiC− mutant complemented with fbiC.
Complementation of F420− mutants with M. bovis fbiC.
A 2,976-bp fragment containing fbiC from M. bovis was PCR cloned, and a 2,750-bp segment which contained the entire Rv1173 homologue gene was subcloned into pSMT3, creating p1173. This plasmid was transformed into mutant FbiC−-3. As shown in the bottom panel of Fig. 3, the ability to make F420 and FO was fully restored by this complementation, confirming that the mutant phenotype resulted specifically from the insertion in fbiC.
Production of histidine-tagged FbiC in E. coli.
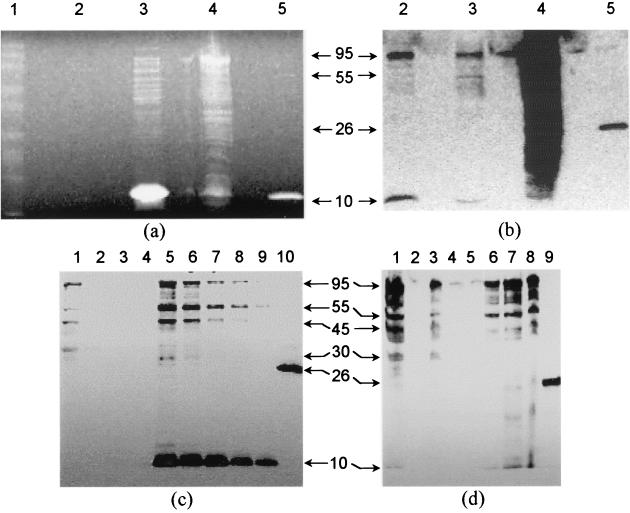
The M. bovis fbiC gene was successfully cloned in frame into pHAT, creating the histidine-tagged expression construct pHAT1173. The fbiC gene in pHAT1173 was found by complete sequencing to be 100% identical to the known M. bovis BCG sequence in the Sanger Centre database, proving that no errors were introduced by Pfu DNA polymerase cloning. As shown in Fig. 4a, when cleared lysate from E. coli carrying pHAT1173 was examined by SDS-PAGE, there appeared to be no major Coomassie blue-stained band that was the expected 97,186-Da size of the HAT-tagged FbiC (92,486-Da FbiC plus 4,700-Da tag and linker). However, a 95-kDa band appeared on the corresponding Western blot of the cleared lysate sample (Fig. 4b, lane 3); the same band was seen in the culture medium taken from some cultures (Fig. 4b, lane 2). The lysate pellet had large amounts of HAT antibody-reactive material, suggesting the presence of insoluble FbiC inclusion bodies. The 95-kDa protein from cleared lysate bound in its native state to Talon resin and eluted with 100 mM imidazole (Fig. 4c and d). We conclude that this protein is the His-tagged FbiC. However, several other bands were present with smaller molecular weights (55, 45, and 10 kDa) which were also very reactive in the HAT Western blot (Fig. 4b, c, and d). Evidence is strong that these bands represent truncated versions of HAT-tagged FbiC, since two independent methods (HAT antibody recognition and Talon resin binding) indicate that the HAT motif is present. Also, the DHFR-positive control (cleared lysate of E. coli JM109 carrying the DHFR gene cloned into the same pHAT vector) shows the absence of such reactive bands, providing evidence that they arise from HAT-tagged fbiC. Examination of Western blots produced from cleared lysate and Talon purification fractions processed with and without protease inhibitors suggested that protein degradation was responsible for the appearance of the smaller Western blot bands. Unprocessed cleared lysate sampled soon after its preparation showed less intense and smaller bands (Fig. 4b, lane 3) than those of Talon-processed samples (Fig. 4c and d). The 10- and 55-kDa bands were the most intense if the imidazole-eluted Talon fractions were processed without protease inhibitors (Fig. 4c, lanes 5 to 9), but when processed with inhibitors, the 95-kDa band was the most intense and very little of the 10-kDa band was visible (Fig. 4d, lanes 6 to 8). This degradation is problematic since yield of the full-size protein is lowered, and the suitability of even the full-sized protein for chemical analysis and enzyme assay is uncertain. The degradation may arise from an aggressive attack on properly folded M. bovis FbiC by E. coli proteases, or it may result from a greater sensitivity to proteases by FbiC that has not been properly folded. Despite the degradation problems, production of a protein of the expected size establishes that the full length of fbiC from M. bovis can code for one protein. This is important since homologues from some organisms code for two separate proteins corresponding to the N-terminal and C-terminal portions of fbiC, as described below.
