Abstract
1. The after-effects of repetitive stimulation of the perforant path fibres to the dentate area of the hippocampal formation have been examined with extracellular micro-electrodes in rabbits anaesthetized with urethane.
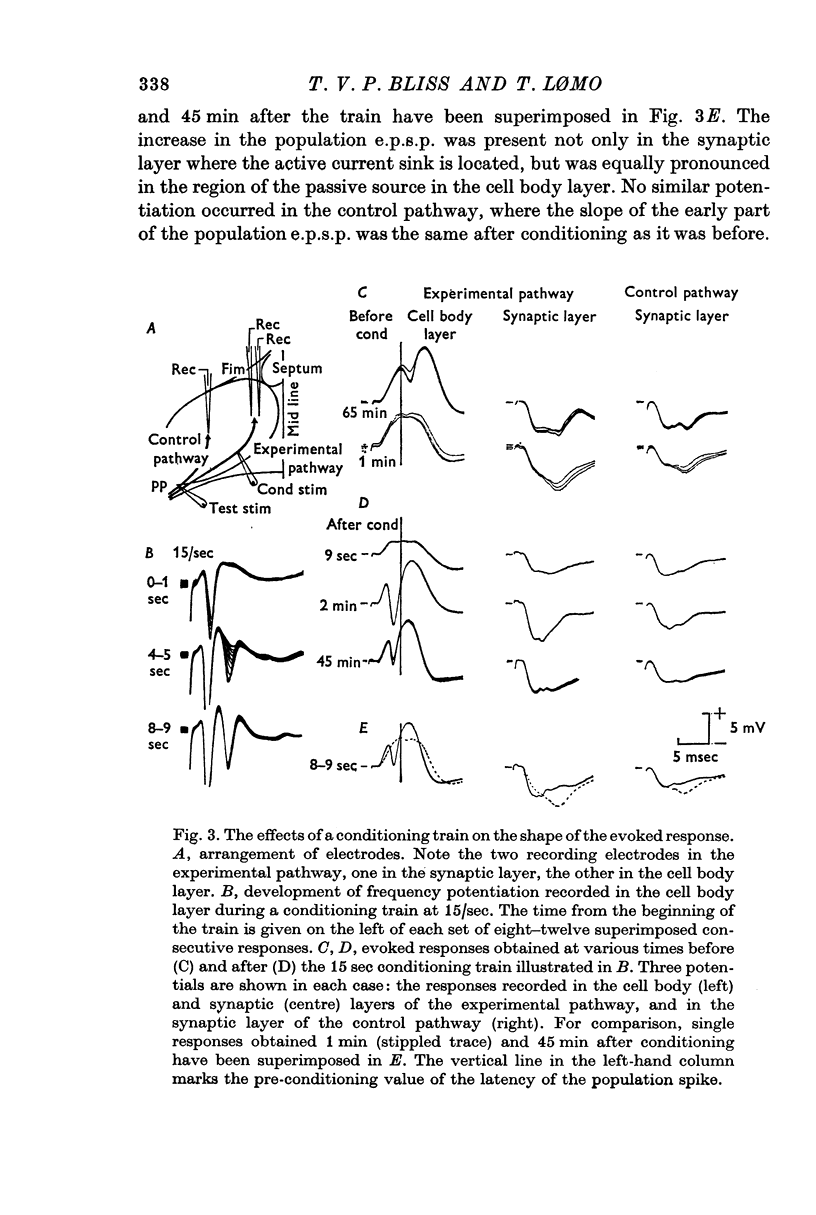
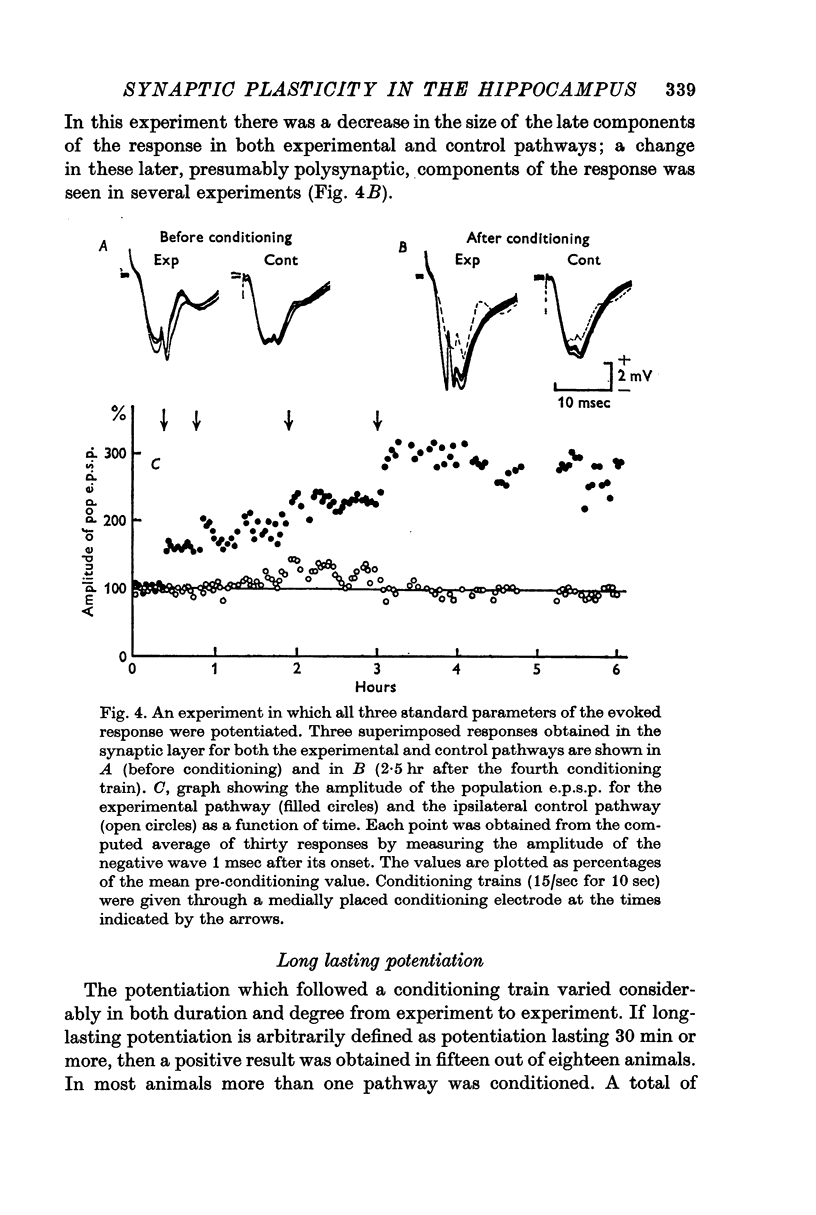
