Abstract
1. The transport of potassium across the distal tubular epithelium was studied in vivo in rats on a normal potassium intake and in rats in which distal tubular potassium secretion was either stimulated by potassium loading or the I.V. administration of a 5% sodium bicarbonate solution or in which potassium secretion was suppressed by dietary deprivation of potassium or sodium.
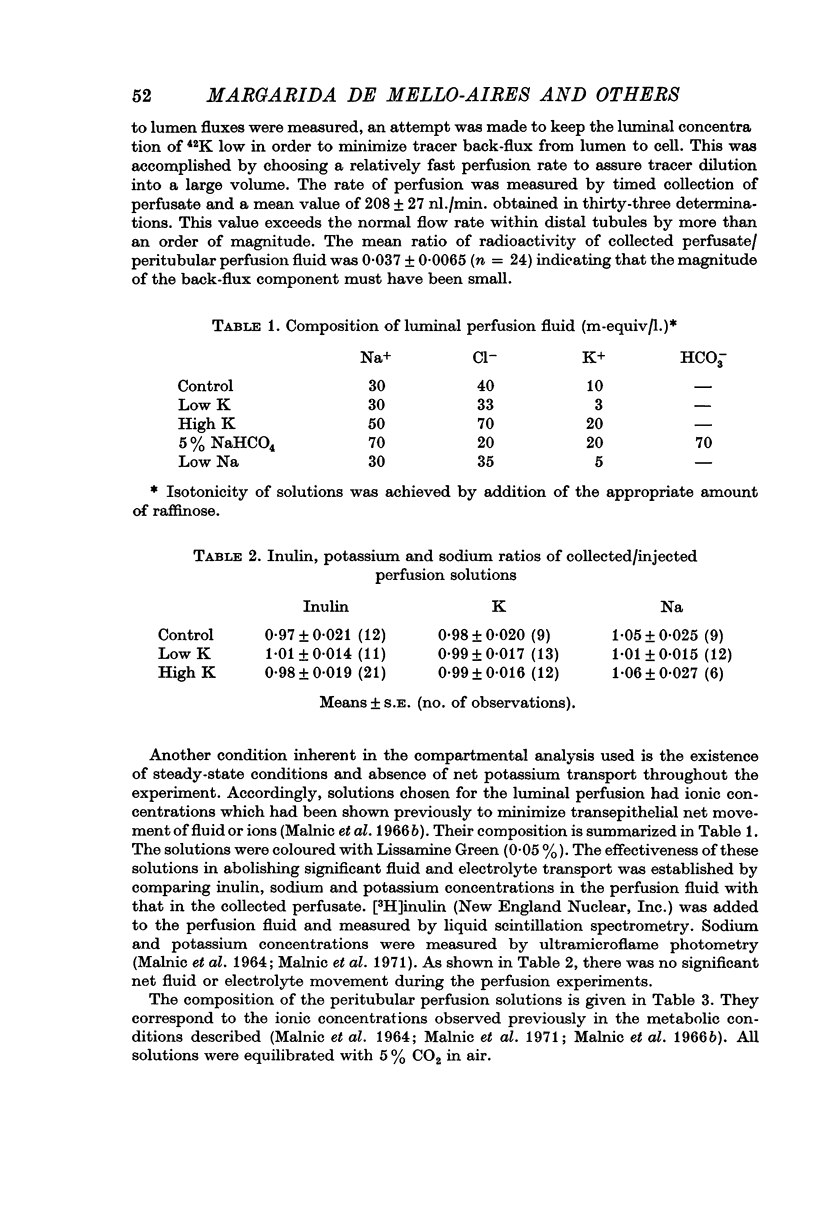
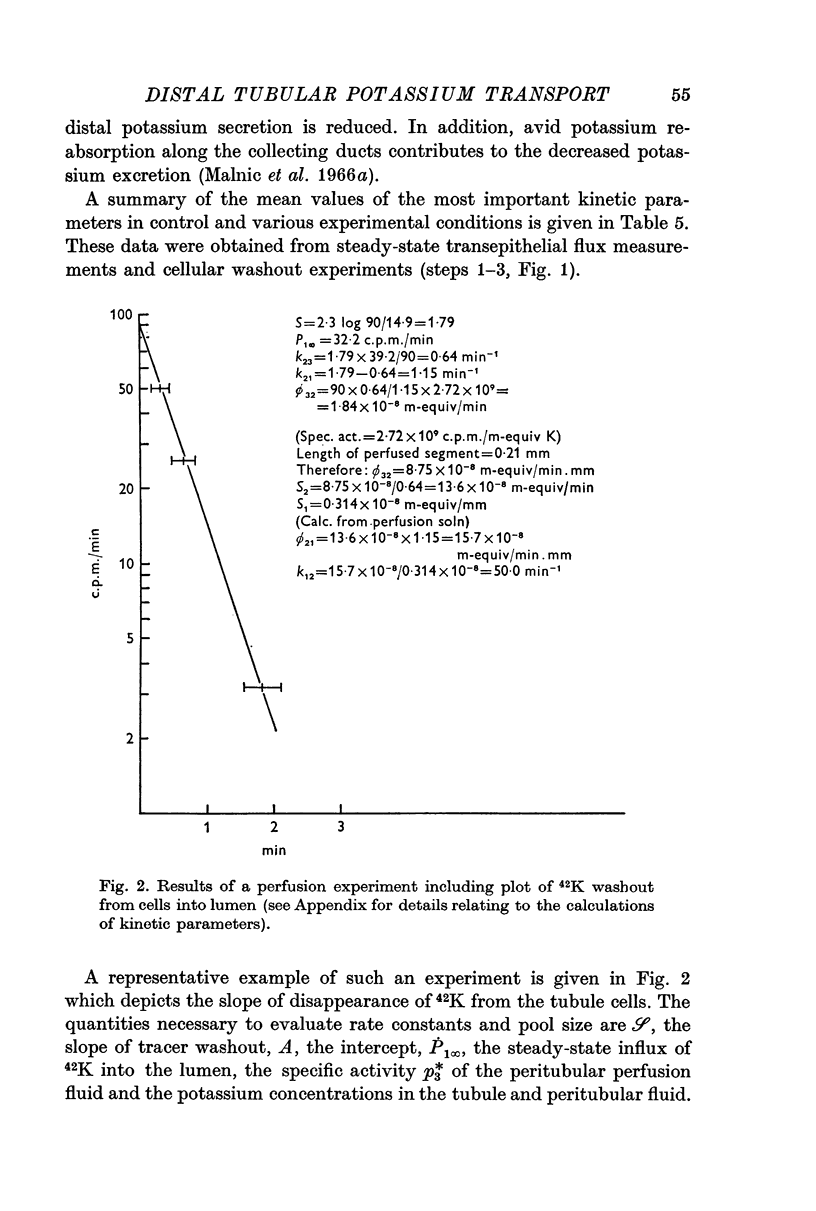
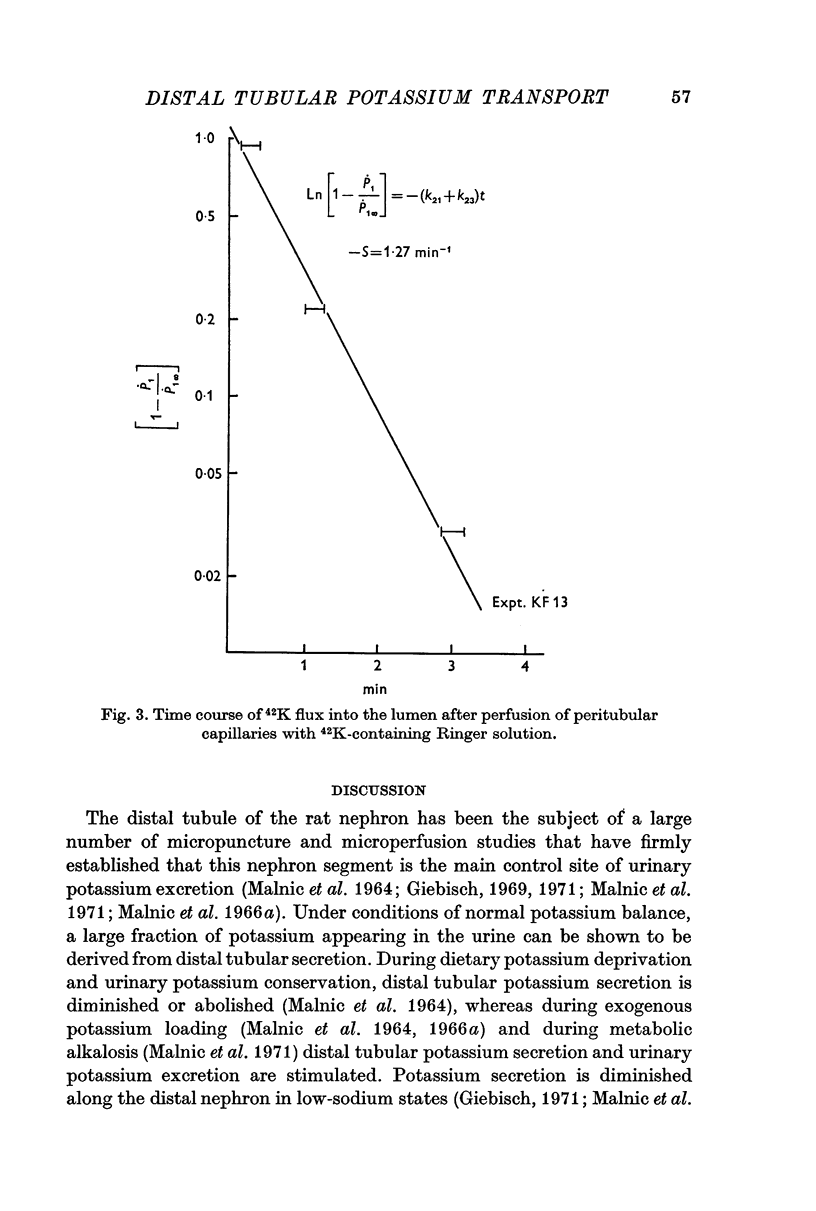
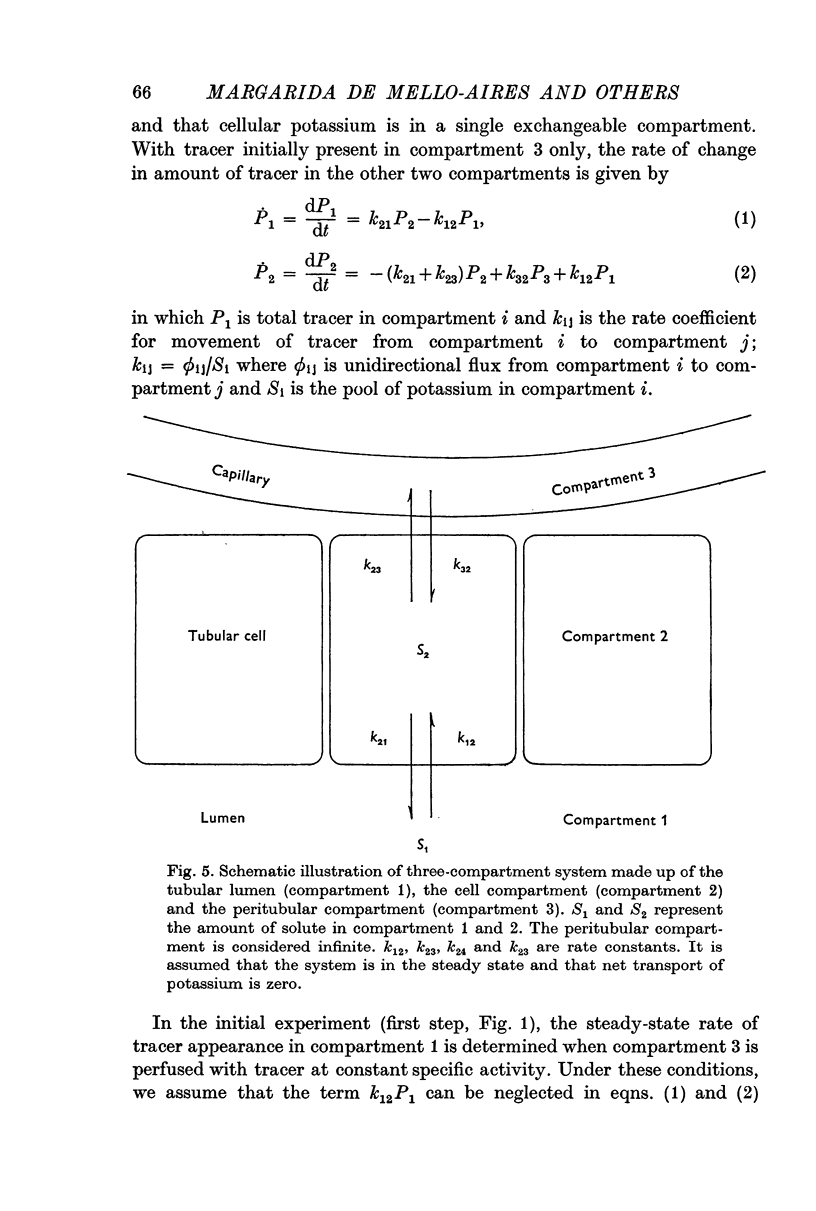
2. 42K was used to measure unidirectional fluxes across the luminal and peritubular cell membranes and to assess the magnitude of cellular potassium partaking in the transport process. This was accomplished by the simultaneous perfusion of the peritubular capillary network with 42K-Ringer and of the distal tubular lumen with initially tracer-free solution. From the steady-state flux and the time course of tracer washout into the lumen after discontinuing the peritubular perfusion, unidirectional fluxes, rate coefficients of ion transfer and cellular transport pools could be measured.
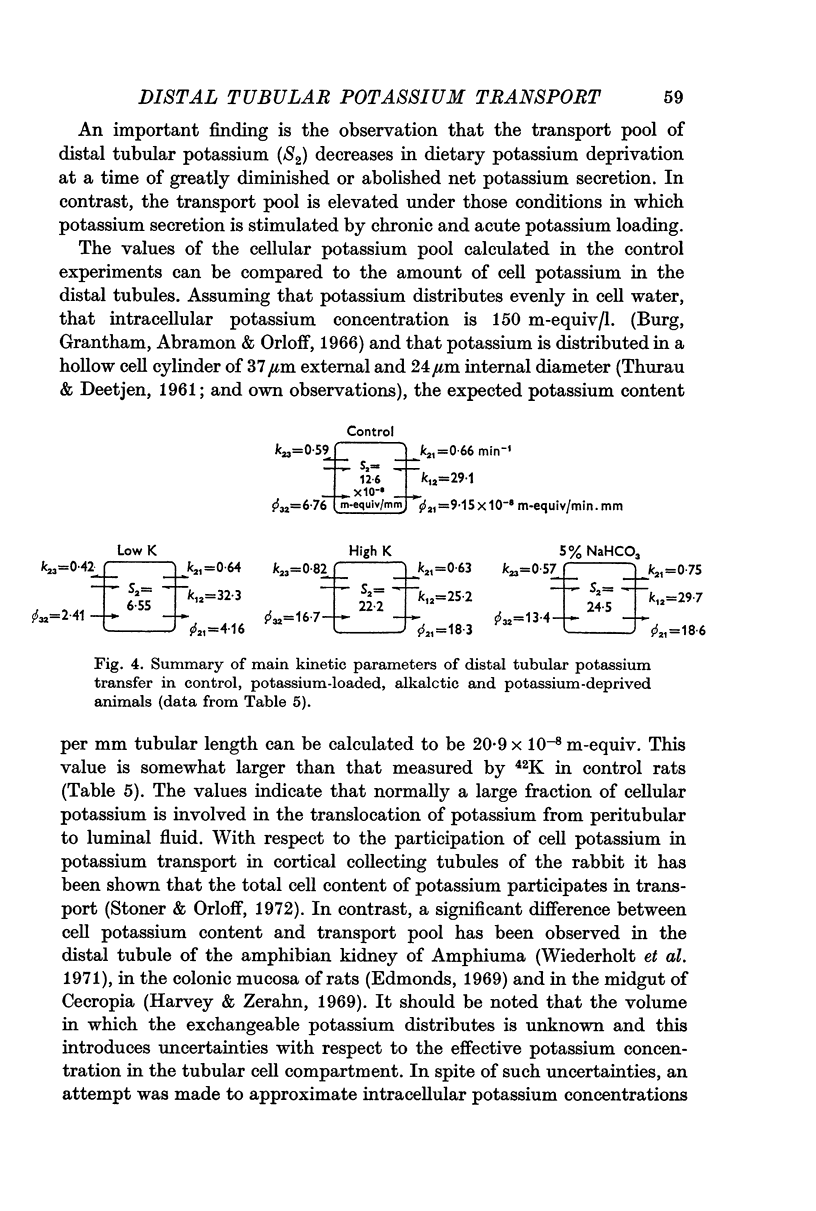
