Abstract
1. The spino-olivocerebellar path ascending through the dorsal funiculus (DF-SOCP) was investigated in decerebrate cats with the cord transected in the third cervical segment except for the dorsal funiculi. The climbing fibre responses evoked in Purkinje cells were studied by recording the mass activity at the cerebellar surface and by recording from single cells.
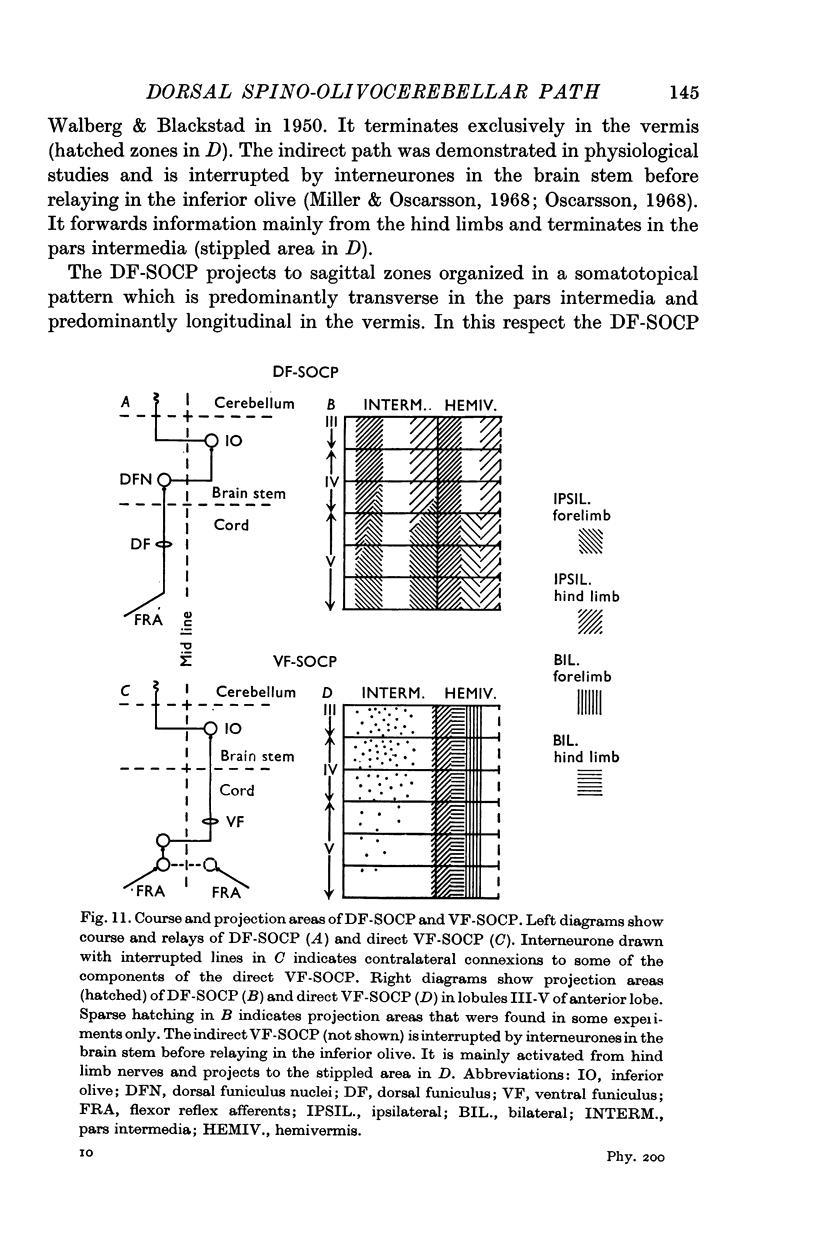
2. The DF-SOCP forms a disynaptic path from the spinal cord to the cerebellar cortex as shown by latency measurements. Anatomical studies have recently demonstrated that the relays are in the rostral part of the dorsal funiculus nuclei and in the dorsal accessory olive.