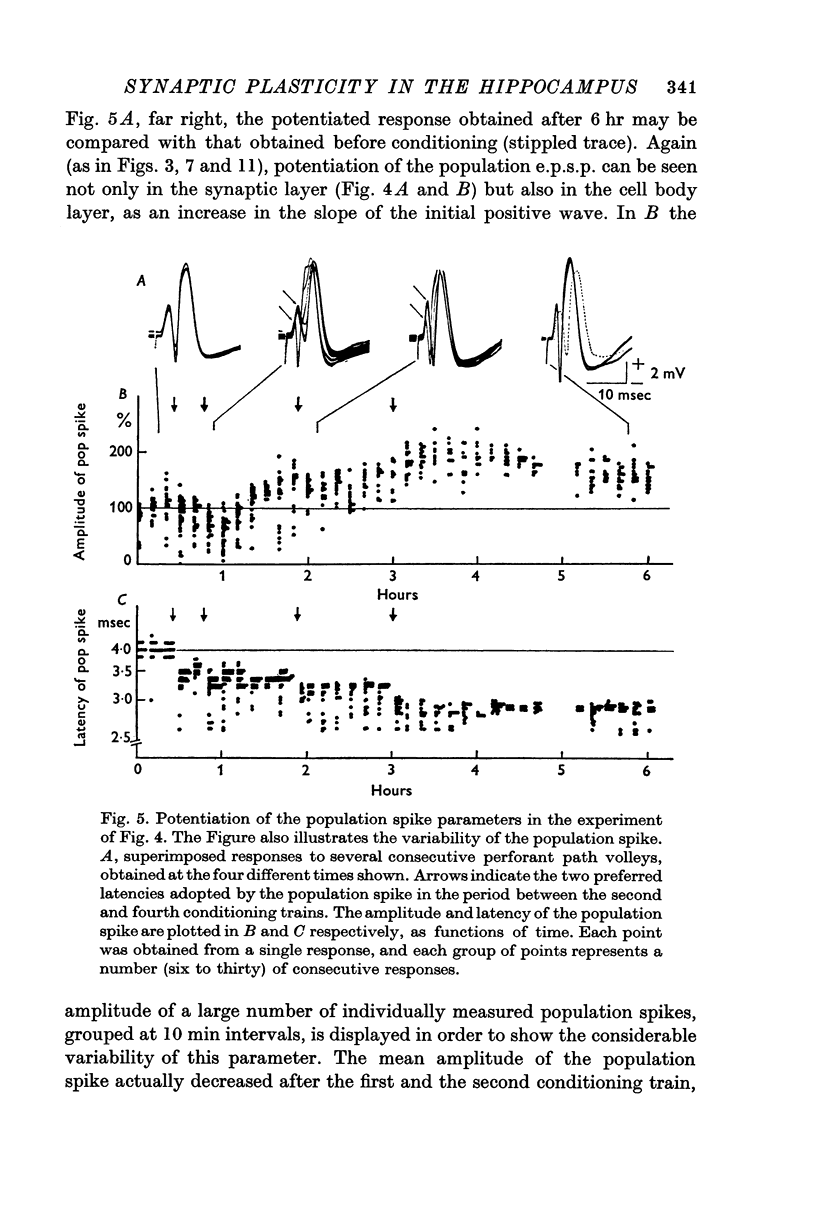
2. In fifteen out of eighteen rabbits the population response recorded from granule cells in the dentate area to single perforant path volleys was potentiated for periods ranging from 30 min to 10 hr after one or more conditioning trains at 10-20/sec for 10-15 sec, or 100/sec for 3-4 sec.