3. Transepithelial movement of potassium involves mixing with a variable cellular potassium transport pool. The latter is significantly elevated in conditions of enhanced distal tubular potassium secretion; cellular potassium labelling is reduced in conditions in which potassium secretion has been suppressed by potassium deprivation.
4. Evidence is presented that changes in the peritubular transport pattern are primarily responsible for modifications of potassium translocation. Thus, stimulation of potassium secretion is associated with increased peritubular potassium uptake; a reduced potassium uptake across the peritubular cell membrane accounts for the fall in potassium secretion in potassium-depleted animals. Whereas passive entry of potassium across the peritubular membrane is augmented in potassium-loaded animals, the induction of metabolic alkalosis by the administration of 5% sodium bicarbonate stimulates active potassium uptake across the peritubular cell membrane. Sodium deprivation stimulates active reabsorptive transfer of potassium from the tubular lumen.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN E. B., Jr, GOOTT B. Intrcellular hydrogen ion changes and potassium movement. Am J Physiol. 1963 May;204:765–770. doi: 10.1152/ajplegacy.1963.204.5.765. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- CONWAY E. J. Nature and significance of concentration relations of potassium and sodium ions in skeletal muscle. Physiol Rev. 1957 Jan;37(1):84–132. doi: 10.1152/physrev.1957.37.1.84. [DOI] [PubMed] [Google Scholar]
- CRABBE J., ROSS E. J., THORN G. W. The significance of the secretion of aldosterone during dietary sodium deprivation in normal subjects. J Clin Endocrinol Metab. 1958 Nov;18(11):1159–1177. doi: 10.1210/jcem-18-11-1159. [DOI] [PubMed] [Google Scholar]
- Duarte C. G., Chomety F., Giebisch G. Effect of amiloride, ouabain, and furosemide on distal tubular function in the rat. Am J Physiol. 1971 Aug;221(2):632–640. doi: 10.1152/ajplegacy.1971.221.2.632. [DOI] [PubMed] [Google Scholar]
- Edmonds C. J. Kinetics of potassium in colonic mucosa of normal and sodium-depleted rats. J Physiol. 1969 Aug;203(3):533–554. doi: 10.1113/jphysiol.1969.sp008878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- GIEBISCH G., BERGER L., PITTS R. F. The extrarenal response to acute acid-base disturbances of respiratory origin. J Clin Invest. 1955 Feb;34(2):231–245. doi: 10.1172/JCI103076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. THE ACTION OF CARDIAC GLYCOSIDES ON ION MOVEMENTS. Pharmacol Rev. 1964 Dec;16:381–407. [PubMed] [Google Scholar]
