Abstract
1. Afferent activity was measured in the vagus nerves in sixteen foetuses within 2-3 days of natural term and in two foetuses 130 and 135 days gestational age, exteriorized by hysterotomy from seventeen ewes given either a spinal anaesthetic (ten) or pentobarbitone sodium (seven).
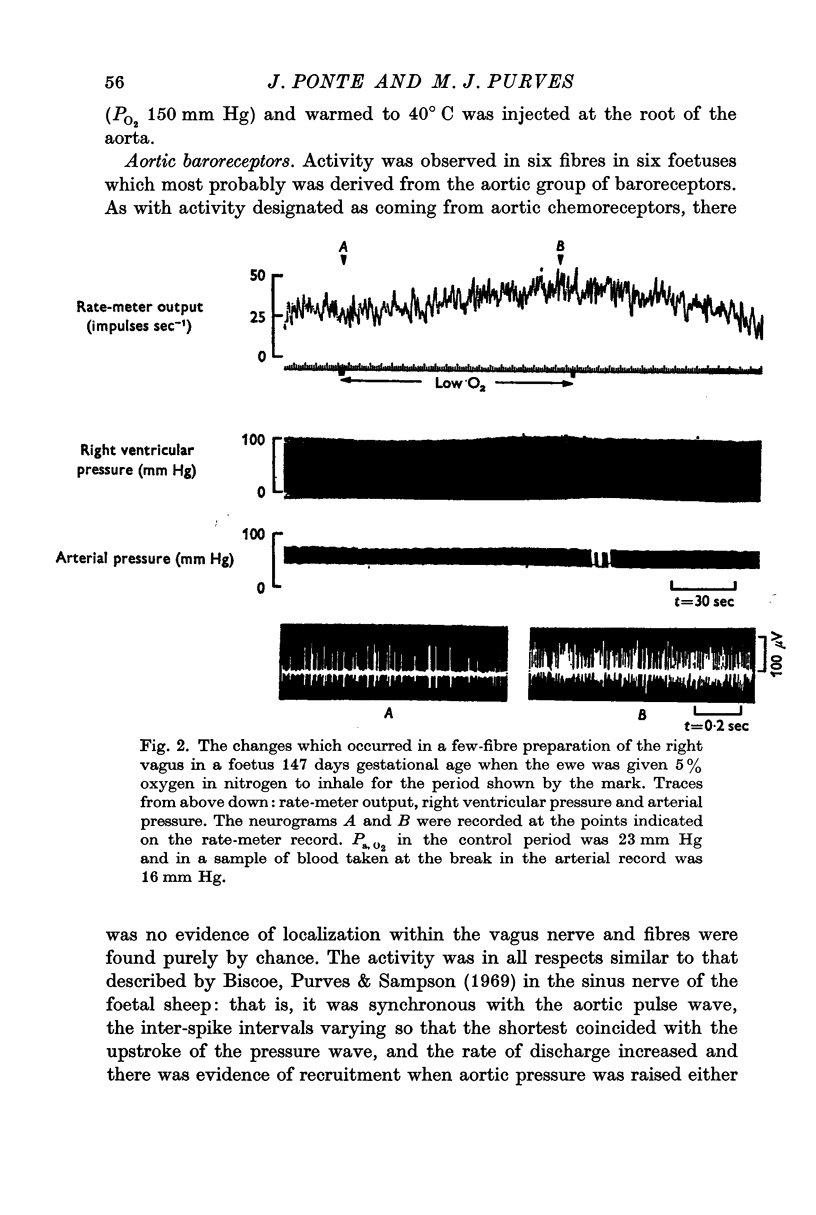