FIG. 4.
Coomassie blue staining and Western blot analyses of FbiC expression and purification fractions. Samples taken from the lysates of an induced culture expressing FbiC were separated in duplicate by SDS-12% PAGE and either stained with Coomassie blue (a) or subjected to Western blotting using a HAT-specific antibody (b). Lane assignments for panels a and b: 1, Benchmark prestained protein ladder; 2, culture supernatant; 3, cleared lysate; 4, lysate pellet; 5, HAT-DHFR cleared lysate (control). (c) Samples taken from TALON purification of FbiC from the cleared lysate of an induced culture in the absence of protease inhibitors were separated and analyzed by Western blotting. Lane assignments for panel c: 1, lysate after TALON adsorption; 2 to 4, sequential wash fractions; 5 to 9, sequential elution fractions; 10, HAT-DHFR cleared lysate (control). (d) Samples from a purification of FbiC in the presence of protease inhibitors were separated and analyzed by Western blotting. Lane assignments for panel d: 1, cleared lysate; 2, blank; 3, lysate after TALON adsorption; 4 and 5, sequential wash fractions; 6 to 8, sequential elution fractions; 9, HAT-DHFR cleared lysate (control). Arrows marked with numbers represent estimated relative molecular masses in kilodaltons (kDa). Samples were loaded at 15 μl per well.
Comparison of M. bovis FbiC with homologues from other F420-producing species.
The arrangement of genes around fbiC from M. bovis, M. leprae, and M. tuberculosis H37Rv is identical to that shown in Fig. 2. BlastP analysis showed that, compared to M. bovis, the translated fbiC sequences from M. tuberculosis and M. leprae were, respectively, 100 and 87% identical at the amino acid level for the full length of the sequence when the low-complexity filter was not used. BlastP analysis of the M. bovis FbiC against protein databases (using a filter to avoid comparison of regions of low complexity, thus providing a conservative measure of protein similarity) revealed that the actinomycetes S. coelicolor (Sanger database) and Thermobifida fusca (JGI database) contained genes coding for proteins with high homology for the full length of the sequence (Table 2 and Fig. 2). BlastP analysis also showed good hits with a variety of shorter sequences. The best of the shorter-sequence hits were almost entirely with Methanobacterium thermoautotrophicum, Methanococcus jannaschii, Methanosarcina barkeri, Archaeoglobus fulgidus, and Halobacterium sp. The cyanobacteria Nostoc punctiforme and a Synechocystis species had good hits as well. The percentages of identity for these segments are shown in Table 2, and examples of the regions of greatest similarity are indicated in Fig. 2. The full genome sequences for all these organisms have been completed and annotated (5, 9, 10, 25, 27, 38, 44, 47) or at least fully sequenced (by the JGI for Methanosarcina barkeri, Nostoc sp., and T. fusca). The methanogens, mycobacteria, S. coelicolor, A. fulgidis, Halobacterium sp., and Synechocystis sp. have been reported to make F420 (12-14, 17, 30, 36, 43), and it has been determined that T. fusca makes F420 (L. Daniels, unpublished data). This agreement in homology is consistent with a role for FbiC in F420 biosynthesis.
TABLE 2.
