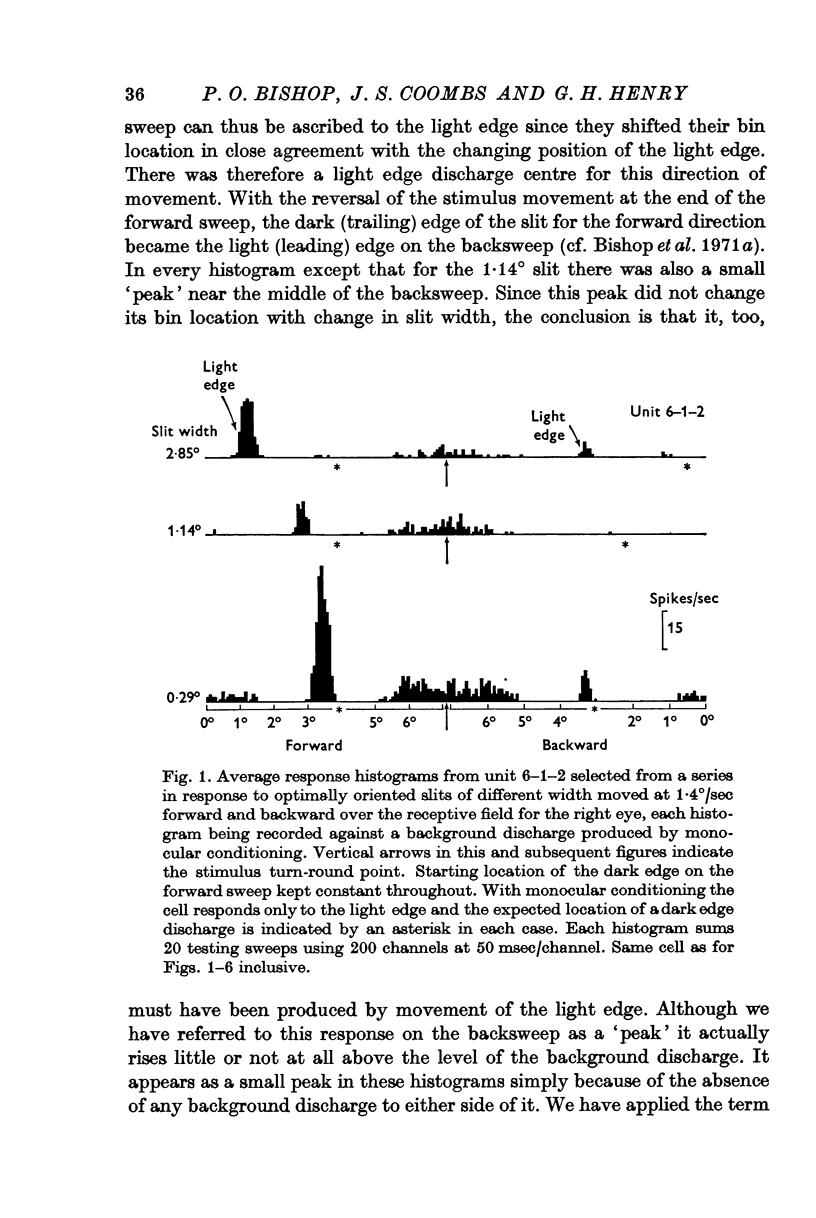
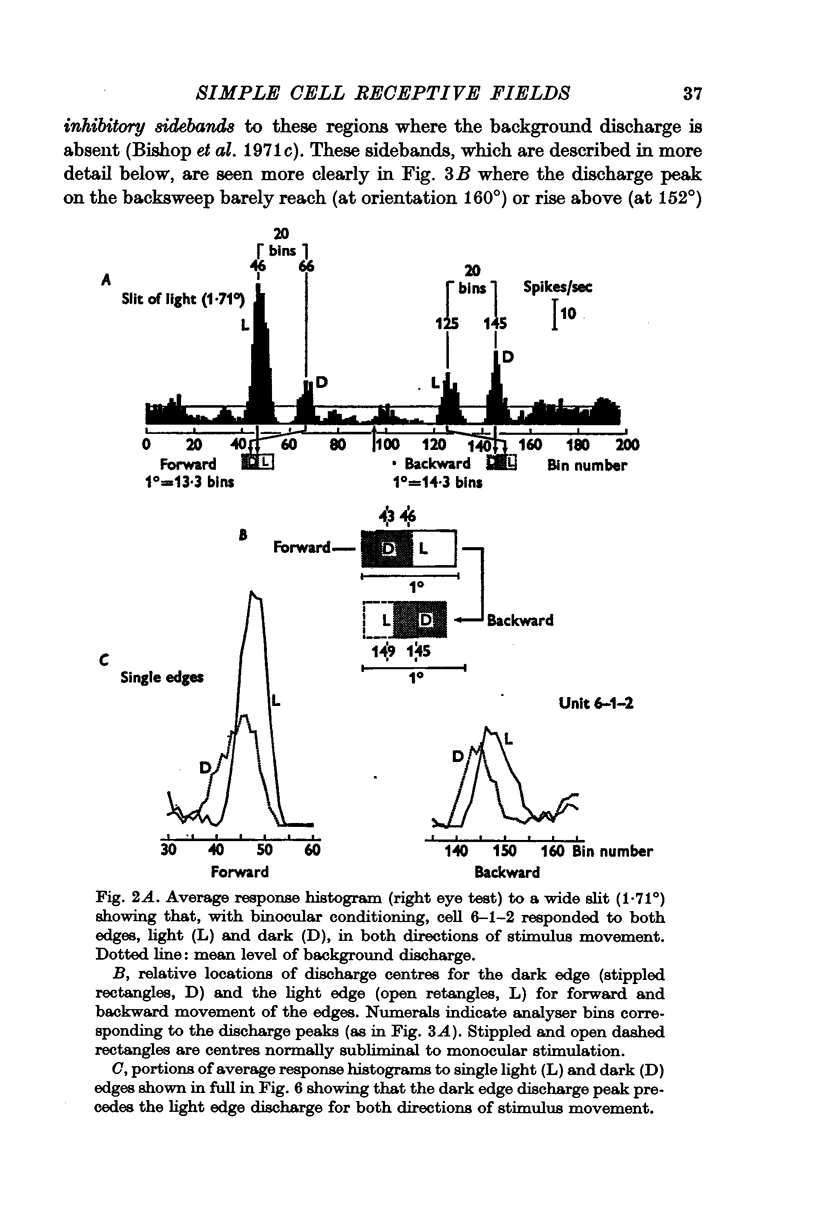
Abstract
1. The excitatory and inhibitory components in the receptive fields of unimodal simple cells in the striate cortex of the cat anaesthetized