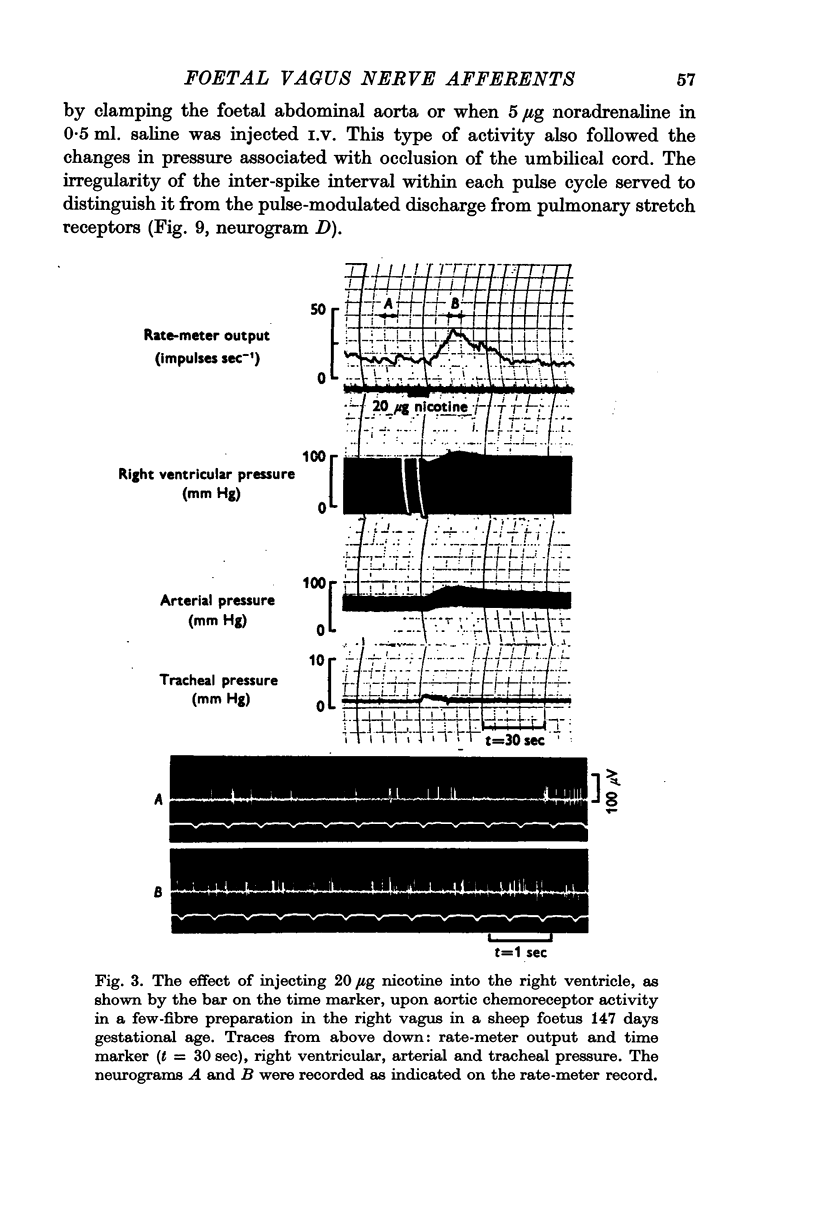
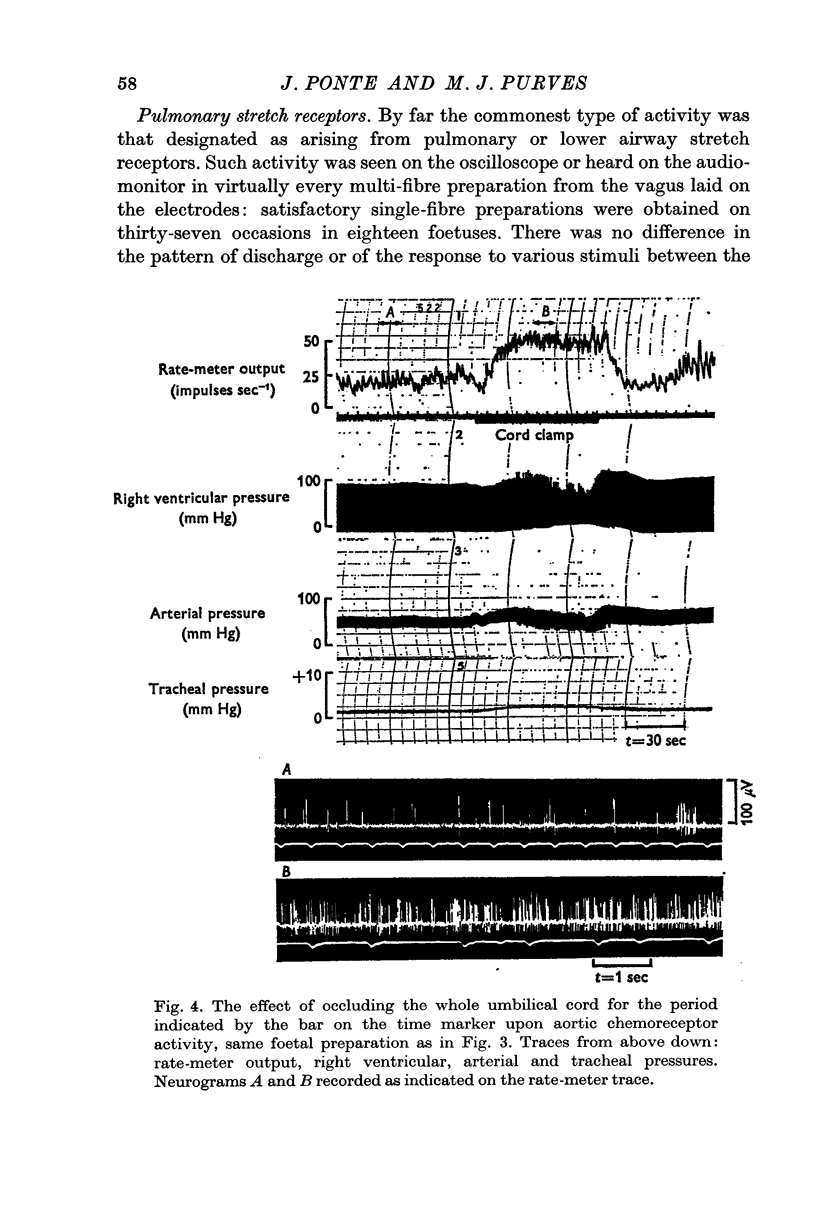
2. Activity from aortic chemoreceptors was observed which increased in rate with foetal hypoxia, NaCN or nicotine and temporary occlusion of the umbilical cord or veins. Activity was abolished or reduced with saline equilibrated with air.