BlastP comparison of homologues with M. bovis BCG FbiCa
| Homologue | Organism | % Identity with BCG FbiC:
|
|
|---|---|---|---|
| N-terminal domain | C-terminal domain | ||
| ML1492b | M. leprae | 84 | -c |
| ML1492d | M. leprae | - | 90 |
| SCD6.07b | S. coelicolor | 59 | 25 |
| SCD6.07d | S. coelicolor | 20 | 63 |
| TfFbiCb, e | T. fusca | 64 | 29 |
| TfFbiCd, e | T. fusca | - | 53 |
| MJ0446 | Methanococcus | 41 | - |
| MJ1431 | Methanococcus | 21 | 50 |
| MTH1198 | M. thermoautotrophicum | 39 | 21 |
| MTH820 | M. thermoautotrophicum | 27 | 45 |
| MsbFbiCae | M. barkeri | 40 | - |
| MsbFbiCbe | M. barkeri | - | 47 |
| AF0797 | A. fulgidus | 40 | - |
| AF0798 | A. fulgidus | 23 | 43 |
| VNG1937c | Halobacterium sp. | 37 | - |
| VNG1938c | Halobacterium sp. | - | 32 |
| SLL1285 | Synechocystis sp. | 35 | - |
| SLL1659 | Synechocystis sp. | 23 | 41 |
| NosFbiCae | Nostoc sp. | 34 | - |
| NosFbiCbe | Nostoc sp. | 24 | 42 |
| jhp0601 | H. pylori | - | 40 |
Default BlastP analysis of homologue sequence versus Mycobacterium tuberculosis NCBI database, using a low-complexity filter. The low-complexity filter results in a conservative identity score, since sequences of low complexity are counted as not identical. Without the filtering, identity is usually higher; e.g., the M. tuberculosis and BCG sequences are 100% identical but are given as 89 and 81% in the BlastP results. Original papers describing genome or partial sequences are as follows: M. tuberculosis (9), M. leprae (10), S. coelicolor (44), A. fulgidus (27), Halobacterium sp. (38), Methanococcus jannaschii (5), Methanobacterium thermoautotrophicum (47), and Synechocystis sp. (25).
The N-terminal half of the protein was used.
-, no homologue found.
The C-terminal half of the protein was used.
Genes found at JGI site, not NCBI.
The sequence comparisons in Fig. 2 and Table 2 are complex, since there is similarity between the N-terminal and C-terminal portions of FbiC. The N-terminal portion of M. bovis FbiC has its best hits with proteins found only in F420 producers but has weak hits with sequences corresponding to the C-terminal portion of FbiC from many F420 producers. In contrast, the C-terminal portion of the M. bovis protein has its best hits with F420 producer proteins (corresponding to the weak hits seen with the N-terminal segment of FbiC) but still has fairly good homology with a family of proteins in bacteria which cannot make F420 (e.g., homology with Helicobacter pylori jhp0601, the most similar of the proteins from microbes that do not make F420.).
Mycobacterium species, T. fusca, and S. coelicolor have one gene for the FbiC N- and C-terminal regions (which should produce one protein, as demonstrated above with the M. bovis HAT-tagged FbiC). However, A. fulgidus, M. barkeri, and Halobacterium sp. have adjacent genes that code for two separate proteins over approximately the same sequence. Methanobacterium thermoautotrophicum, Methanococcus jannaschii, and the cyanobacteria have two nonadjacent genes that correspond to this region. We propose that FbiC should refer to the intact, full-length sequence and that the two domains of this large protein be called FbiC (N terminal) and FbiC (C terminal).
Since the transposon insertion sites in fbiC that cause a 5-deazaflavin− phenotype are found in both N-terminal and C-terminal regions, it is possible that both domains are important for F420 biosynthesis. Alternatively, the insertions in the C-terminal region may interfere with the activity coded for by the N-terminal portion. This must be experimentally examined by making individual mutations in the two domains coded for by different genes in one of the F420 producers. It is possible that the function of the C-terminal domain in F420 producers is not unique for the production of F420. Due to the essentiality of F420 for the methanogens and Archaeoglobus (it is central to their energy generation pathways) and the economic barriers to using F420 as a supplement in media, it is worth determining if Halobacterium, Nostoc, or Synechocystis species can be used to investigate the roles of these two separate genes in F420 biosynthesis.