3. The population response was analysed in terms of three parameters: the amplitude of the population excitatory post-synaptic potential (e.p.s.p.), signalling the depolarization of the granule cells, and the amplitude and latency of the population spike, signalling the discharge of the granule cells.
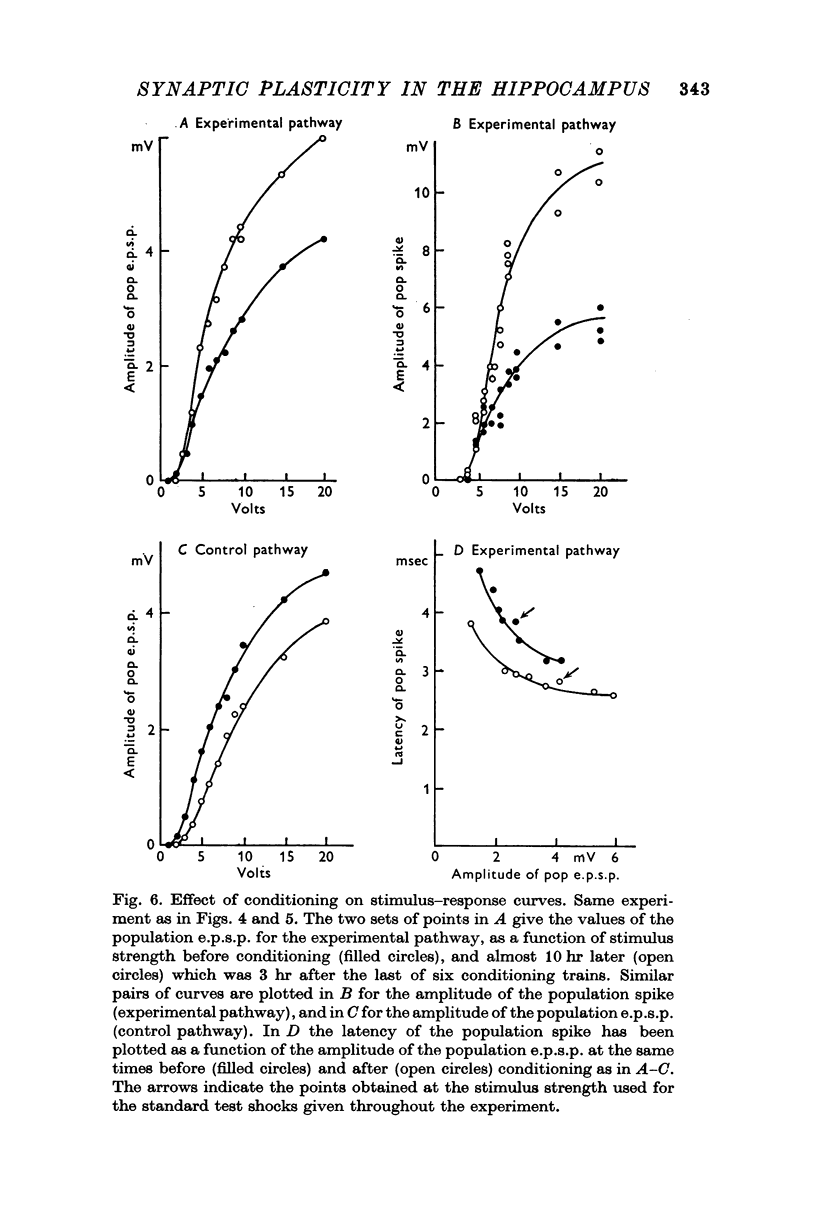
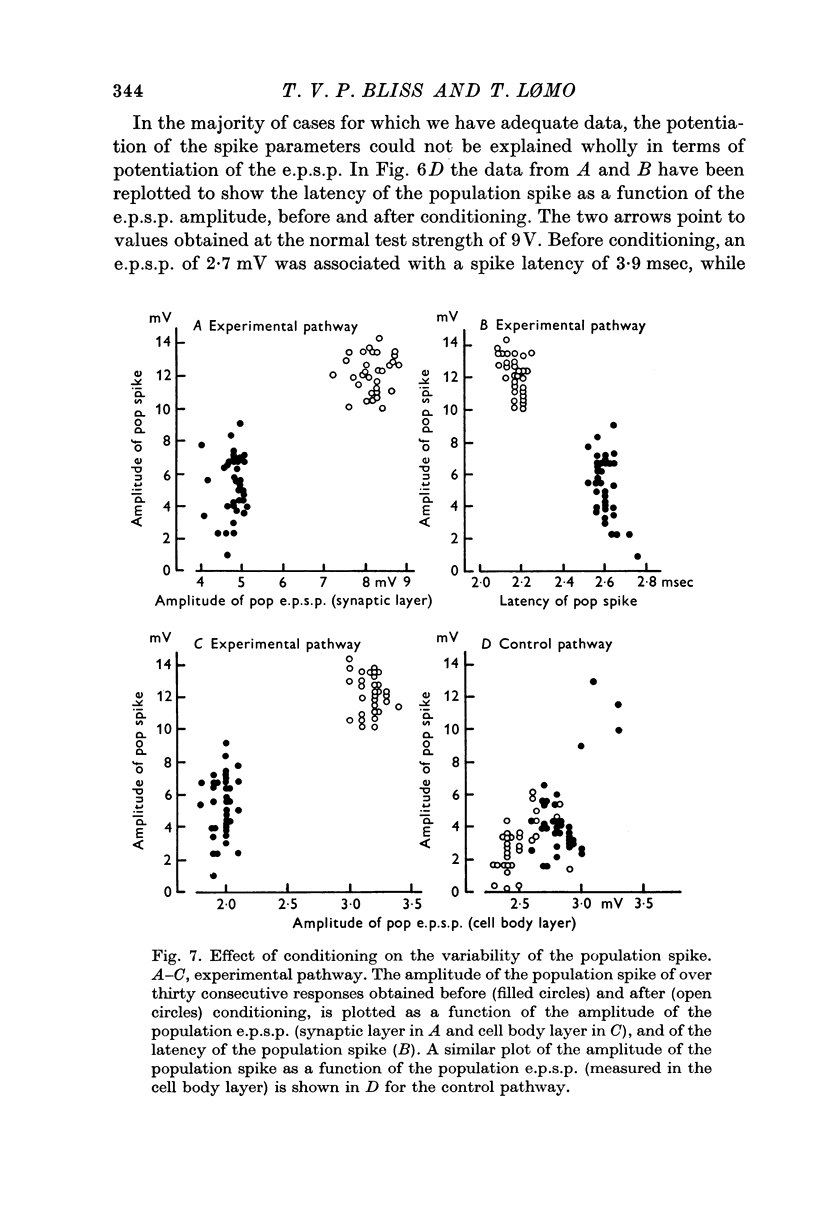
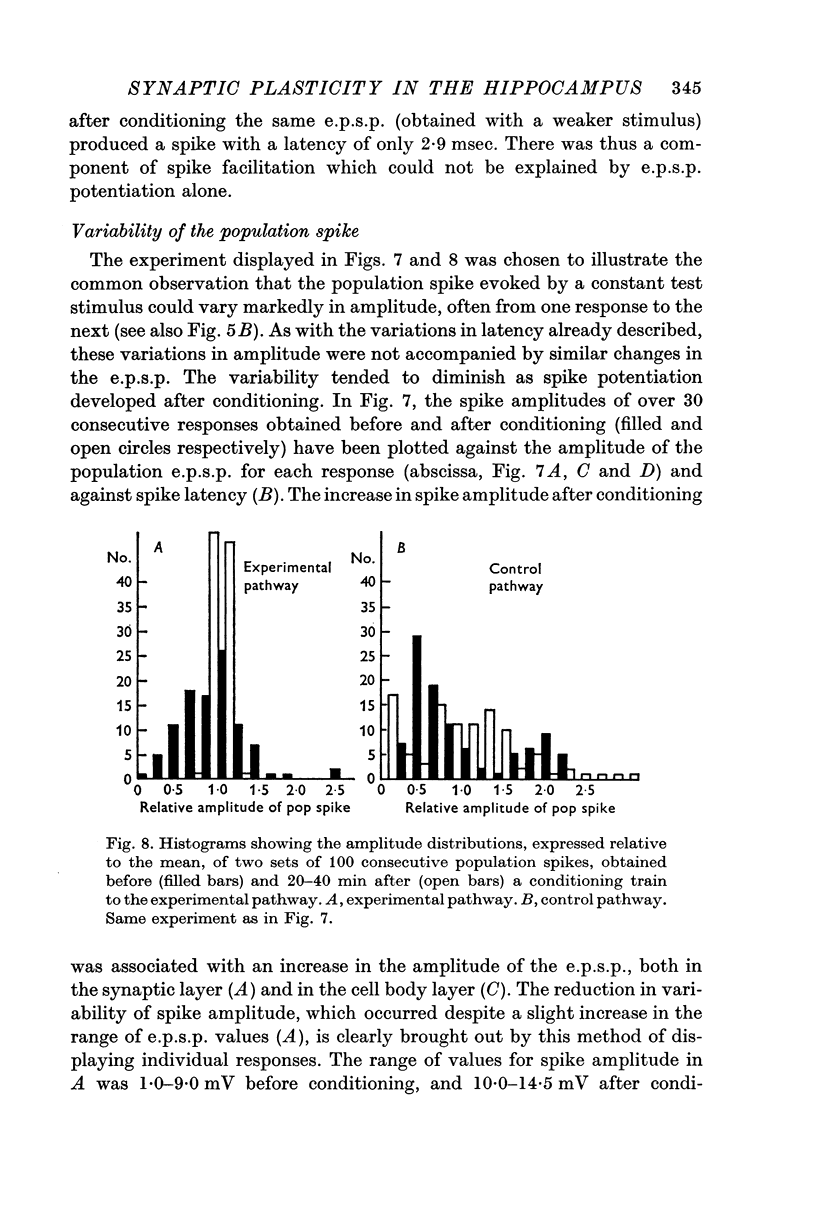
4. All three parameters were potentiated in 29% of the experiments; in other experiments in which long term changes occurred, potentiation was confined to one or two of the three parameters. A reduction in the latency of the population spike was the commonest sign of potentiation, occurring in 57% of all experiments. The amplitude of the population e.p.s.p. was increased in 43%, and of the population spike in 40%, of all experiments.
