Abstract
1. The effect of different polyvalent metal ions in the external solution upon the threshold membrane potential for spike initiation in crayfish axons has been studied by means of intracellular micro-electrodes. The metal ions tested included six divalent cations (Ca2+, Mg2+, Sr2+, Ba2+, Co2+, and Ni2+) and three trivalent cations (La3+, Y3+ and Eu3+).
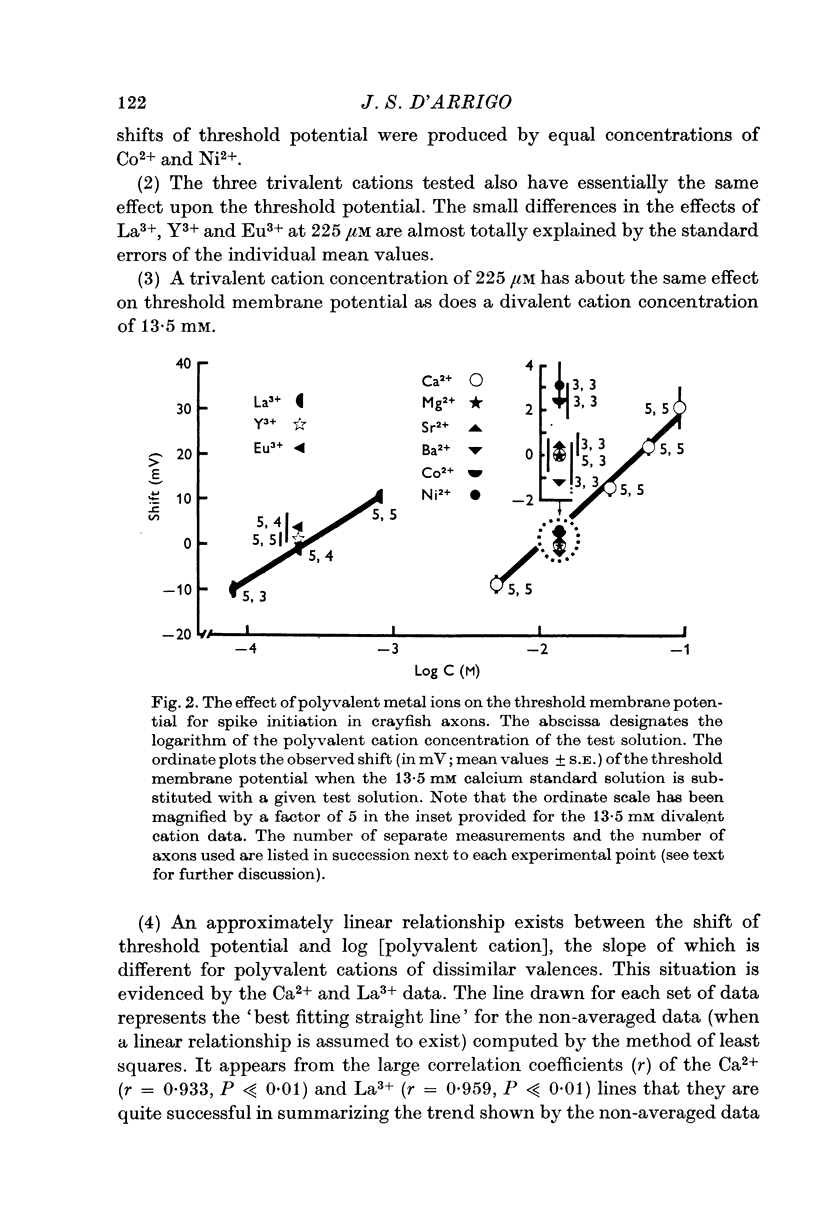
2. Identical extracellular concentrations of different cations with the same valence had essentially the same effect on threshold membrane potential. However, a very low concentration of trivalent cations (about 225 μM) was found to be equivalent to a much higher divalent cation concentration (13·5 mM) as measured by their effects on threshold potential.
3. Upon a tenfold increase in concentration, the threshold potential for spike initiation was shifted in a positive direction by 30·6 mV with divalent cations and by 20·8 mV with trivalent cations.