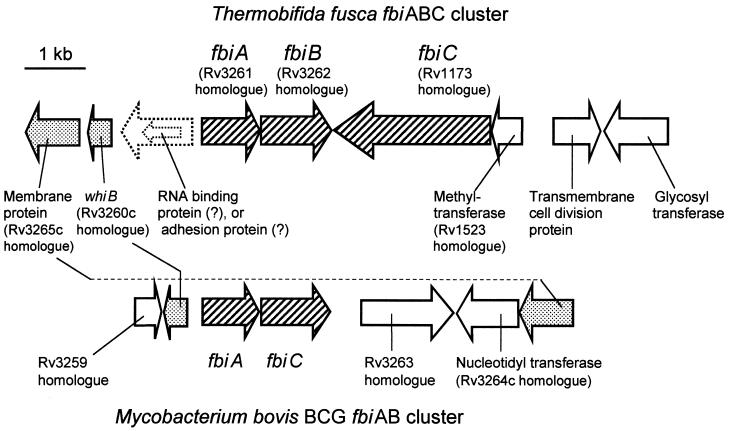
As shown in Fig. 5, fbiC in T. fusca is located in a cluster with fbiA and fbiB. As in M. bovis, M. tuberculosis, and M. leprae, a whiB homologue is located upstream of fbiA, except that in T. fusca another gene may be between the two. T. fusca is the only organism we have found where these three functionally related genes (fbiABC) are clustered. It is possible that such an arrangement is seen among some other actinomycetes, but in S. coelicolor (the only other nonmycobacterium actinomycete that has been fully sequenced), and in the three Mycobacterium species for which we have genome sequences, these three genes are not adjacent.
FIG. 5.
Comparison of gene arrangement of the fbiABC cluster in Thermobifida fusca with the fbiAB region in M. bovis. Rv numbers refer to genes identified in M. tuberculosis H37Rv.
We have compared the N-terminal half and the C-terminal half of the M. bovis FbiC with homologous sequences from 10 of the known F420 producers for which this sequence is available, using multiple alignment (data not shown). Several regions of very high identity are seen, especially an AGxlxiPFTTGlLvGIGE segment in the N-terminal comparison, and the tiPGTAAEiLxDxvR segment in the C-terminal comparison (amino acids always found are shown in uppercase letters, amino acids found most of the time are in lowercase letters, and x indicates that no one amino acid predominates). A group of three cysteines is seen in both N-terminal (txxCxxxCxYCxf) and C-terminal (NiNfTxiCxxxCxFCxF) regions.
Consideration of the role of FbiC in F420 biosynthesis.
The reaction catalyzed by FbiC must play a role in the early portion of the pathway shown in Fig. 1B, between the pyrimidinedione and FO, since no FO is made by the FbiC− mutants. The N-terminal portion of FbiC shows no great similarity to any protein of known function, but the most-similar known enzymes identified by BlastP or COGS are BioB and ThiH, which participate in biotin and thiamine biosynthesis, respectively. However, these are not close matches, and M. tuberculosis has a putative BioB (Rv1589) with much greater homology to known BioB enzymes. No ThiH homologue could be identified in Mycobacterium, but it is likely that a putative ThiO (Rv0415) fulfills this function in M. tuberculosis (4, 35). Database comparison of the C-terminal portion of FbiC showed the best hits with hypothetical proteins, especially with probable iron-sulfur proteins (expect value [E], ∼10−40), and with very slight homology with two known proteins, ThiH and uroporphyrinogen decarboxylase (UroD or HemE). M. tuberculosis has a HemE homologue (Rv2678c) with high similarity to known HemE enzymes. We conclude that the inability of FbiC− mutants to make 5-deazaflavins does not result from interruption of biotin, thiamine, or heme biosynthetic genes and that the specific reaction catalyzed by Mycobacterium species FbiC is not clear based on sequence homologies. The development of assays for the reactions thought to be present in the early portion of the F420 biosynthesis pathway shown in Fig. 1 will be very informative.