with nitrous oxide have been described using slits of light and single light-dark edges as stimuli.
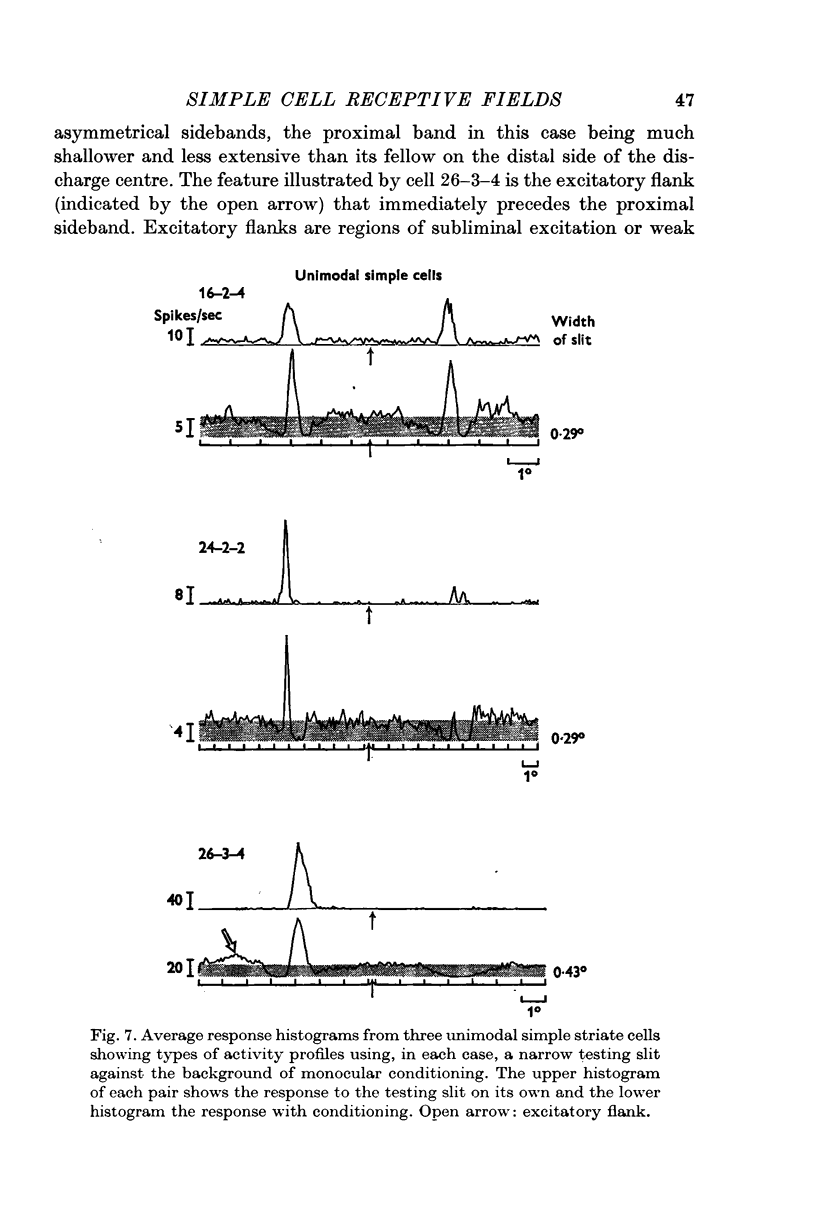
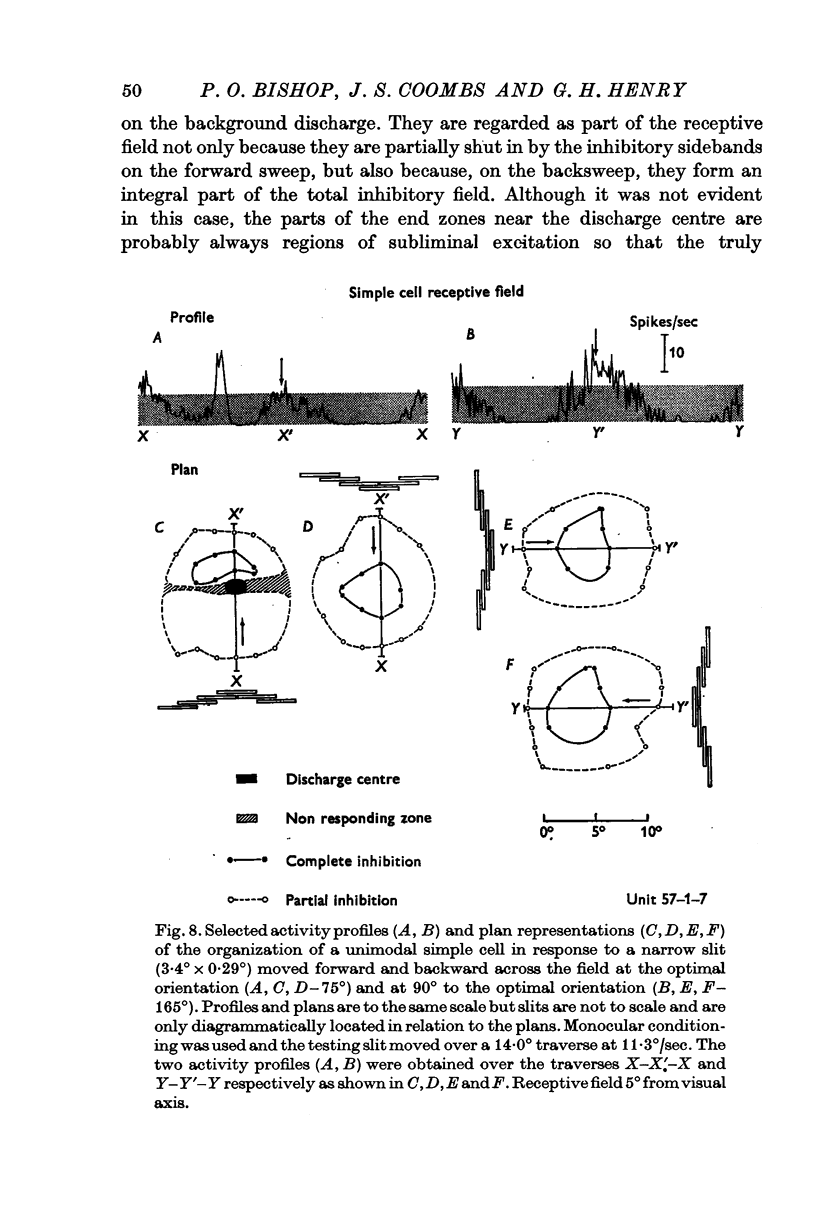
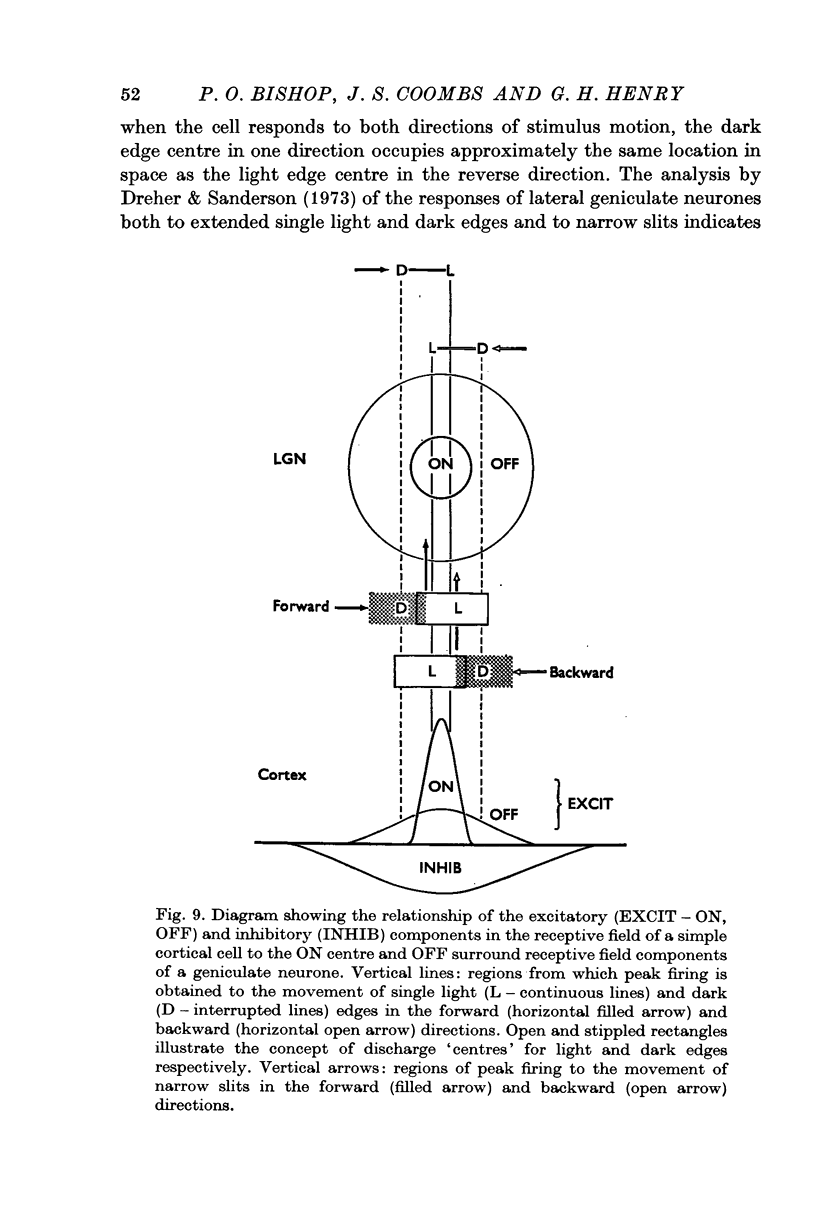
2. There is a small excitatory region (excitatory complex) centrally located in the receptive field that is made up of various combinations and spatial arrangements of subliminal excitatory and discharge subregions or centres.
3. The subliminal excitatory centres were revealed by a binocular facilitation technique. The excitability of the cell was raised by repeated stimulation via one eye while the neurone was tested with single edges via the other eye.