4. It is shown that a hypothesis involving screening of negative charges at the axonal membrane surface, based on Gouy—Chapman theory, predicts these various experimental results rather closely.
5. It is concluded that a high negative charge density, sufficient to render a screening mechanism possible, exists at the surface of crayfish axons in the region of the sodium `gates'.
6. The density of surface charges is calculated to be approximately 1e-/43 Å2. This calculation is discussed in connexion with the possible molecular identity of the sodium `gates' in crayfish axons and other excitable systems.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaustein M. P., Goldman D. E. The action of certain polyvalent cations on the voltage-clamped lobster axon. J Gen Physiol. 1968 Mar;51(3):279–291. doi: 10.1085/jgp.51.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Wright E. M. Biological membranes: the physical basis of ion and nonelectrolyte selectivity. Annu Rev Physiol. 1969;31:581–646. doi: 10.1146/annurev.ph.31.030169.003053. [DOI] [PubMed] [Google Scholar]
- Elul R. Fixed charge in the cell membrane. J Physiol. 1967 Apr;189(3):351–365. doi: 10.1113/jphysiol.1967.sp008173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. The effect of calcium on the myelinated nerve fibre. J Physiol. 1957 Jul 11;137(2):245–260. doi: 10.1113/jphysiol.1957.sp005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMANN D. E. A MOLECULAR STRUCTURAL BASIS FOR THE EXCITATION PROPERTIES OF AXONS. Biophys J. 1964 May;4:167–188. doi: 10.1016/s0006-3495(64)86776-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAME D. C. The electrical double layer and the theory of electrocapillarity. Chem Rev. 1947 Dec;41(3):441–501. doi: 10.1021/cr60130a002. [DOI] [PubMed] [Google Scholar]
- Gilbert D. L., Ehrenstein G. Effect of divalent cations on potassium conductance of squid axons: determination of surface charge. Biophys J. 1969 Mar;9(3):447–463. doi: 10.1016/S0006-3495(69)86396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. L., Ehrenstein G. Use of a fixed charge model to determine the pK of the negative sites on the external membrane surface. J Gen Physiol. 1970 Jun;55(6):822–825. [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Hladky S. B. Ion transport across thin lipid membranes: a critical discussion of mechanisms in selected systems. Q Rev Biophys. 1972 May;5(2):187–282. doi: 10.1017/s0033583500000883. [DOI] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H., Tashiro N. Effects of divalent cations on spike generation in the longitudinal somatic muscle of the earthworm. J Exp Biol. 1970 Feb;52(1):79–94. doi: 10.1242/jeb.52.1.79. [DOI] [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Current-voltage relations in the lobster giant axon membrane under voltage clamp conditions. J Gen Physiol. 1962 Jul;45:1217–1238. doi: 10.1085/jgp.45.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLINS L. J. An analysis of conductance changes in squid axon. J Gen Physiol. 1959 May 20;42(5):1013–1035. doi: 10.1085/jgp.42.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G., Ciani S. M. Surface charge and the conductance of phospholipid membranes. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1268–1275. doi: 10.1073/pnas.67.3.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R. U., Finkelstein A. The effect of surface charge on the voltage-dependent conductance induced in thin lipid membranes by monazomycin. J Gen Physiol. 1972 Sep;60(3):285–306. doi: 10.1085/jgp.60.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner F. F. Kinetics of excitable membranes. Voltage amplification in a diffusion regime. J Gen Physiol. 1970 Aug;56(2):272–296. doi: 10.1085/jgp.56.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J. R. Surface charge of giant axons of squid and lobster. Biophys J. 1968 Apr;8(4):470–489. doi: 10.1016/S0006-3495(68)86501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens W. G. Hydrogen ion and the activation of electrically excitable membranes. Nature. 1969 Nov 8;224(5219):547–549. doi: 10.1038/224547a0. [DOI] [PubMed] [Google Scholar]
- Wang J. H. A possible role of phospholipid in nerve excitation. Proc Natl Acad Sci U S A. 1970 Oct;67(2):916–920. doi: 10.1073/pnas.67.2.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L. Y. Quantum theory of nerve excitation. Bull Math Biophys. 1971 Jun;33(2):187–194. doi: 10.1007/BF02579471. [DOI] [PubMed] [Google Scholar]