Acknowledgments
We thank Ken Stover, Paul Warrener, David Sherman, and Ying Yuan of the PathoGenesis Corporation for the gift of PA-824. We gratefully acknowledge the gift from David Wilson of a culture of Thermobifida fusca. We thank Brigitte Gicquel and Vladimir Pelicic at the Institut Pasteur, Paris, France, for providing pPR29. We are very appreciative of the many hours of excellent technical work contributed by Seong-Ae Kang. We thank Diana Downs for informative discussions about genes involved in biotin and thiamine biosynthesis. Preliminary sequence data were obtained from the Department of Energy Joint Genome Institute (JGI) at http://spider.jgi-psf.org/JGI_microbial/html/.
This work was supported by National Institutes of Health grant GM56177 and U.S. Department of Agriculture grant 4132008 to L.D.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Bair, T. B., D. W. Isabelle, and L. Daniels. 2001. Structures of coenzyme F420 in Mycobacterium species. Arch. Microbiol. 176:37-43. [DOI] [PubMed] [Google Scholar]
- 3.Bardarov, S., J. Kriakov, C. Carriere, S. Yu, C. Vaamonde, R. A. McAdam, B. R. Bloom, G. F. Hatfull, and W. R. Jacobs, Jr. 1997. Conditionally replicating mycobacteriophages: a system for transposon delivery to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 94:10961-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begley, T. P., D. M. Downs, S. E. Ealick, F. W. McLafferty, A. P. Van Loon, S. Taylor, N. Campobasso, H. J. Chiu, C. Kinsland, J. J. Reddick, and J. Xi. 1999. Thiamin biosynthesis in prokaryotes. Arch. Microbiol. 171:293-300. [DOI] [PubMed] [Google Scholar]
- 5.Bult, C. J., O. White, G. J. Olsen, L. Zhou, R. D. Fleischmann, G. G. Sutton, J. A. Blake, L. M. FitzGerald, R. A. Clayton, J. D. Gocayne, A. R. Kerlavage, B. A. Dougherty, J. F. Tomb, M. D. Adams, C. I. Reich, R. Overbeek, E. F. Kirkness, K. G. Weinstock, J. M. Merrick, A. Glodek, J. L. Scott, N. S. M. Geoghagen, J. F. Weidman, J. L. Fuhrmann, E. A. Presley, D. Nguyen, T. R. Utterback, J. M. Kelley, J. D. Peterson, P. W. Sadow, M. C. Hanna, M. D. Cotton, M. A. Hurst, K. M. Roberts, B. P. Kaine, M. Borodovsky, H.-P. Klenk, C. M. Fraser, H. O. Smith, C. R. Woese, and J. C. Venter. 1996. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science 273:1058-1073. [DOI] [PubMed] [Google Scholar]
- 6.Cheeseman, P., A. Toms-Wood, and R. S. Wolfe. 1972. Isolation and properties of a fluorescent compound, factor 420, from Methanobacterium strain M.o.H. J. Bacteriol. 112:527-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi, K.-P., T. B. Bair, Y.-M. Bae, and L. Daniels. 2001. Use of transposon Tn5367 mutagenesis and a nitroimidazopyran-based selection system to demonstrate a requirement for fbiA and fbiB in coenzyme F420 biosynthesis by Mycobacterium bovis BCG. J. Bacteriol. 183:7058-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coats, J. H., G. P. Li, M. S. Kuo, and D. A. Yurek. 1989. Discovery, production, and biological assay of an unusual flavenoid cofactor involved in lincomycin biosynthesis. J. Antibiot. (Tokyo) 42:472-474. [DOI] [PubMed] [Google Scholar]
- 9.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 10.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 11.Cousins, F. B. 1960. The prosthetic group of a chromoprotein from mycobacteria. Biochim. Biophys. Acta 40:532-534. [DOI] [PubMed] [Google Scholar]
- 12.Daniels, L., N. Bakhiet, and K. Harmon. 1985. Widespread distribution of a 5-deazaflavin cofactor in Actinomycetes and related bacteria. Syst. Appl. Microbiol. 6:12-17. [Google Scholar]
