Abstract
1. The relation between discharge frequency and angular head position was determined for a population of regularly discharging single first-order vestibular neurones in the eighth nerve of the barbiturate anaesthetized cat.
2. Each axon had a characteristic head position which was maximally excitatory to it, and a diametrically opposed head position which was minimally excitatory.
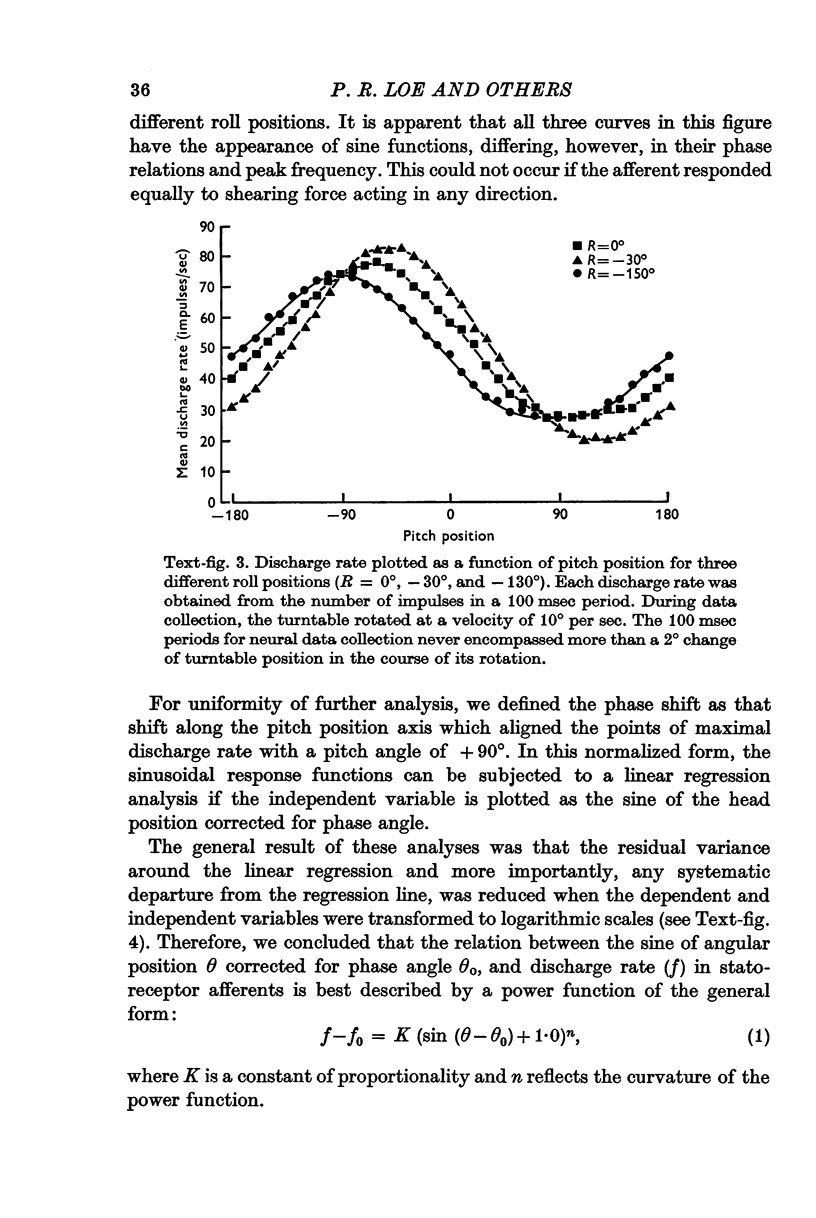
3. After correction for phase shifts introduced by the orientation of preferred excitability, discharge rate in statoreceptor afferents varied as a power function of the sine of angular head position with exponents ranging from 0·9 to 1·6.