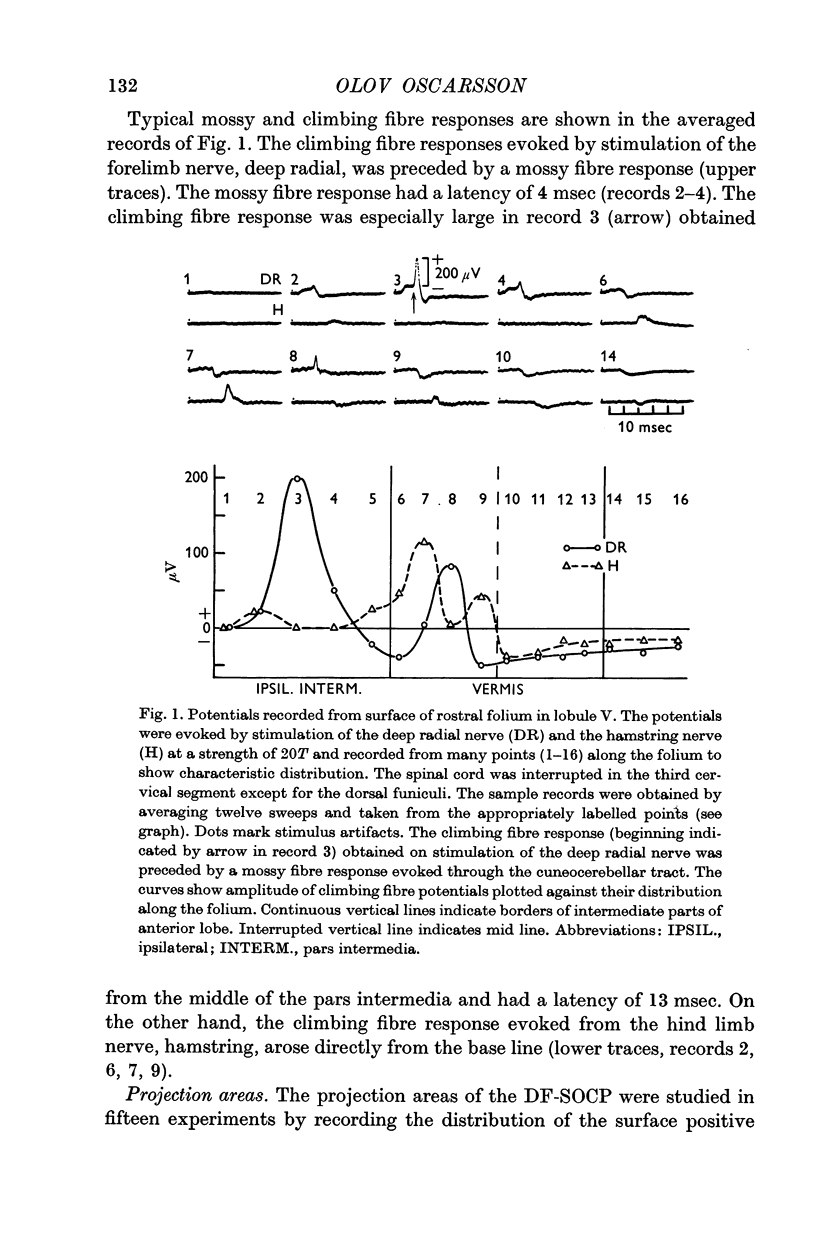
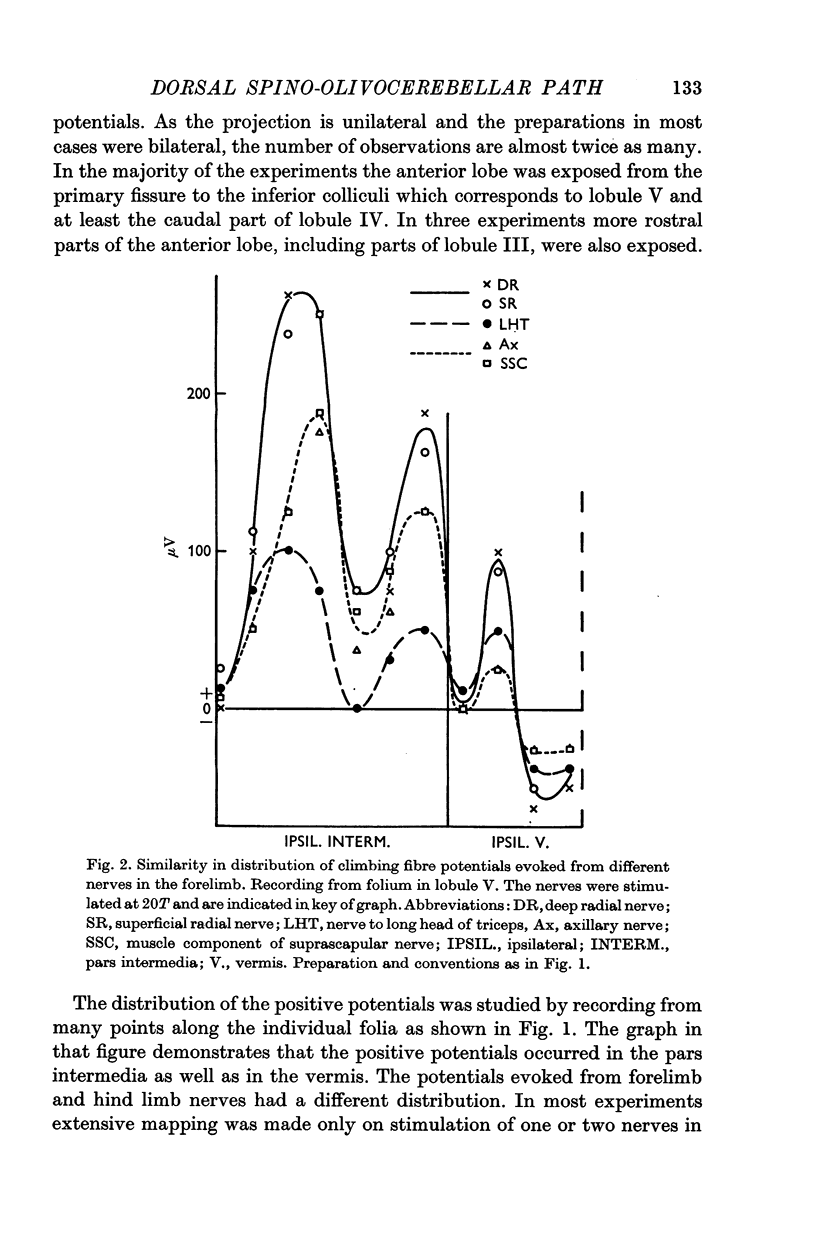
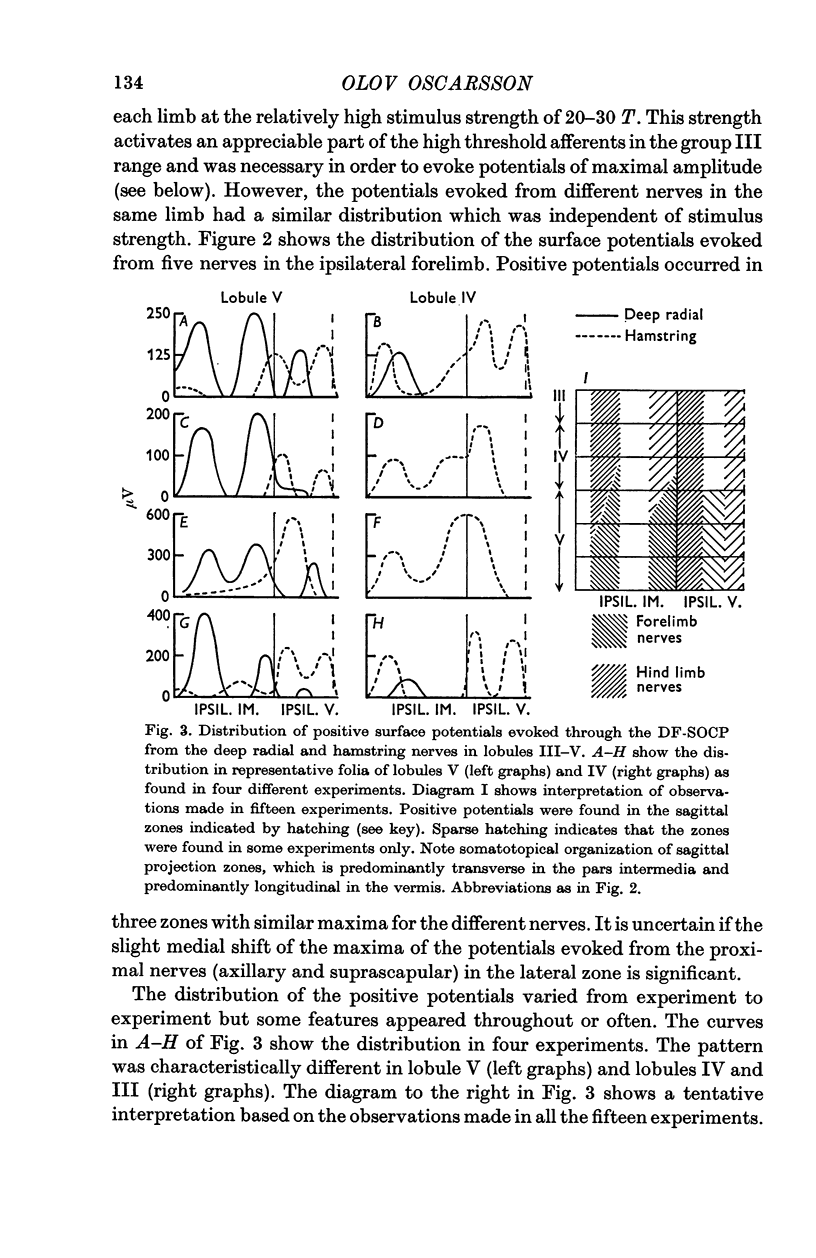
3. The DF-SOCP projects to sagittal zones in the pars intermedia and vermis of the anterior lobe. The somatotopical organization is predominantly transverse in the pars intermedia and predominantly longitudinal in the vermis.