- Giebisch G. Functional organization of proximal and distal tubular electrolyte transport. Nephron. 1969;6(3):260–281. doi: 10.1159/000179733. [DOI] [PubMed] [Google Scholar]
- Giebisch G., Malnic G., Klose R. M., Windhager E. E. Effect of ionic substitutions on distal potential differences in rat kidney. Am J Physiol. 1966 Sep;211(3):560–568. doi: 10.1152/ajplegacy.1966.211.3.560. [DOI] [PubMed] [Google Scholar]
- Harvey W. R., Zerahn K. Kinetics and route of active K-transport in the isolated midgut of Hyalophora cecropia. J Exp Biol. 1969 Apr;50(2):297–306. doi: 10.1242/jeb.50.2.297. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D. SOME FURTHER OBSERVATIONS ON THE SODIUM EFFLUX IN FROG MUSCLE. J Physiol. 1965 May;178:305–325. doi: 10.1113/jphysiol.1965.sp007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALNIC G., KLOSE R. M., GIEBISCH G. MICROPUNCTURE STUDY OF RENAL POTASSIUM EXCRETION IN THE RAT. Am J Physiol. 1964 Apr;206:674–686. doi: 10.1152/ajplegacy.1964.206.4.674. [DOI] [PubMed] [Google Scholar]
- Malnic G., De Mello Aires M., Giebisch G. Potassium transport across renal distal tubules during acid-base disturbances. Am J Physiol. 1971 Oct;221(4):1192–1208. doi: 10.1152/ajplegacy.1971.221.4.1192. [DOI] [PubMed] [Google Scholar]