3. Activity from baroreceptors was observed which was synchronous with the arterial pulse and which followed changes in arterial pressure.
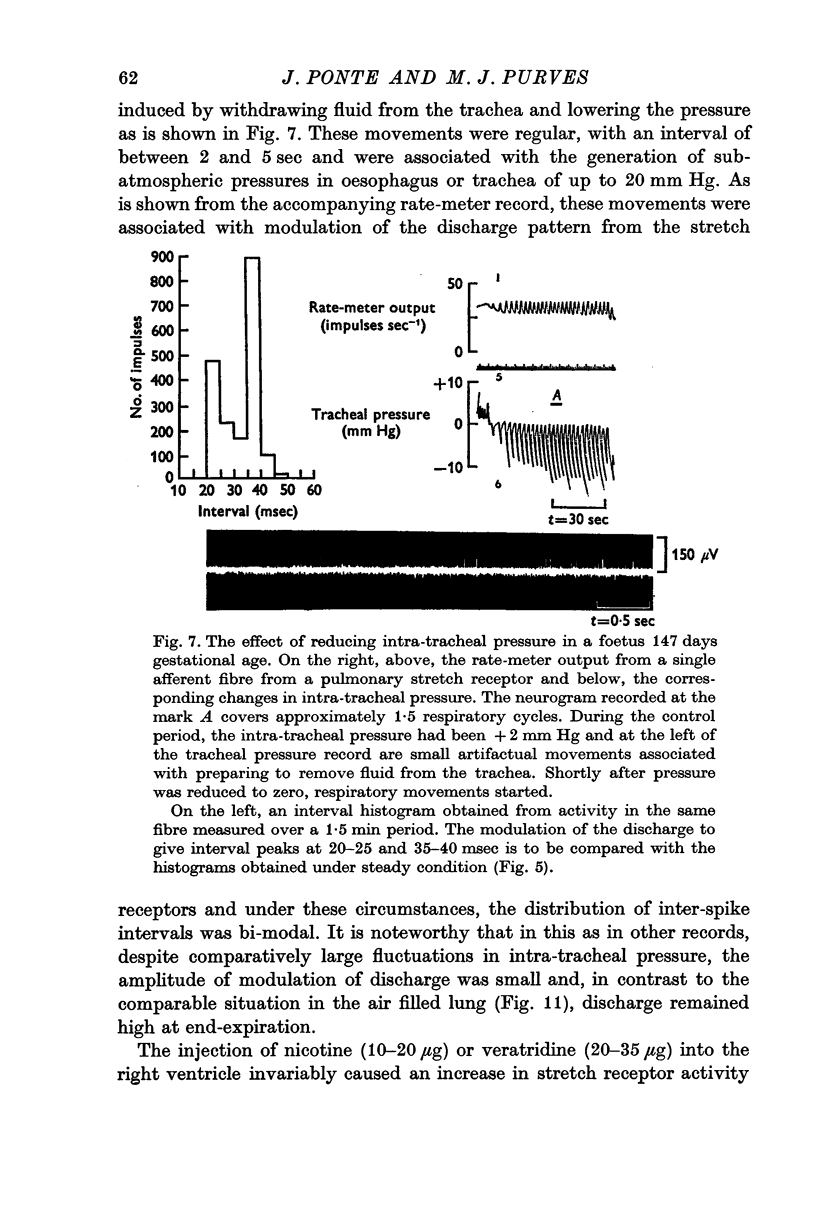
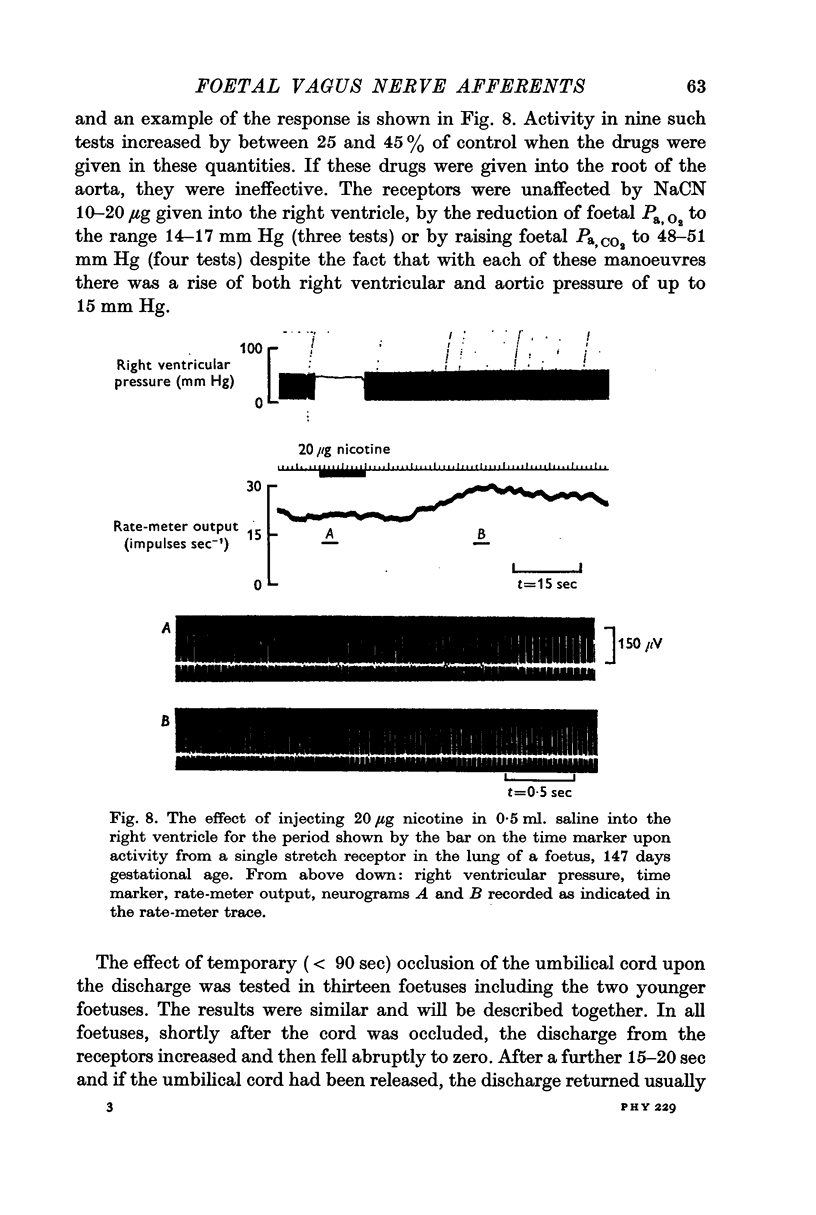
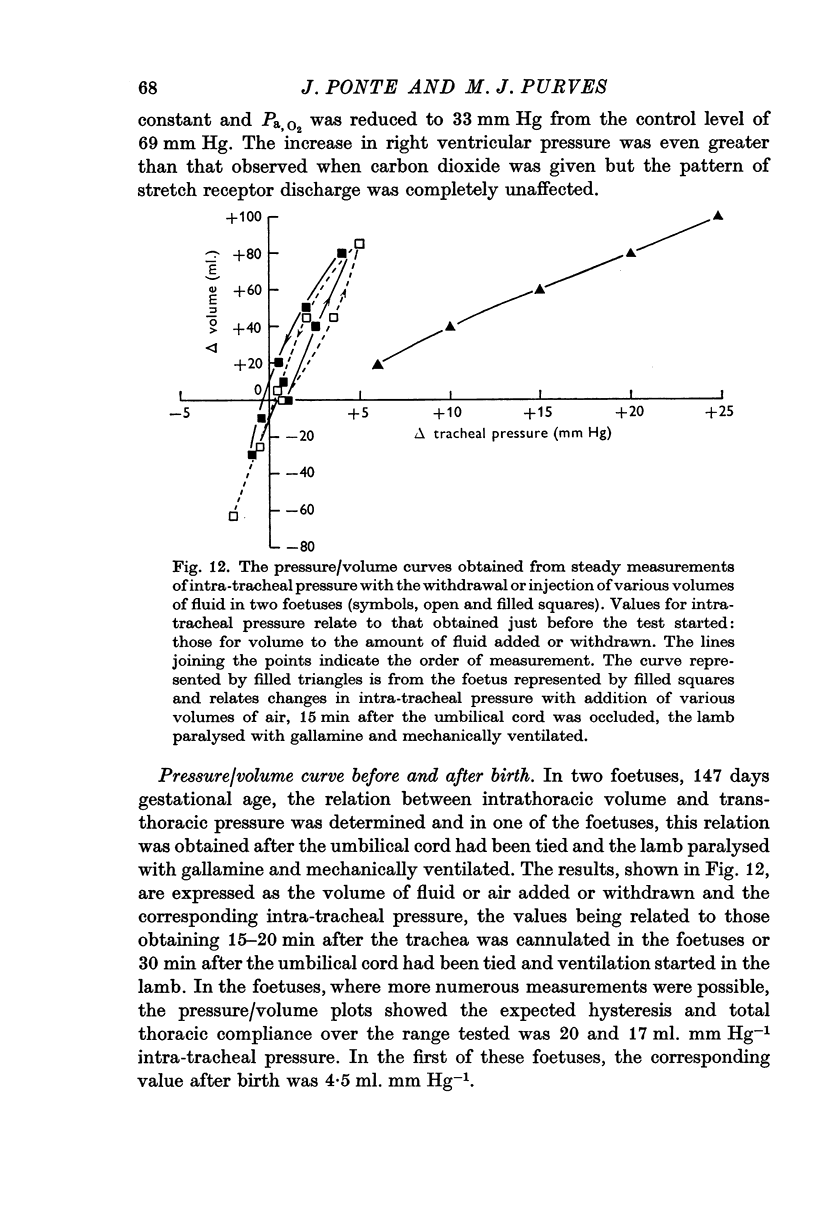
4. Activity designated as arising from stretch receptors in the lungs or lower airways was observed which consisted of a regular discharge at 15-45 impulses sec-1, which varied with intra-tracheal pressure, which increased with negative intrathoracic pressure generated by spontaneous respiratory movements, which, when the umbilical cord was occluded, at first increased in rate and then fell to zero at or about the occasion of the first breath. After breathing had been established, activity assumed the rhythmical pattern and showed evidence of slow adaptation of receptors seen in the adult. This activity was frequently modulated by the heart beat and after breathing had started it was reduced when carbon dioxide was raised.