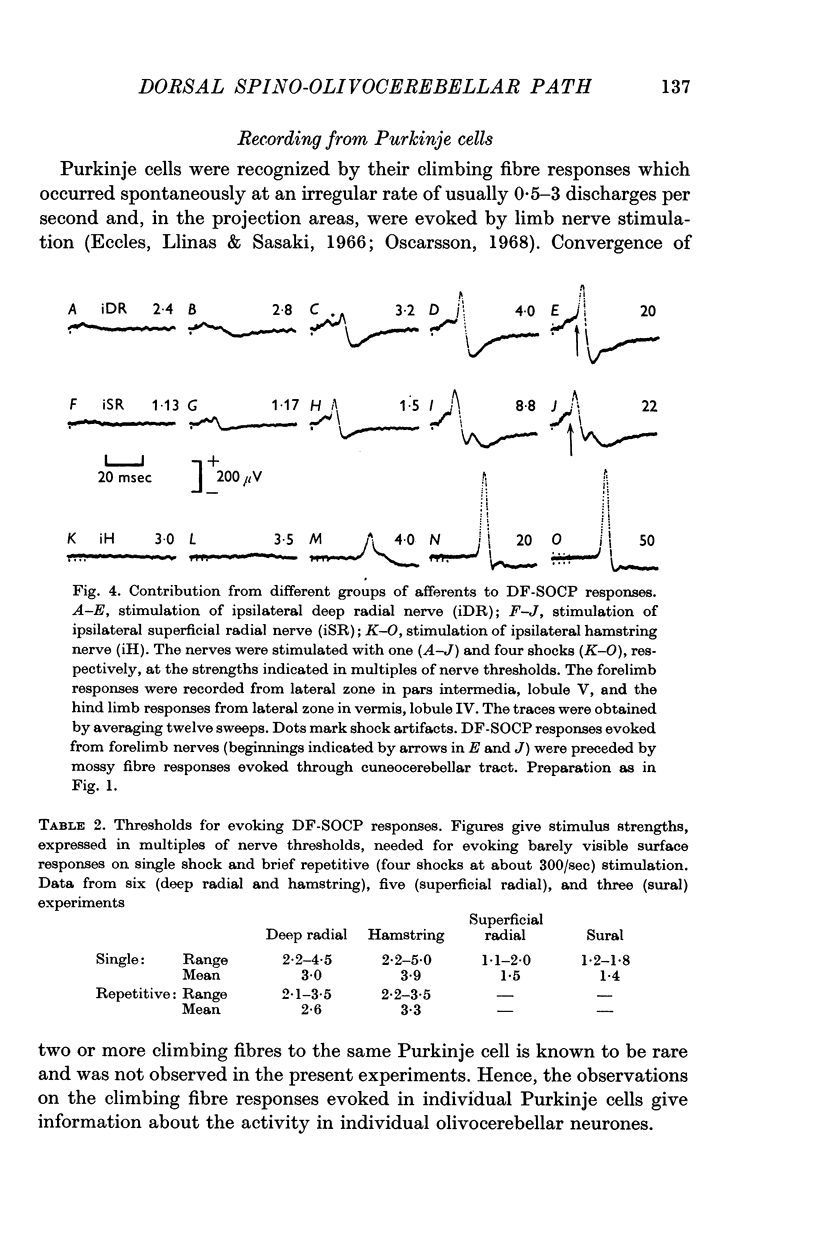
4. The olivary neurones in the DF-SOCP are activated by the flexor reflex afferents from wide receptive fields. The fields are restricted to one ipsilateral limb and the majority of the olivary neurones could be activated from all the nerves tested in this limb.