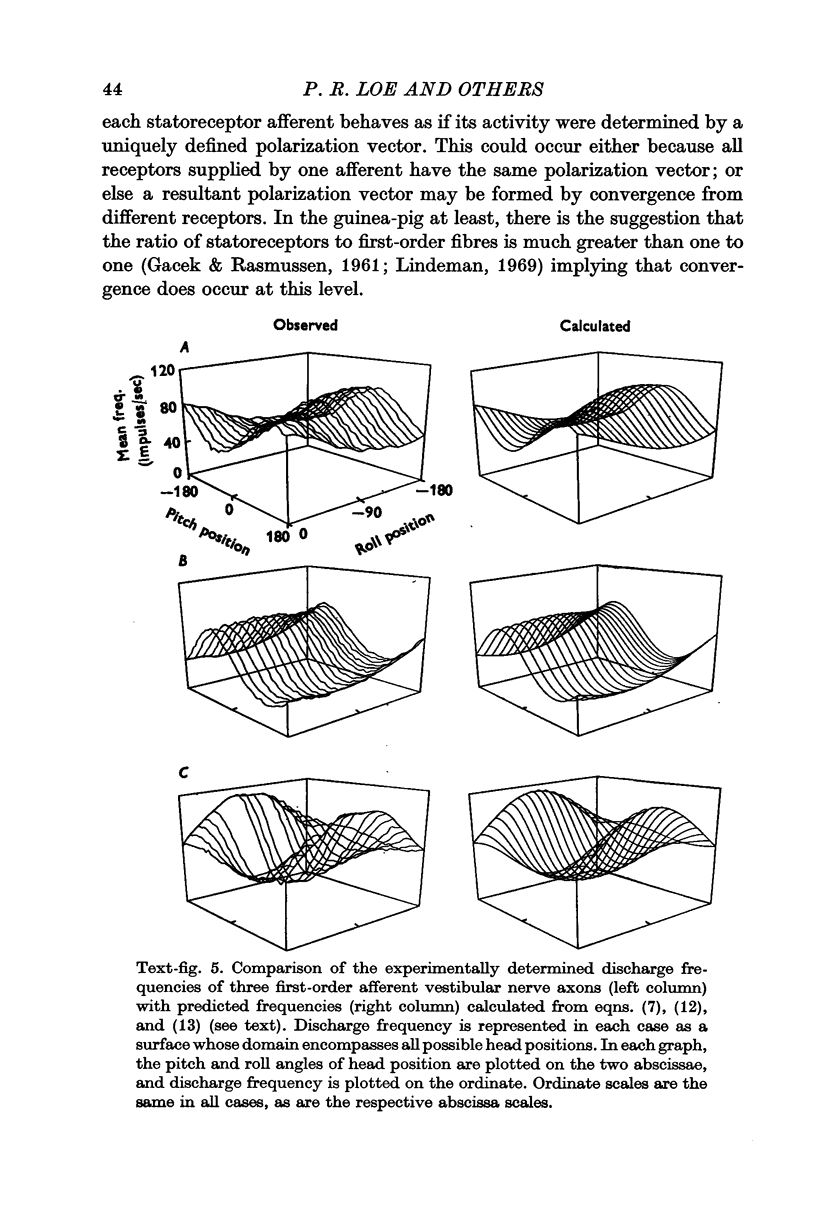
4. Experimentally determined discharge rates were compared with the predictions of a computer simulation model incorporating the idea that shearing force acting on morphologically polarized receptors is the adequate stimulus for macular receptor cells.
5. This approach permitted the identification of a population of first-order vestibular afferents whose discharge frequency varied with head position as did the magnitude of shear force computed for individual receptors, each most excited in a particular head position.