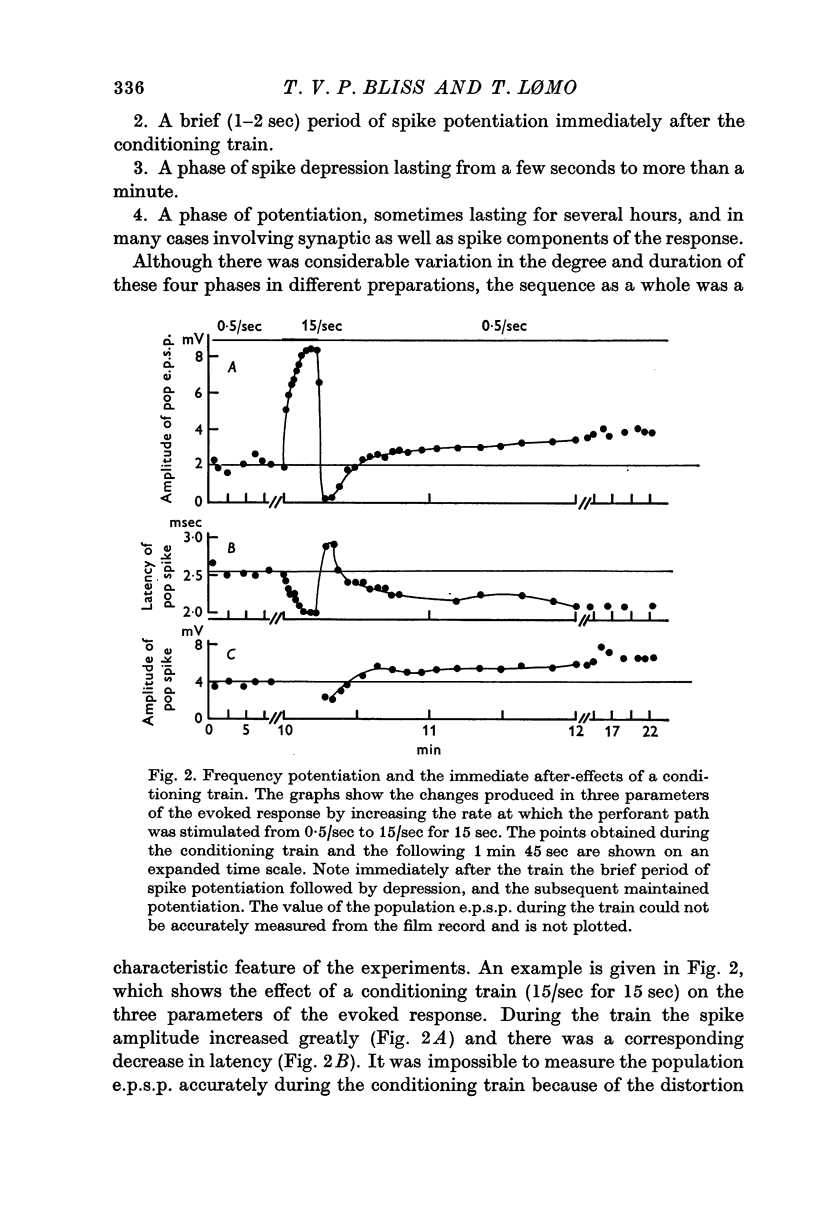
5. During conditioning at 10-20/sec there was massive potentiation of the population spike (`frequency potentiation'). The spike was suppressed during stimulation at 100/sec. Both frequencies produced long-term potentiation.
6. The results suggest that two independent mechanisms are responsible for long-lasting potentiation: (a) an increase in the efficiency of synaptic transmission at the perforant path synapses; (b) an increase in the excitability of the granule cell population.