5. Natural stimulation of receptors evoked excitation in about half of the olivary neurones investigated. This excitation was elicited by pressure against deep structures. Inhibitory effects were rarely observed.
6. The dorsal and ventral spino-olivocerebellar paths are compared.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M., Harvey R. J. Responses to a spino-olivo-cerebellar pathway in the cat. J Physiol. 1968 Jan;194(1):147–168. doi: 10.1113/jphysiol.1968.sp008399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODAL A., WALBERG F., BLACKSTAD T. Termination of spinal afferents to inferior olive in cat. J Neurophysiol. 1950 Nov;13(6):431–454. doi: 10.1152/jn.1950.13.6.431. [DOI] [PubMed] [Google Scholar]
- Ebbesson S. O. A connection between the dorsal column nuclei and the dorsal accessory alive. Brain Res. 1968 May;8(2):393–397. doi: 10.1016/0006-8993(68)90062-0. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Intracellularly recorded responses of the cerebellar Purkinje cells. Exp Brain Res. 1966;1(2):161–183. doi: 10.1007/BF00236869. [DOI] [PubMed] [Google Scholar]
- GRANT G. Projection of the external cuneate nucleus onto the cerebellum in the cat: an experimental study using silver methods. Exp Neurol. 1962 Mar;5:179–195. doi: 10.1016/0014-4886(62)90032-8. [DOI] [PubMed] [Google Scholar]
- Grant G., Oscarsson O. Mass discharges evoked in the olivocerebellar tract on stimulation of muscle and skin nerves. Exp Brain Res. 1966;1(4):329–337. doi: 10.1007/BF00237705. [DOI] [PubMed] [Google Scholar]
- HOLMQVIST B., OSCARSSON O., ROSEN I. Functional organization of the cuneocrebellar tract in the cat. Acta Physiol Scand. 1963 Jun-Jul;58:216–235. doi: 10.1111/j.1748-1716.1963.tb02643.x. [DOI] [PubMed] [Google Scholar]
- KUYPERS H. G., TUERK J. D. THE DISTRIBUTION OF THE CORTICAL FIBRES WITHIN THE NUCLEI CUNEATUS AND GRACILIS IN THE CAT. J Anat. 1964 Apr;98:143–162. [PMC free article] [PubMed] [Google Scholar]
- LARSELL O. The cerebellum of the cat and the monkey. J Comp Neurol. 1953 Aug;99(1):135–199. doi: 10.1002/cne.900990110. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. ASCENDING SPINAL HINDLIMB PATHWAYS IN THE CAT. Prog Brain Res. 1964;12:135–163. doi: 10.1016/s0079-6123(08)60621-4. [DOI] [PubMed] [Google Scholar]
- Morest D. K. Experimental study of the projections of the nucleus of the tractus solitarius and the area postrema in the cat. J Comp Neurol. 1967 Aug;130(4):277–300. doi: 10.1002/cne.901300402. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of a dorsal spino-olivocerebellar path. Brain Res. 1967 Aug;5(4):531–534. doi: 10.1016/0006-8993(67)90029-7. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the ventral spino-olivocerebellar path. J Physiol. 1968 May;196(2):453–478. doi: 10.1113/jphysiol.1968.sp008518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O., Uddenberg N. Somatotopic termination of spino-olivocerebellar path. Brain Res. 1966 Dec;3(2):204–207. doi: 10.1016/0006-8993(66)90080-1. [DOI] [PubMed] [Google Scholar]
- Provini L., Redman S., Strata P. Somatotopic organization of mossy and climbing fibres to the anterior lobe of cerebellum activated by the sensorimotor cortex. Brain Res. 1967 Oct;6(2):378–381. doi: 10.1016/0006-8993(67)90205-3. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Functional organization of long, second-order afferents in the dorsal funiculus. Exp Brain Res. 1968;4(4):377–382. doi: 10.1007/BF00235702. [DOI] [PubMed] [Google Scholar]
- WALBERG F. Corticofugal fibres to the nuclei of the dorsal columns; an experimental study in the cat. Brain. 1957 Jun;80(2):273–287. doi: 10.1093/brain/80.2.273. [DOI] [PubMed] [Google Scholar]