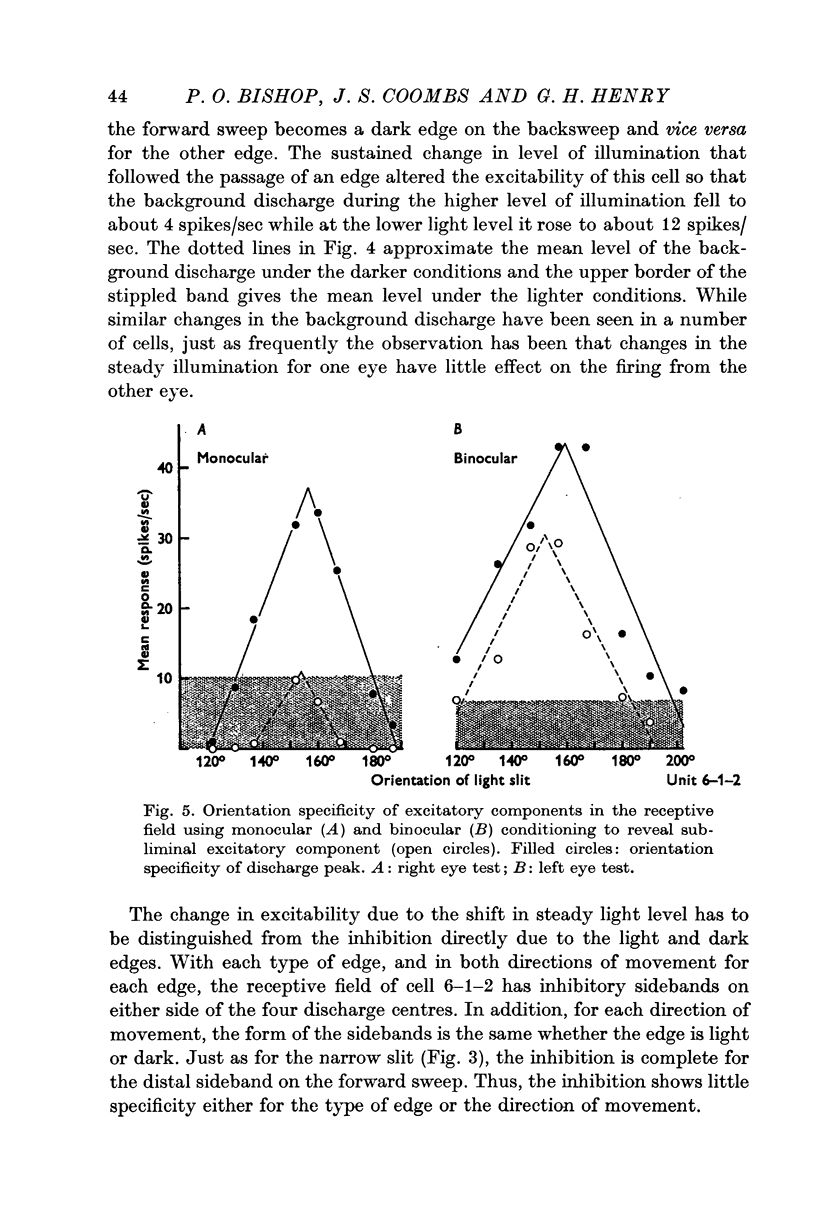
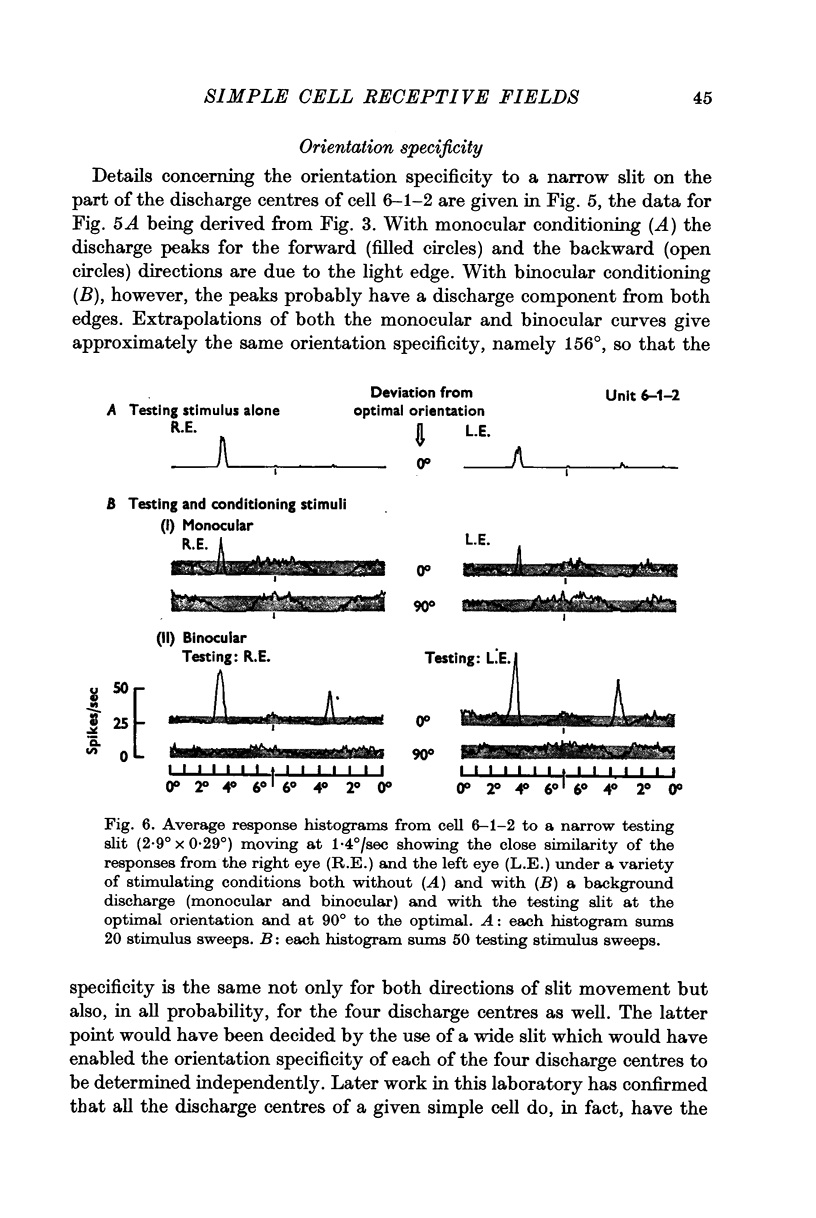
4. The subliminal excitatory and discharge centres are each specifically activated by only one type of edge, light-dark or dark-light, and then only in one direction of motion. All the subregions in the excitatory complex have the same optimal stimulus orientation.
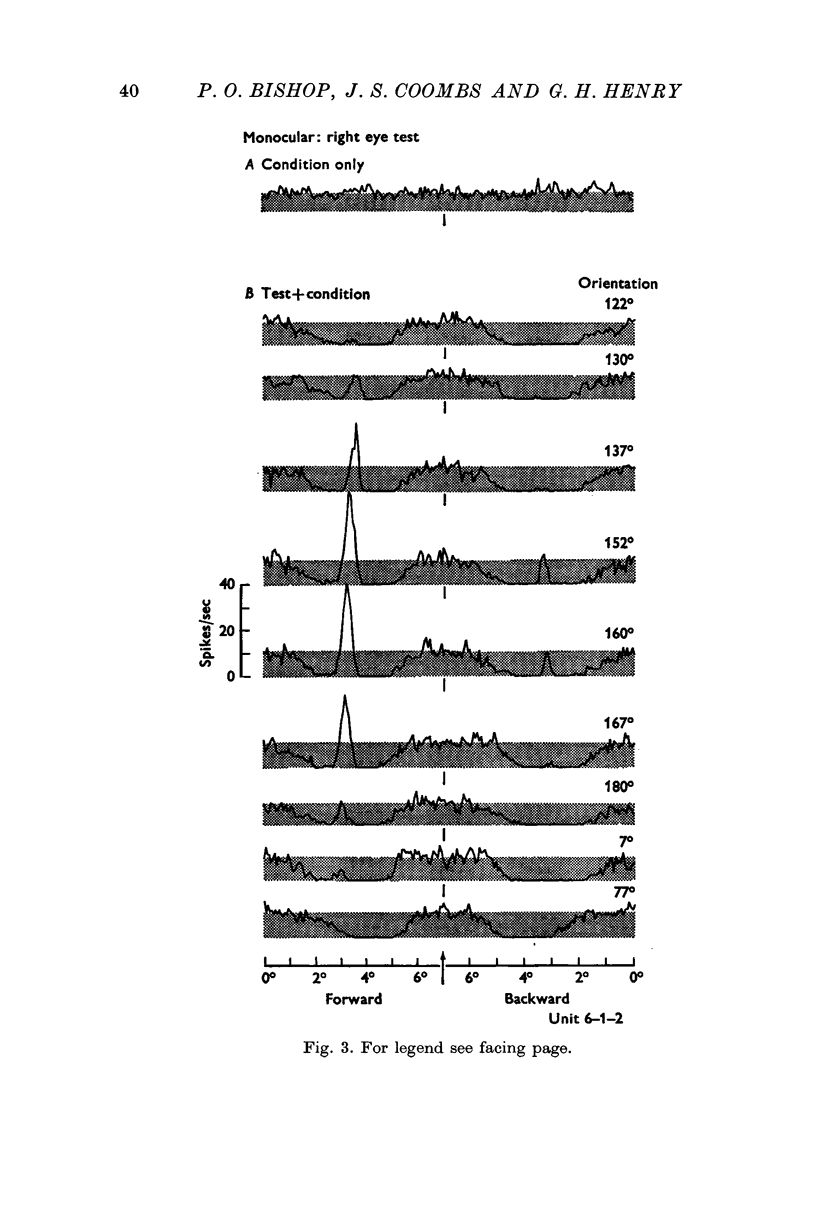
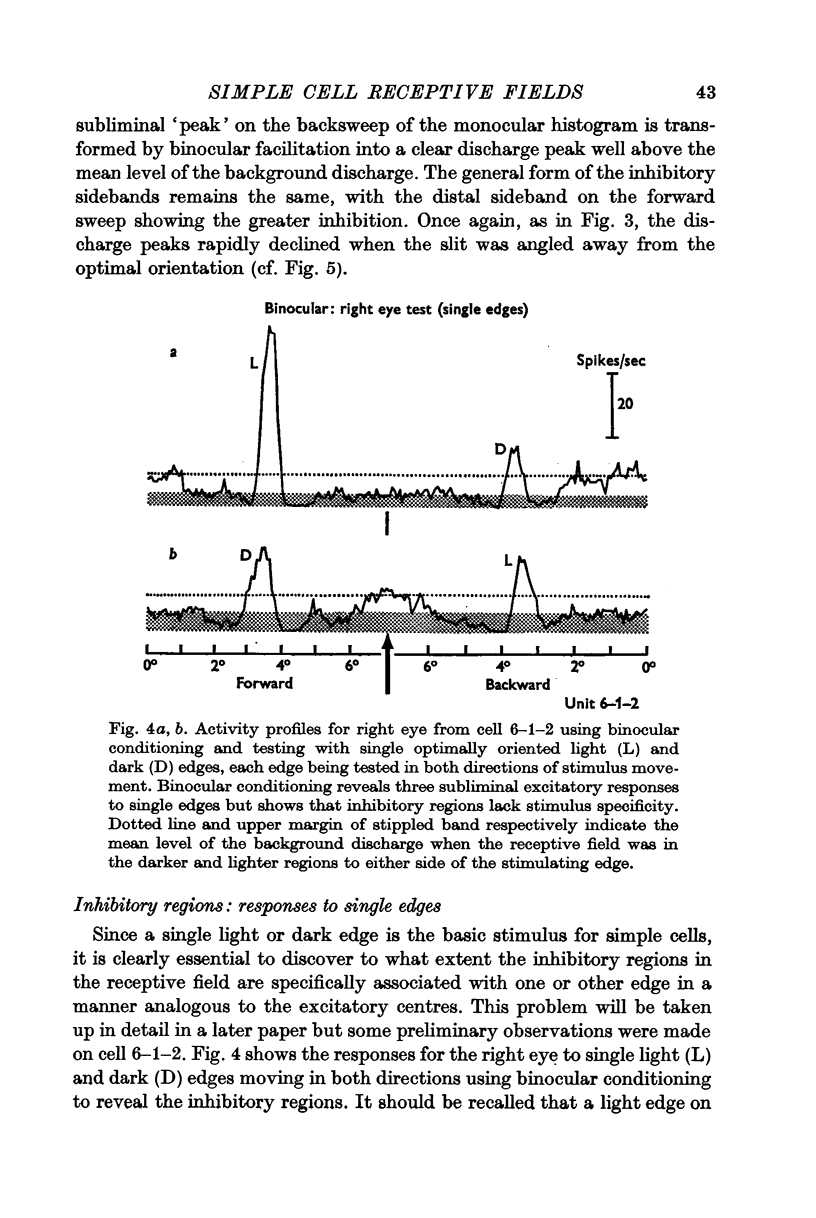
5. Inhibitory components in the receptive field were identified by stimulating the cell with bars of light and single edges against an artificial background discharge produced by repeated stimulation separately applied either to the same eye (monocular conditioning) or to the other eye (binocular conditioning). There are powerful inhibitory sidebands to either side of the excitatory complex and these inhibitory regions merge to include the excitatory complex when stimulus orientation is angled away from the optimal.
6. Excitation is highly stimulus specific whereas inhibition is non-specific.
7. The organization of the two receptive fields of a binocularly discharged cell can be closely similar.
8. The attempt is made to translate the concept of subliminal excitatory and discharge centres into specific neural mechanisms involving both the geniculo-cortical input and various intracortical circuits.