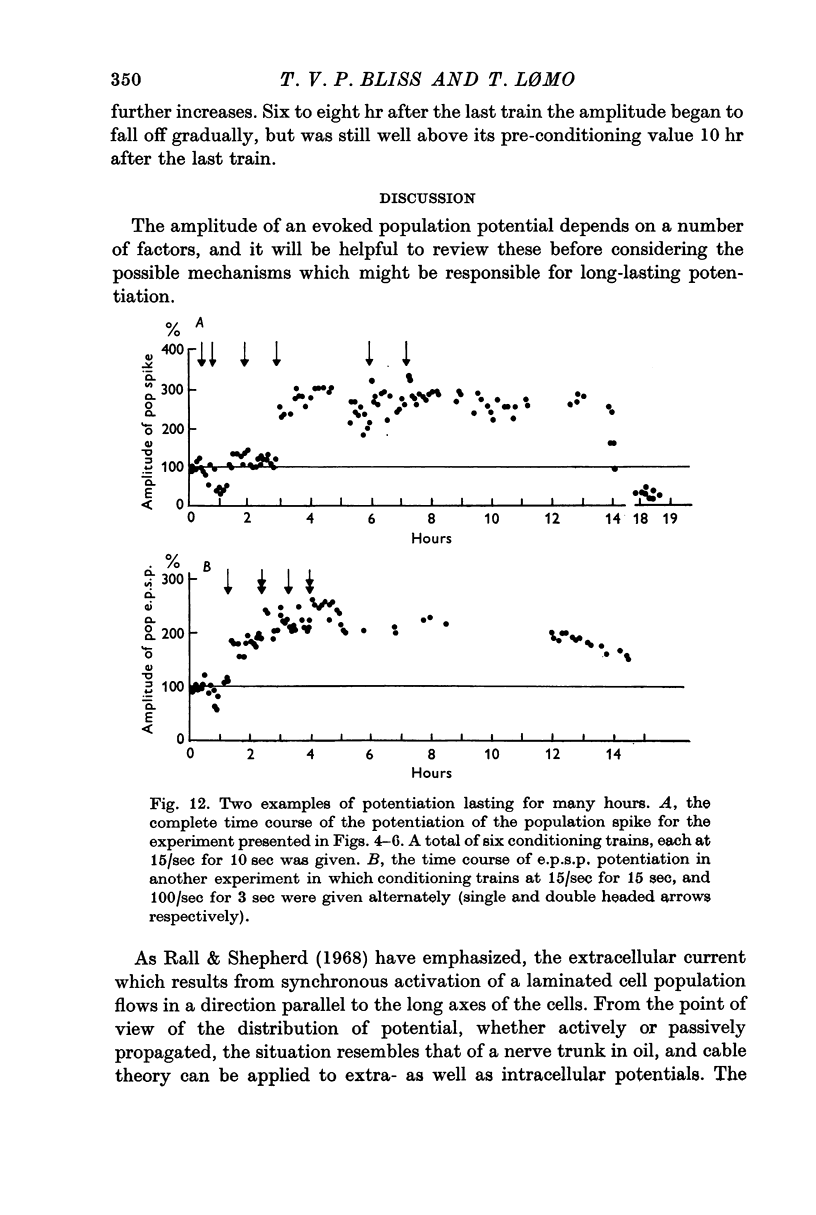
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Bliss T. V., Skrede K. K. Unit analysis of hippocampal polulation spikes. Exp Brain Res. 1971;13(2):208–221. doi: 10.1007/BF00234086. [DOI] [PubMed] [Google Scholar]
- Andersen P., Holmqvist B., Voorhoeve P. E. Entorhinal activation of dentate granule cells. Acta Physiol Scand. 1966 Apr;66(4):448–460. doi: 10.1111/j.1748-1716.1966.tb03223.x. [DOI] [PubMed] [Google Scholar]
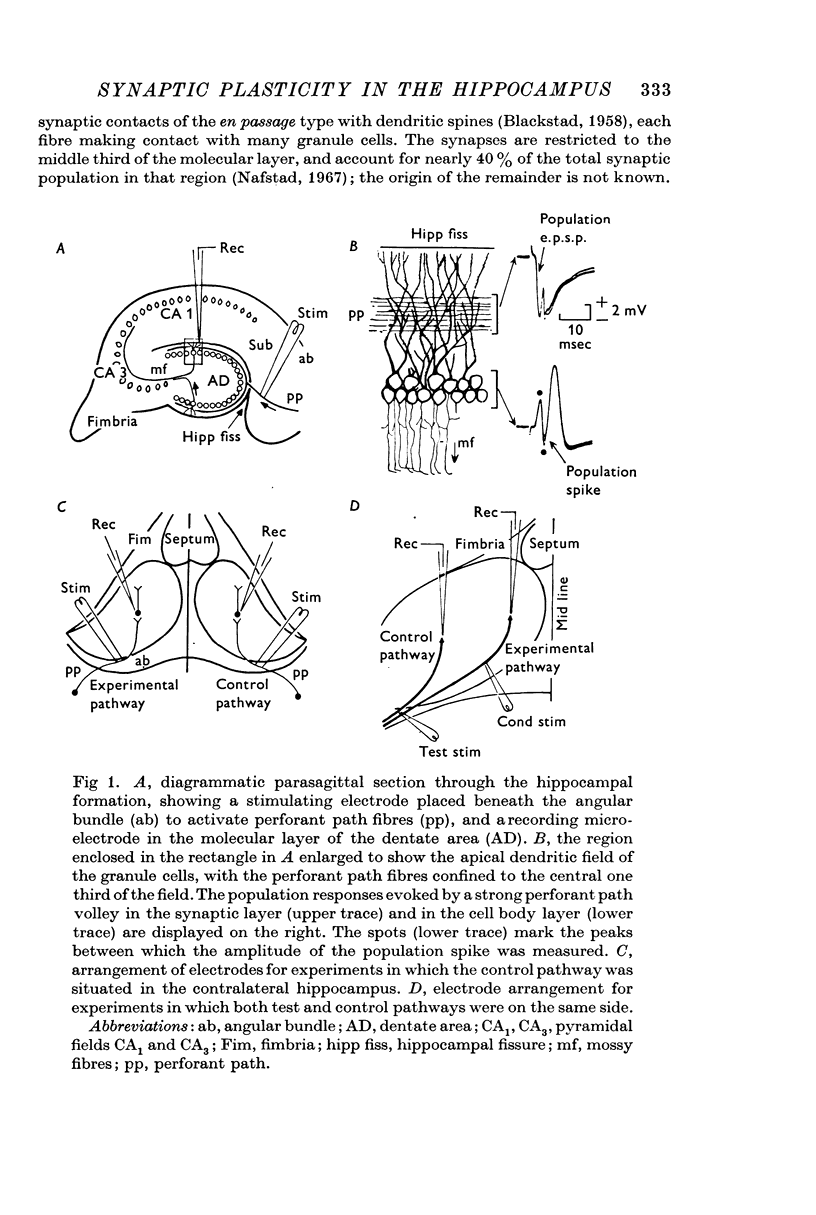
- BLACKSTAD T. W. On the termination of some afferents to the hippocampus and fascia dentata; an experimental study in the rat. Acta Anat (Basel) 1958;35(3):202–214. doi: 10.1159/000141409. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Gardner-Medwin A. R. Long-lasting increases of synaptic influence in the unanesthetized hippocampus. J Physiol. 1971 Jul;216(1):32P–33P. [PubMed] [Google Scholar]
- Bliss T. V., Gardner-Medwin A. R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Plasticity in a monosynaptic cortical pathway. J Physiol. 1970 Apr;207(2):61P–61P. [PubMed] [Google Scholar]
- Douglas R. J. The hippocampus and behavior. Psychol Bull. 1967 Jun;67(6):416–422. doi: 10.1037/h0024599. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. The effect of inhibitory nerve impulses on a crustacean muscle fibre. J Physiol. 