6. The majority of the spatial orientations of maximal sensitivity defined a surface which is tilted by approximately 30° with reference to the Horsley—Clarke horizontal plane, implying that most statoreceptor afferents are maximally sensitive to position changes when the cat's head is at or near its normal position.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FLOCK A. STRUCTURE OF THE MACULA UTRICULI WITH SPECIAL REFERENCE TO DIRECTIONAL INTERPLAY OF SENSORY RESPONSES AS REVEALED BY MORPHOLOGICAL POLARIZATION. J Cell Biol. 1964 Aug;22:413–431. doi: 10.1083/jcb.22.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluur E. The interaction between the utricle and the saccule. Acta Otolaryngol. 1970 Jan-Feb;69(1):17–24. doi: 10.3109/00016487009123332. [DOI] [PubMed] [Google Scholar]
- GACEK R. R., NOMURA Y., BALOGH K. ACETYLCHOLINESTERASE ACTIVITY IN THE EFFERENT FIBERS OF THE STATO-ACOUSTIC NERVE. Acta Otolaryngol. 1965 Jun;59:541–553. doi: 10.3109/00016486509124585. [DOI] [PubMed] [Google Scholar]
- GACEK R. R., RASMUSSEN G. L. Fiber analysis of the statoacoustic nerve of guinea pig, cat, and monkey. Anat Rec. 1961 Apr;139:455–463. doi: 10.1002/ar.1091390402. [DOI] [PubMed] [Google Scholar]
- GLEISNER L., HENRIKSSON N. G. EFFERENT AND AFFERENT ACTIVITY PATTERN IN THE VESTIBULAR NERVE OF THE FROG. Acta Otolaryngol Suppl. 1963;192:SUPPL 192–192:90+. doi: 10.3109/00016486409134632. [DOI] [PubMed] [Google Scholar]
- GRAYBIEL A., PATTERSON J. L., Jr Thresholds of stimulation of the otolith organs as indicated by the oculogravic illusion. J Appl Physiol. 1955 May;7(6):666–670. doi: 10.1152/jappl.1955.7.6.666. [DOI] [PubMed] [Google Scholar]
- Gacek R. R. The course and central termination of first order neurons supplying vestibular endorgans in the cat. Acta Otolaryngol Suppl. 1969;254:1–66. [PubMed] [Google Scholar]
- Goldberg J. M., Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol. 1971 Jul;34(4):635–660. doi: 10.1152/jn.1971.34.4.635. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN O., ROBERTS T. D. M. The equilibrium function of the otolith organs of the thornback ray (Raja clavata). J Physiol. 1949 Dec;110(3-4):392–415. doi: 10.1113/jphysiol.1949.sp004448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman H. H. Studies on the morphology of the sensory regions of the vestibular apparatus with 45 figures. Ergeb Anat Entwicklungsgesch. 1969;42(1):1–113. [PubMed] [Google Scholar]
- Pratt L. L. A histochemical study of the course and distribution of the efferent vestibular fibers to the maculae of the saccule and utricle. Laryngoscope. 1969 Sep;79(9):1515–1545. [PubMed] [Google Scholar]
- RUPERT A., MOUSHEGIAN G., GALAMBOS R. Microelectrode studies of primary vestibular neurons in cat. Exp Neurol. 1962 Feb;5:100–109. doi: 10.1016/0014-4886(62)90026-2. [DOI] [PubMed] [Google Scholar]
- SPOENDLIN H. H. ORGANIZATION OF THE SENSORY HAIRS IN THE GRAVITY RECEPTORS IN UTRICULE AND SACCULE OF THE SQUIRREL MONKEY. Z Zellforsch Mikrosk Anat. 1964 May 15;62:701–706. doi: 10.1007/BF00341855. [DOI] [PubMed] [Google Scholar]
- Spoendlin H., Lichtensteiger W. The adrenergic innervation of the labyrinth. Acta Otolaryngol. 1966 May;61(5):423–434. [PubMed] [Google Scholar]
- Vidal J., Jeannerod M., Lifschitz W., Levitan H., Rosenberg J., Segundo J. P. Static and dynamic properties of gravity-sensitive receptors in the cat vestibular system. Kybernetik. 1971 Dec;9(6):205–215. doi: 10.1007/BF00289582. [DOI] [PubMed] [Google Scholar]