- 13.Eirich, L. D., G. D. Vogels, and R. S. Wolfe. 1979. Distribution of coenzyme F420 and properties of its hydrolytic fragments. J. Bacteriol. 140:20-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eirich, L. D., G. D. Vogels, and R. S. Wolfe. 1978. Proposed structure for coenzyme F420 from Methanobacterium. Biochemistry 17:4583-4593. [DOI] [PubMed] [Google Scholar]
- 15.Eisenreich, W., B. Schwarzkopf, and A. Bacher. 1991. Biosynthesis of nucleotides, flavins, and deazaflavins in Methanobacterium thermoautotrophicum. J. Biol. Chem. 266:9622-9631. [PubMed] [Google Scholar]
- 16.Eker, A. P., J. K. C. Hessels, and J. van de Velde. 1988. Photoreactivating enzyme from the green alga Scenedesmus acutus. Evidence for the presence of two different flavin chromophores. Biochemistry 27:1758-1765. [Google Scholar]
- 17.Eker, A. P., P. Kooiman, J. K. Hessels, and A. Yasui. 1990. DNA photoreactivating enzyme from the cyanobacterium Anacystis nidulans. J. Biol. Chem. 265:8009-8015. [PubMed] [Google Scholar]
- 18.Gorris, L. G. M., and C. van der Drift. 1988. Separation and quantitation of cofactors from methanogenic bacteria by high-performance liquid chromatography: optimum and routine analysis. J. Microbiol. Methods 8:175-190. [Google Scholar]
- 19.Graupner, M., and R. H. White. 2001. Biosynthesis of the phosphodiester bond in coenzyme F420 in the methanoarchaea. Biochemistry 40:10859-10872. [DOI] [PubMed] [Google Scholar]
- 20.Hartzell, P. L., G. Zvilius, J. C. Escalante-Semerena, and M. I. Donnelly. 1985. Coenzyme F420 dependence of the methylenetetrahydromethanopterin dehydrogenase of Methanobacterium thermoautotrophicum. Biochem. Biophys. Res. Commun. 133:884-890. [DOI] [PubMed] [Google Scholar]
- 21.Husson, R. N., B. E. James, and R. A. Young. 1990. Gene replacement and expression of foreign DNA in mycobacteria. J. Bacteriol. 172:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson, F. S., L. Daniels, J. A. Fox, C. T. Walsh, and W. H. Orme-Johnson. 1982. Purification and properties of an 8-hydroxy-5-deazaflavin-reducing hydrogenase from Methanobacterium thermoautotrophicum. J. Biol. Chem. 257:3385-3388. [PubMed] [Google Scholar]
- 23.Jaenchen, R., P. Schonheit, and R. K. Thauer. 1984. Studies on the biosynthesis of coenzyme F420 in methanogenic archaea. Arch. Microbiol. 137:362-365. [DOI] [PubMed] [Google Scholar]
- 24.Jones, J. B., and T. C. Stadtman. 1980. Reconstitution of a formate-NADP+ oxidoreductase from formate dehydrogenase and a 5-deazaflavin-linked NADP+ reductase isolated from Methanococcus vannielii. J. Biol. Chem. 255:1049-1053. [PubMed] [Google Scholar]
- 25.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakazaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3:109-136. [DOI] [PubMed] [Google Scholar]
- 26.Kiener, A., I. Husain, A. Sancar, and C. Walsh. 1989. Purification and properties of Methanobacterium thermoautotrophicum DNA photolyase. J. Biol. Chem. 264:13880-13887. [PubMed] [Google Scholar]
- 27.Klenk, H. P., R. A. Clayton, J. F. Tomb, O. White, K. E. Nelson, K. A. Ketchum, R. J. Dodson, M. Gwinn, E. K. Hickey, J. D. Peterson, D. L. Richardson, A. R. Kerlavage, D. E. Graham, N. C. Kyrpides, R. D. Fleischmann, J. Quackenbush, N. H. Lee, G. G. Sutton, S. Gill, E. F. Kirkness, B. A. Dougherty, K. McKenney, M. D. Adams, B. Loftus, and J. C. Venter. 1997. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature 390:364-370. [DOI] [PubMed] [Google Scholar]