1953 Aug;121(2):374–389. doi: 10.1113/jphysiol.1953.sp004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- Gottlieb D. I., Cowan W. M. On the distribution of axonal terminals containing spheroidal and flattened synaptic vesicles in the hippocampus and dentate gyrus of the rat and cat. Z Zellforsch Mikrosk Anat. 1972;129(3):413–429. doi: 10.1007/BF00307297. [DOI] [PubMed] [Google Scholar]
- Hjorth-Simonsen A., Jeune B. Origin and termination of the hippocampal perforant path in the rat studied by silver impregnation. J Comp Neurol. 1972 Feb;144(2):215–232. doi: 10.1002/cne.901440206. [DOI] [PubMed] [Google Scholar]
- Lomo T. Patterns of activation in a monosynaptic cortical pathway: the perforant path input to the dentate area of the hippocampal formation. Exp Brain Res. 1971;12(1):18–45. [PubMed] [Google Scholar]
- Lomo T. Potentiation of monosynaptic EPSPs in the perforant path-dentate granule cell synapse. Exp Brain Res. 1971;12(1):46–63. [PubMed] [Google Scholar]
- Nafstad P. H. An electron microscope study on the termination of the perforant path fibres in the hippocampus and the fascia dentata. Z Zellforsch Mikrosk Anat. 1967;76(4):532–542. doi: 10.1007/BF00339754. [DOI] [PubMed] [Google Scholar]
- Olds J. Learning and the hippocampus. Rev Can Biol. 1972;31(Suppl):215–238. [PubMed] [Google Scholar]
- Rall W., Shepherd G. M. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968 Nov;31(6):884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]