- Malnic G., Giebisch G. Some electrical properties of distal tubular epithelium in the rat. Am J Physiol. 1972 Oct;223(4):797–808. doi: 10.1152/ajplegacy.1972.223.4.797. [DOI] [PubMed] [Google Scholar]
- Malnic G., Klose R. M., Giebisch G. Microperfusion study of distal tubular potassium and sodium transfer in rat kidney. Am J Physiol. 1966 Sep;211(3):548–559. doi: 10.1152/ajplegacy.1966.211.3.548. [DOI] [PubMed] [Google Scholar]
- Malnic G., Klose R. M., Giebisch G. Micropuncture study of distal tubular potassium and sodium transport in rat nephron. Am J Physiol. 1966 Sep;211(3):529–547. doi: 10.1152/ajplegacy.1966.211.3.529. [DOI] [PubMed] [Google Scholar]
- McCance R. A. The effect of salt deficiency in man on the volume of the extracellular fluids, and on the composition of sweat, saliva, gastric juice and cerebrospinal fluid. J Physiol. 1938 Mar 14;92(2):208–218. doi: 10.1113/jphysiol.1938.sp003595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWAN R. C., AXELROD D. R., SEIP M., PITTS R. F. Distribution of sodium bicarbonate infused into nephrectomized dogs. J Clin Invest. 