- 28.Kunow, J., D. Linder, K. O. Stetter, and R. K. Thauer. 1994. F420H2: quinone oxidoreductase from Archaeoglobus fulgidus. Characterization of a membrane-bound multisubunit complex containing FAD and iron-sulfur clusters. Eur. J. Biochem. 223:503-511. [DOI] [PubMed] [Google Scholar]
- 29.Kuo, M. S., D. A. Yurek, J. H. Coats, and G. P. Li. 1989. Isolation and identification of 7,8-didemethyl-8-hydroxy-5-deazariboflavin, an unusual cosynthetic factor in streptomycetes, from Streptomyces lincolnensis. J. Antibiot. (Tokyo) 42:475-478. [DOI] [PubMed] [Google Scholar]
- 30.Lin, X. L., and R. H. White. 1986. Occurrence of coenzyme F420 and its gamma-monoglutamyl derivative in nonmethanogenic archaebacteria. J. Bacteriol. 168:444-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma, K., and R. K. Thauer. 1990. Purification and properties of N5,N10-methylene-trahydromethanopterin reductase from Methanobacterium thermoautotrophicum (strain Marburg). Eur. J. Biochem. 191:187-193. [DOI] [PubMed] [Google Scholar]
- 32.Mao, Y., M. Varoglu, and D. H. Sherman. 1999. Molecular characterization and analysis of the biosynthetic gene cluster for the antitumor antibiotic mitomycin C from Streptomyces lavendulae NRRL 2564. Chem. Biol. 6:251-263. [DOI] [PubMed] [Google Scholar]
- 33.McAdam, R. A., T. R. Weisbrod, J. Martin, J. D. Scuderi, A. M. Brown, J. D. Cirillo, B. R. Bloom, and W. R. Jacobs, Jr. 1995. In vivo growth characteristics of leucine and methionine auxotrophic mutants of Mycobacterium bovis BCG generated by transposon mutagenesis. Infect. Immun. 63:1004-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormick, J. R. D., and G. O. Morton. 1982. Identity of cosynthetic Factor 1 of Streptomyces aureofaciens and fragment FO from coenzyme F420 of Methanobacterium sp. J. Am. Chem. Soc. 104:4014-4015. [Google Scholar]
- 35.Miranda-Rios, J., C. Morera, H. Taboada, A. Davalos, S. Encarnacion, J. Mora, and M. Soberon. 1997. Expression of thiamin biosynthetic genes (thiCOGE) and production of symbiotic terminal oxidase cbb3 in Rhizobium etli. J. Bacteriol. 179:6887-6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moller-Zinkan, D., G. Borner, and R. K. Thauer. 1989. Function of methanofuran, tetrahydromethopterin and coenzyme F420 in Archaeoglobus fulgidus. Arch. Microbiol. 152:362-368. [Google Scholar]
- 37.Naraoka, T., K. Mamoi, K. Fukasawa, and M. Goto. 1984. Isolation and identification of a naturally occurring 7,8-didemethyl-8-hydroxy-5-deazaflavin derivative from Mycobacterium avium. Biochim. Biophys. Acta 797:377-380. [Google Scholar]
- 38.Ng, W. V., S. P. Kennedy, G. G. Mahairas, B. Berquist, M. Pan, H. D. Shukla, S. R. Lasky, N. S. Baliga, V. Thorsson, J. Sbrogna, S. Swartzell, D. Weir, J. Hall, T. A. Dahl, R. Welti, Y. A. Goo, B. Leithauser, K. Keller, R. Cruz, M. J. Danson, D. W. Hough, D. G. Maddocks, P. E. Jablonski, M. P. Krebs, C. M. Angevine, H. Dale, T. A. Isenbarger, R. F. Peck, M. Pohlschroder, J. L. Spudich, K. H. Jung, M. Alam, T. Freitas, S. Hou, C. J. Daniels, P. P. Dennis, A. D. Omer, H. Ebhardt, T. M. Lowe, P. Liang, M. Riley, L. Hood, and S. DasSarma. 2000. Genome sequence of Halobacterium species NRC-1. Proc. Natl. Acad. Sci. USA 97:12176-12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O Gaora, P. 1998. Expression of genes in mycobacteria, p. 472. In T. Parish and N. G. Stoker (ed.), Mycobacteria protocols, vol. 101. Humana Press, Totowa, N.J.