9. These new developments call for only minor modifications to the model we have proposed for the organization of the receptive field.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. O., Coombs J. S., Henry G. H. Interaction effects of visual contours on the discharge frequency of simple striate neurones. J Physiol. 1971 Dec;219(3):659–687. doi: 10.1113/jphysiol.1971.sp009682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Responses to visual contours: spatio-temporal aspects of excitation in the receptive fields of simple striate neurones. J Physiol. 1971 Dec;219(3):625–657. doi: 10.1113/jphysiol.1971.sp009681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Dreher B., Henry G. H. Simple striate cells: comparison of responses to stationary and moving stimuli. J Physiol. 1972 Dec;227(2):15P–17P. [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H., Smith C. J. Binocular interaction fields of single units in the cat striate cortex. J Physiol. 1971 Jul;216(1):39–68. doi: 10.1113/jphysiol.1971.sp009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H. Striate neurons: receptive field concepts. Invest Ophthalmol. 1972 May;11(5):346–354. [PubMed] [Google Scholar]
- Garey L. J., Powell T. P. An experimental study of the termination of the lateral geniculo-cortical pathway in the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):41–63. doi: 10.1098/rspb.1971.0080. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Coombs J. S. Inhibitory and sub-liminal excitatory receptive fields of simple units in cat striate cortex. Vision Res. 1969 Oct;9(10):1289–1296. doi: 10.1016/0042-6989(69)90116-3. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O. Simple cells of the striate cortex. Contrib Sens Physiol. 1971;5:1–46. doi: 10.1016/b978-0-12-151805-9.50007-9. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O. Striate neurons: receptive field organization. Invest Ophthalmol. 1972 May;11(5):357–368. [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
- Joshua D. E., Bishop P. O. Binocular single vision and depth discrimination. Receptive field disparities for central and peripheral vision and binocular interaction on peripheral single units in cat striate cortex. Exp Brain Res. 1970;10(4):389–416. doi: 10.1007/BF02324766. [DOI] [PubMed] [Google Scholar]
- Kinston W. J., Vadas M. A., Bishop P. O. Multiple projection of the visual field to the medical portion of the dorsal lateral geniculate nucleus and the adjacent nuclei of the thalamus of the cat. J Comp Neurol. 1969 Jul;136(3):295–315. doi: 10.1002/cne.901360304. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Levick W. R., Cleland B. G., Dubin M. W. Lateral geniculate neurons of cat: retinal inputs and physiology. Invest Ophthalmol. 1972 May;11(5):302–311. [PubMed] [Google Scholar]
- Rodieck R. W., Pettigrew J. D., Bishop P. O., Nikara T. Residual eye movements in receptive-field studies of paralyzed cats. Vision Res. 1967 Jan;7(1):107–110. doi: 10.1016/0042-6989(67)90031-4. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res. 1965 Dec;5(11):583–601. doi: 10.1016/0042-6989(65)90033-7. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Response of cat retinal ganglion cells to moving visual patterns. J Neurophysiol. 1965 Sep;28(5):819–832. doi: 10.1152/jn.1965.28.5.819. [DOI] [PubMed] [Google Scholar]
- Sanderson K. J., Bishop P. O., Darian-Smith I. The properties of the binocular receptive fields of lateral geniculate neurons. Exp Brain Res. 1971;13(2):178–207. doi: 10.1007/BF00234085. [DOI] [PubMed] [Google Scholar]
- Sanderson K. J., Darian-Smith I., Bishop P. O. Binocular corresponding receptive fields of single units in the cat dorsal lateral geniculate nucleus. Vision Res. 1969 Oct;9(10):1297–1303. doi: 10.1016/0042-6989(69)90117-5. [DOI] [PubMed] [Google Scholar]
- Singer W. Inhibitory binocular interaction in the lateral geniculate body of the cat. Brain Res. 1970 Feb 17;18(1):165–170. doi: 10.1016/0006-8993(70)90463-4. [DOI] [PubMed] [Google Scholar]
- Toyama K., Matsunami K. Synaptic action of specific visual inpulses upon cat's parastriate cortex. Brain Res. 1968 Sep;10(3):473–476. doi: 10.1016/0006-8993(68)90220-5. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Konishi M., Creutzfeldt O. D. Postsynaptic potentials in the cat's visual cortex following electrical stimulation of afferent pathways. Exp Brain Res. 1966;1(3):272–283. doi: 10.1007/BF00234347. [DOI] [PubMed] [Google Scholar]