1955 Dec;34(12):1795–1801. doi: 10.1172/JCI103234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer A., Windhager E. E. Effect of peritubular oncotic pressure changes on proximal tubular fluid reabsorption. Am J Physiol. 1970 Apr;218(4):1188–1193. doi: 10.1152/ajplegacy.1970.218.4.1188. [DOI] [PubMed] [Google Scholar]
- Sullivan W. J. Electrical potential differences across distal renal tubules of Amphiuma. Am J Physiol. 1968 May;214(5):1096–1103. doi: 10.1152/ajplegacy.1968.214.5.1096. [DOI] [PubMed] [Google Scholar]
- TOUSSAINT C., VEREERSTRAETEN P. Effects of blood pH changes on potassium excretion in the dog. Am J Physiol. 1962 Apr;202:768–772. doi: 10.1152/ajplegacy.1962.202.4.768. [DOI] [PubMed] [Google Scholar]
- USSING H. H., WINDHAGER E. E. NATURE OF SHUNT PATH AND ACTIVE SODIUM TRANSPORT PATH THROUGH FROG SKIN EPITHELIUM. Acta Physiol Scand. 1964 Aug;61:484–504. [PubMed] [Google Scholar]
- WHITTEMBURY G. SODIUM EXTRUSION AND POTASSIUM UPTAKE IN GUINEA PIG KIDNEY CORTEX SLICES. J Gen Physiol. 1965 Mar;48:699–717. doi: 10.1085/jgp.48.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederholt M., Sullivan W. J., Giebisch G. Potassium and sodium transport across single distal tubules of Amphiuma. J Gen Physiol. 1971 May;57(5):495–525. doi: 10.1085/jgp.57.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]