5. Respiratory movements with a frequency of 10-20/min occurred spontaneously in foetuses from ewes given a spinal anaesthetic and were associated with a modulation of the stretch receptor discharge. They could be induced by withdrawing fluid from the trachea and so lowering both intra-tracheal pressure and receptor discharge and were abolished by raising intra-tracheal pressure. These changes were abolished by sectioning the vagi just caudal to the nodose ganglion.
6. These results indicate that there are excitatory and inhibitory pathways in the vagus nerves which are active in the foetus and which may well be concerned with the limitation of respiratory movements of the foetus and the onset of maintained respiration at birth.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHERNE W., DAWKINS M. J. THE REMOVAL OF FLUID FROM THE PULMONARY AIRWAYS AFTER BIRTH IN THE RABBIT, AND THE EFFECT ON THIS OF PREMATURITY AND PRE-NATAL HYPOXIA. Biol Neonat. 1964;7:214–229. doi: 10.1159/000239925. [DOI] [PubMed] [Google Scholar]
- Adams F. H., Yanagisawa M., Kuzela D., Martinek H. The disappearance of fetal lung fluid following birth. J Pediatr. 1971 May;78(5):837–843. doi: 10.1016/s0022-3476(71)80356-6. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J., Sampson S. R. Types of nervous activity which may be recorded from the carotid sinus nerve in the sheep foetus. J Physiol. 1969 May;202(1):1–23. doi: 10.1113/jphysiol.1969.sp008792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHINARD F. P., ENNS T. Transcapillary pulmonary exchange of water in the dog. Am J Physiol. 1954 Aug;178(2):197–202. doi: 10.1152/ajplegacy.1954.178.2.197. [DOI] [PubMed] [Google Scholar]
- COMLINE R. S., SILVER I. A., SILVER M. FACTORS RESPONSIBLE FOR THE STIMULATION OF THE ADRENAL MEDULLA DURING ASPHYXIA IN THE FOETAL LAMB. J Physiol. 1965 May;178:211–238. doi: 10.1113/jphysiol.1965.sp007624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSS K. W., KLAUS M., TOOLEY W. H., WEISSER K. The response of the new-born baby to inflation of the lungs. J Physiol. 1960 Jun;151:551–565. doi: 10.1113/jphysiol.1960.sp006459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALYMDE B., HAZZLEDINE J. L., HOWE A. REFLEX RESPIRATORY AND PERIPHERAL VASCULAR RESPONSES TO STIMULATION OF THE ISOLATED PERFUSED AORTIC ARCH CHEMORECEPTORS OF THE DOG. J Physiol. 1965 Mar;177:300–322. doi: 10.1113/jphysiol.1965.sp007593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS H. L., FOWLER W. S., LAMBERT E. H. Effect of volume and rate of inflation and deflation on transpulmonary pressure and response of pulmonary stretch receptors. Am J Physiol. 1956 Dec;187(3):558–566. doi: 10.1152/ajplegacy.1956.187.3.558. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., MALCOLM J. L. The effect of localized cooling on conduction in cat nerves. J Physiol. 1955 Oct 28;130(1):53–71. doi: 10.1113/jphysiol.1955.sp005392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Duncan S. L., Lewis B. V., Merlet C. L., Owen-Thomas J. B., Reeves J. T. Cyanide stimulation of the systemic arterial chemoreceptors in foetal lambs. J Physiol. 1969 Mar;201(1):117–128. doi: 10.1113/jphysiol.1969.sp008746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Duncan S. L., Lewis B. V., Merlet C. L., Owen-Thomas J. B., Reeves J. T. Hypoxaemia and aortic chemoreceptor function in foetal lambs. J Physiol. 1969 Mar;201(1):105–116. doi: 10.1113/jphysiol.1969.sp008745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Fox H. E., Leduc B. M., Liggins G. C., Richards R. T. Respiratory movements and paradoxical sleep in the foetal lamb. J Physiol. 1970 Sep;210(1):47P–48P. [PubMed] [Google Scholar]