- 40.Pelicic, V., M. Jackson, J. M. Reyrat, W. R. Jacobs, Jr., B. Gicquel, and C. Guilhot. 1997. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 94:10955-10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purwantini, E., and L. Daniels. 1998. Molecular analysis of the gene encoding F420-dependent glucose-6-phosphate dehydrogenase from Mycobacterium smegmatis. J. Bacteriol. 180:2212-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purwantini, E., and L. Daniels. 1996. Purification of a novel coenzyme F420-dependent glucose-6-phosphate dehydrogenase from Mycobacterium smegmatis. J. Bacteriol. 178:2861-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purwantini, E., T. Gillis, and L. Daniels. 1997. Presence of F420-dependent glucose-6-phosphate dehydrogenase in Mycobacterium and Nocardia species, but absence in Streptomyces and Corynebacterium species and methanogenic Archaea. FEMS Microbiol. Lett. 146:129-134. [DOI] [PubMed] [Google Scholar]
- 44.Redenbach, M., H. M. Kieser, D. Denapaite, A. Eichner, J. Cullum, H. Kinashi, and D. A. Hopwood. 1996. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol. Microbiol. 21:77-96. [DOI] [PubMed] [Google Scholar]
- 45.Reuke, B., S. Korn, W. Eisenreich, and A. Bacher. 1992. Biosynthetic precursors of deazaflavins. J. Bacteriol. 174:4042-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhodes, P. M., N. Winskill, E. J. Friend, and M. Warren. 1981. Biochemical and genetic comparison of Streptomyces rimosus mutants impaired in oxytetracycline biosynthesis. J. Gen. Microbiol. 124:329-338. [Google Scholar]
- 47.Smith, D. R., L. A. Doucette-Stamm, C. Deloughery, H. Lee, J. Dubois, T. Aldredge, R. Bashirzadeh, D. Blakely, R. Cook, K. Gilbert, D. Harrison, L. Hoang, P. Keagle, W. Lumm, B. Pothier, D. Qiu, R. Spadafora, R. Vicaire, Y. Wang, J. Wierzbowski, R. Gibson, N. Jiwani, A. Caruso, D. Bush, H. Safer, D. Patwell, S. Prabhaker, S. McDougall, G. Shimer, A. Goyal, S. Pietrokovski, G. M. Church, C. J. Daniels, J.-I. Mao, P. Rice, J. Nolling, and J. N. Reeve. 1997. Complete genome sequence of Methanobacterium thermoautotrophicum deltaH: functional analysis and comparative genomics. J. Bacteriol. 179:7135-7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stover, C. K., P. Warrener, D. R. VanDevanter, D. R. Sherman, T. M. Arain, M. H. Langhorne, S. W. Anderson, J. A. Towell, Y. Yuan, D. N. McMurray, B. N. Kreiswirth, C. E. Barry, and W. R. Baker. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962-966. [DOI] [PubMed] [Google Scholar]
- 49.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Widdel, F., and R. S. Wolfe. 1989. Expression of secondary alcohol dehydrogenase in methanogenic bacteria and purification of the F420-specific enzyme from Methanogenium thermophilum strain TCI. Arch. Microbiol. 152:322-328. [Google Scholar]