- Dawes G. S., Fox H. E., Leduc B. M., Liggins G. C., Richards R. T. Respiratory movements and rapid eye movement sleep in the foetal lamb. J Physiol. 1972 Jan;220(1):119–143. doi: 10.1113/jphysiol.1972.sp009698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Lewis B. V., Milligan J. E., Roach M. R., Talner N. S. Vasomotor responses in the hind limbs of foetal and new-born lambs to asphyxia and aortic chemoreceptor stimulation. J Physiol. 1968 Mar;195(1):55–81. doi: 10.1113/jphysiol.1968.sp008446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz D. N., Iggo A. Conduction failure in myelinated and non-myelinated axons at low temperatures. J Physiol. 1968 Dec;199(2):319–345. doi: 10.1113/jphysiol.1968.sp008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howatt W. F., Humphreys P. W., Normand I. C., Strang L. B. Ventilation of liquid by the fetal lamb during asphyxia. J Appl Physiol. 1965 May;20(3):496–502. doi: 10.1152/jappl.1965.20.3.496. [DOI] [PubMed] [Google Scholar]
- Hughes D. T., Parker H. R., Williams J. V. The response of foetal sheep and lambs to pulmonary inflation. J Physiol. 1967 Apr;189(2):177–187. doi: 10.1113/jphysiol.1967.sp008162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys P. W., Normand I. C., Reynolds E. O., Strang L. B. Pulmonary lymph flow and the uptake of liquid from the lungs of the lamb at the start of breathing. J Physiol. 1967 Nov;193(1):1–29. doi: 10.1113/jphysiol.1967.sp008340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE K. D., MAYOU R. A., TORRANCE R. W. THE EFFECT OF BLOOD PRESSURE UPON CHEMORECEPTOR DISCHARGE TO HYPOXIA, AND THE MODIFICATION OF THIS EFFECT BY THE SYMPATHETIC-ADRENAL SYSTEM. Q J Exp Physiol Cogn Med Sci. 1964 Apr;49:171–183. doi: 10.1113/expphysiol.1964.sp001717. [DOI] [PubMed] [Google Scholar]
- LILIENFIELD L. S., FREIS E. D., PARTENOPE E. A., MOROWITZ H. J. Transcapillary migration of heavy water and thiocyanate ion in the pulmonary circulation of normal subjects and patients with congestive heart failure. J Clin Invest. 1955 Jan;34(1):1–8. doi: 10.1172/JCI103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlet C., Hoerter J., Devilleneuve C., Tchobroutsky C. Mise en évidence de mouvements respiratoires chez le foetus d'agneau in utero au cours du dernier mois de la gestation. C R Acad Sci Hebd Seances Acad Sci D. 1970 May 20;270(20):2462–2464. [PubMed] [Google Scholar]
- Mills E. Activity of aortic chemoreceptors during electrical stimulation of the stellate ganglion in the cat. J Physiol. 1968 Nov;199(1):103–114. doi: 10.1113/jphysiol.1968.sp008641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa M. E., Purves M. J. The effect of CO 2 upon discharge from slowly adapting stretch receptors in the lungs of rabbits. Respir Physiol. 1972 Oct;16(2):197–212. doi: 10.1016/0034-5687(72)90051-5. [DOI] [PubMed] [Google Scholar]
- PAINTAL A. S. The conduction velocities of respiratory and cardiovascular afferent fibres in the vagus nerve. J Physiol. 1953 Aug;121(2):341–359. doi: 10.1113/jphysiol.1953.sp004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves M. J., James I. M. Observations on the control of cerebral blood flow in the sheep fetus and newborn lamb. Circ Res. 1969 Dec;25(6):651–667. doi: 10.1161/01.res.25.6.651. [DOI] [PubMed] [Google Scholar]
- SELLER M. J., SPECTOR R. G. THE EFFECTS OF ANOXIA ON THE NEWBORN AND ADULT RAT LUNG. J Pathol Bacteriol. 1964 Jul;88:309–311. [PubMed] [Google Scholar]
- WYSS O. A. The mode of functioning of the respiratory centre. Helv Physiol Pharmacol Acta Suppl 1. 1954 Dec;10:5–25. [PubMed] [Google Scholar]


